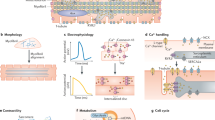
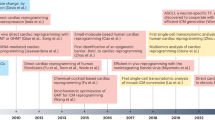
Abstract
Human pluripotent stem cells (hPSCs), in particular embryonic stem cells and induced pluripotent stem cells, have received enormous attention in cardiovascular regenerative medicine owing to their ability to expand and differentiate into functional cardiomyocytes and other cardiovascular cell types. Despite the potential applications of hPSCs for tissue regeneration in patients suffering from cardiovascular disease, whether hPSC-based therapies can be safe and efficacious remains inconclusive, with strong evidence from clinical trials lacking. Critical factors limiting therapeutic efficacy are the degree of maturity and purity of the hPSC-derived differentiated progeny, and the tumorigenic risk associated with residual undifferentiated cells. In this Review, we discuss recent advances in cardiac-cell differentiation from hPSCs and in the direct reprogramming of non-myocyte cells for cardiovascular regenerative applications. We also discuss approaches for the delivery of cells to diseased tissue, and how such advances are contributing to progress in cardiac tissue engineering for tackling heart disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Zhao, M. T. et al. Molecular and functional resemblance of differentiated cells derived from isogenic human iPSCs and SCNT-derived ESCs. Proc. Natl Acad. Sci. USA 114, E11111–E11120 (2017).
Neofytou, E., O’Brien, C. G., Couture, L. A. & Wu, J. C. Hurdles to clinical translation of human induced pluripotent stem cells. J. Clin. Invest. 125, 2551–2557 (2015).
Burridge, P. W., Sharma, A. & Wu, J. C. Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annu. Rev. Genet. 49, 461–484 (2015).
Kawamura, M. et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–S37 (2012).
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012).
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Ong, S. G. et al. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation 132, 762–771 (2015).
Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016).
Zwi, L. et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 120, 1513–1523 (2009).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Burridge, P. W. et al. Chemically defined culture and cardiomyocytes differentiation of human pluripotent stem cells. Current Protoc. Human Genet. 15, 21–23 (2015).
Gepstein, L. et al. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells 28, 2151–2161 (2010).
Kuppusamy, K. T. et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc. Natl Acad. Sci. USA 112, E2785–E2794 (2015).
Wen, J. Y. et al. Maturation-based model of arrhythmogenic right ventricular dysplasia using patient-specific induced pluripotent stem cells. Circ. J. 79, 1402–1408 (2015).
Kadota, S., Pabon, L., Reinecke, H. & Murry, C. E. In vivo maturation of human induced pluripotent stem cell-derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Rep. 8, 278–289 (2017).
Cho, G. S., Tampakakis, E., Andersen, P. & Kwon, C. Use of a neonatal rat system as a bioincubator to generate adult-like mature cardiomyocytes from human and mouse pluripotent stem cells. Nat. Protoc. 12, 2097–2109 (2017).
Ribeiro, A. J. et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA 112, 12705–12710 (2015).
Boothe, S. D. et al. The effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem. Biophys. 74, 527–535 (2016).
Dvir, T. et al. Nanowired three-dimensional cardiac patches. Nat. Nanotech. 6, 720–725 (2011).
Tiburcy, M. et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135, 1832–1847 (2017).
Sayed, N., Liu, C. & Wu, J. C. Translation of human induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol. 67, 2161–2176 (2016).
Riegler, J., Gillich, A., Shen, Q., Gold, J. D. & Wu, J. C. Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function. Circulation 130, S77–S86 (2014).
Levenberg, S., Golub, J. S., Amit, M., Itskovitz-Eldor, J. & Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 4391–4396 (2002).
James, D. et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFβ inhibition is Id1 dependent. Nat. Biotechnol. 28, 161–166 (2010).
Nourse, M. B. et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler. Thromb. Vasc. Biol. 30, 80–89 (2010).
Lu, S. J. et al. Robust generation of hemangioblastic progenitors from human embryonic stem cells. Regen. Med. 3, 693–704 (2008).
Samuel, R. et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 110, 12774–12779 (2013).
Kusuma, S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl Acad. Sci. USA 110, 12601–12606 (2013).
Song, W., Kaufman, D. S. & Shen, W. Efficient generation of endothelial cells from human pluripotent stem cells and characterization of their functional properties. J. Biomed. Mater. Res. A 104, 678–687 (2016).
Kane, N. M. et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 30, 1389–1397 (2010).
Lian, X. et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 3, 804–816 (2014).
Zhang, J. et al. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc. Natl Acad. Sci. USA 114, E6072–E6078 (2017).
Rufaihah, A. J. et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 31, e72–e79 (2011).
Li, Z. et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE 4, e8443 (2009).
Masumoto, H. et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 4, 6716 (2014).
Ye, L. et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761 (2014).
Rufaihah, A. J. et al. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am. J. Transl. Res. 5, 21–35 (2013).
Li, R. K., Jia, Z. Q., Weisel, R. D., Merante, F. & Mickle, D. A. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J. Mol. Cell. Cardiol. 31, 513–522 (1999).
Cheung, C. & Sinha, S. Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. J. Mol. Cell. Cardiol. 51, 651–664 (2011).
Ayoubi, S., Sheikh, S. P. & Eskildsen, T. V. Human induced pluripotent stem cell-derived vascular smooth muscle cells: differentiation and therapeutic potential. Cardiovasc. Res. 113, 1282–1293 (2017).
Mauritz, C. et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur. Heart J. 32, 2634–2641 (2011).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Skelton, R. J. et al. CD13 and ROR2 permit isolation of highly enriched cardiac mesoderm from differentiating human embryonic stem cells. Stem Cell Rep. 6, 95–108 (2016).
Menasche, P. et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. Eur. Heart J. 36, 743–750 (2015).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Friedrich, E. B., Walenta, K., Scharlau, J., Nickenig, G. & Werner, N. CD34–/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ. Res. 98, e20–e25 (2006).
Patel, J. et al. Functional definition of progenitors versus mature endothelial cells reveals key SoxF-dependent differentiation process. Circulation 135, 786–805 (2017).
Feng, Q. et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28, 704–712 (2010).
Cimato, T. et al. Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression. Circulation 119, 2170–2178 (2009).
Prasain, N. et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 32, 1151–1157 (2014).
Bao, X. et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat. Biomed. Eng. 1, 0003 (2016).
Bao, X. et al. Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions. Nat. Protoc. 12, 1890–1900 (2017).
Zhao, J. et al. Efficient differentiation of TBX18+/WT1+ epicardial-like cells from human pluripotent stem cells using small molecular compounds. Stem Cells Dev. 26, 528–540 (2017).
Cano, E. et al. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proