Abstract
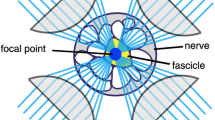
Technologies for peripheral nerve stimulation have conventionally relied on the anatomic placement of electrodes adjacent to subsets of sensory fibres or motor fibres that selectively target an end effector. Here, we demonstrate the use of optogenetics to directly target the innervating fibres of an end effector by relying on retrograde transfection of adeno-associated virus serotype 6 to restrict axonal opsin expression to the desired fibre targets. By using an in vivo screen in rats, we identify the first channelrhodopsins as well as a halorhodopsin that respond to red light in the peripheral nerve. Combining two channelrhodopsins with spectrally distinct activation profiles allowed us to drive opposing muscle activity via two-colour illumination of the same mixed nerve. We also show halorhodopsin-mediated reductions in electrically evoked muscle tremor spectrally optimized for deep peripheral nerves. Our non-invasive peripheral neurostimulator with targeted multi-fascicle resolution enables scientific and clinical exploration, such as motor control in paralysis, biomimetic sensation feedback for amputees and targeted inhibition of muscle tremor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Navarro, X. et al. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 10, 229–258 (2005).
Raspopovic, S. et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 6, 222ra19 (2014).
Boretius, T. et al. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 26, 62–69 (2010).
Musick, K. M., Chew, D. J., Fawcett, J. W. & Lacour, S. P. PDMS microchannel regenerative peripheral nerve interface. In 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER) 649–652 (2013).
Maimon, B. et al. Assessment of nerve regeneration through a novel microchannel array. Int. J. Phys. Med. Rehabil. 4, 332 (2016).
Dyer, K. R. & Duncan, I. D. The intraneural distribution of myelinated fibres in the equine recurrent laryngeal nerve. Brain 110, 1531–1543 (1987).
Stewart, J. D. Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve 28, 525–541 (2003).
Thompson, A. C., Stoddart, P. R. & Jansen, E. D. Optical stimulation of neurons. Curr. Mol. Imaging 3, 162–177 (2014).
Peterson, E. J. & Tyler, D. J. Motor neuron activation in peripheral nerves using infrared neural stimulation. J. Neural Eng. 11, 16001 (2014).
Iyer, S. M. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol. 32, 274–278 (2014).
Llewellyn, M. E., Thompson, K. R., Deisseroth, K. & Delp, S. L. Orderly recruitment of motor units under optical control in vivo. Nat. Med. 16, 1161–1165 (2010).
Towne, C., Montgomery, K. L., Iyer, S. M., Deisseroth, K. & Delp, S. L. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS ONE 8, e72691 (2013).
Lin, J. Y. et al. A user’s guide to channelrhodopsin variants: features, limitations and future developments. Exp. Physiol. 96, 19–25 (2011).
Stujenske, J. M., Spellman, T. & Gordon, J. A. Modeling the spatiotemporal dynamics of light and heat propagation for in vivo optogenetics. Cell. Rep. 12, 525–534 (2015).
Maimon, B. et al. Transdermal optogenetic peripheral nerve stimulation. J. Neural Eng. 14, 034002 (2017).
Lin, J. Y., Knutsen, P. M., Muller, A., Kleinfeld, D. & Tsien, R. Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Fenno, L., Yizhar, O., & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Prigge, M. et al. Color-tuned channelrhodopsins for multiwavelength optogenetics. J. Biol. Chem. 287, 31804–31812 (2012).
Lin, J. Y., Lin, M. Z., Steinbach, P. & Tsien, R. Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Mason, M. R. et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol. Ther. 18, 715–724 (2010).
Hareendran, S. et al. Adeno-associated virus (AAV) vectors in gene therapy: immune challenges and strategies to circumvent them. Rev. Med. Virol. 23, 399–413 (2013).
Mingozzi, F. et al. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122, 23–36 (2013).
Wang, H. et al. High-speed mapping of synaptic connectivity using photostimulation in channelrhodopsin-2 transgenic mice. Proc. Natl Acad. Sci. USA 104, 8143–8148 (2007).
Zepetnek, J. E. T., de Gordon, T., Stein, R. B. & Zung, H. V. Comparison of force and EMG measures in normal and reinnervated tibialis anterior muscles of the rat. Can. J. Physiol. Pharmacol. 69, 1774–1783 (1991).
Schmalbruch, H. Fiber composition of the rat sciatic nerve. Anat. Rec. 215, 71–81 (1986).
Mense, S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl. Int. 105, 214–219 (2008).
Mohan, R., Tosolini, A. P. & Morris, R. Targeting the motor end plates in the mouse hindlimb gives access to a greater number of spinal cord motor neurons: an approach to maximize retrograde transport. Neuroscience 274, 318–330 (2014).
Tozburun, S., Cilip, C. M., Lagoda, G. A., Burnett, A. L. & Fried, N. M. Continuous-wave infrared optical nerve stimulation for potential diagnostic applications. J. Biomed. Opt. 15, 55012 (2010).
Gray, S. J. et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther. 22, 1143–1153 (2011).
Hofherr, A., Fakler, B. & Klöcker, N. Selective Golgi export of Kir2.1 controls the stoichiometry of functional Kir2.x channel heteromers. J. Cell. Sci. 118, 1935–1943 (2005).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Alusi, S. H., Glickman, S., Aziz, T. Z. & Bain, P. G. Tremor in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 66, 131–134 (1999).
O'Suilleabhain, P. E. & Matsumoto, J. Y. Time-frequency analysis of tremors. Brain 121, 2127–2134 (1998).
Harmaline induced tremors. PsychoGenics http://www.psychogenics.com/harmaline.html (accessed 2 June 2017).
McGie, S. C., Zariffa, J., Popovic, M. R. & Nagai, M. K. Short-term neuroplastic effects of brain-controlled and muscle-controlled electrical stimulation. Neuromodulation Technol. Neural Interface 18, 233–240 (2015).
Arcourt, A. et al. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 93, 179–193 (2017).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Chang, R. B., Strochlic, D. E., Williams, E. K., Umans, B. D. & Liberles, S. D. Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633 (2015).
Bashkatov, A. N. et al. In vivo and in vitro study of control of rat skin optical properties by acting of osmotical liquid. Biomed. Photon. Optoelectronic Imaging 4224, 300–311 (2000).
Bashkatov, A. N., Genina, E. A. & Tuchin, V. V. Optical properties of skin, subcutaneous, and muscle tissues: a review. J. Innov. Opt. Health Sci. 4, 9–38 (2011).
Islam, M. S. et al. Extracting structural features of rat sciatic nerve using polarization-sensitive spectral domain optical coherence tomography. J. Biomed. Opt. 17, 56012 (2012).
Hendriks, B. H. W. et al. Nerve detection with optical spectroscopy for regional anesthesia procedures. J. Transl. Med. 13, 380 (2015).
Yaroslavsky, A. N. et al. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 47, 2059 (2002).
Pennes, H. H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1, 93–122 (1948).
Ward, S. R. & Lieber, R. L. Density and hydration of fresh and fixed human skeletal muscle. J. Biomech. 38, 2317–2320 (2005).
Hasgall, P. et al. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues Version 3.0 (IT’IS Foundation, 2015); https://doi.org/10.13099/VIP21000-03-0
Fagher, B. & Monti, M. Thermogenic effect of two β-adrenoceptor blocking drugs, propranolol and carvedilol, on skeletal muscle in rats. A microcalorimetric study. Thermochim. Acta 251, 183–189 (1995).
Allen, J. T., Allen, J. T. & Bowman, H. F. The simultaneous measurement of thermal conductivity, thermal diffusivity, and perfusion in small volumes of tissue. J. Biomech. Eng. 106, 192 (1984).
Rins, M., Diez, I., Calpena, A. C., & Obach, R. Skin density in the hairless rat. Evidence of regional differences. Eur. J. Drug. Metab. Pharmacokinet. Spec. No. 3, 456–457 (1991).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Acknowledgements
We thank C. Towne for his assistance in crafting the motivation for this project. We thank E. Boyden for his assistance and consultation on this project. Furthermore, we thank K. Cormier, C. Condon, M. Brown, as well as the entire Koch Histology Core for their countless hours of painstaking histology efforts. In addition, we thank N. Klapoetke, O. Shemesh, K. Piatkevich, E. Revol, P. Calvaresi, C. Varela, A. Harding, M. Fahmi, C. Lee, H.-J. Suk, D. Park, M. Diaz, A. Schneider and S. Osseiran for their advice, guidance and assistance. This work was funded by the MIT Media Lab Consortium.
Author information
Authors and Affiliations
Contributions
B.E.M. and H.M.H. contributed to idea conception, study design and data analysis. B.E.M. oversaw experiment conduction and data analysis for in vivo experiments including histology and fluence modelling. K.S. contributed to experiment conduction, data collection and histology processing. S.S. performed in vitro screen experiments along with corresponding data processing. A.N.Z. contributed to idea conception and experiment planning as well as assisted with Monte Carlo modelling. B.E.M. wrote the manuscript with all authors contributing to editing the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary figures.
Supplementary Video 1
Two-colour independent peripheral nerve stimulation (transdermal and exposed nerves), as well as high-frequency CsChrimson activation and JAWS tremor inhibition.
Rights and permissions
About this article
Cite this article
Maimon, B.E., Sparks, K., Srinivasan, S. et al. Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation. Nat Biomed Eng 2, 485–496 (2018). https://doi.org/10.1038/s41551-018-0255-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0255-5
This article is cited by
-
Towards translational optogenetics
Nature Biomedical Engineering (2022)
-
Colocalized, bidirectional optogenetic modulations in freely behaving mice with a wireless dual-color optoelectronic probe
Nature Communications (2022)
-
Bioresorbable thin-film silicon diodes for the optoelectronic excitation and inhibition of neural activities
Nature Biomedical Engineering (2022)
-
Near-infrared manipulation of multiple neuronal populations via trichromatic upconversion
Nature Communications (2021)
-
Optical vagus nerve modulation of heart and respiration via heart-injected retrograde AAV
Scientific Reports (2021)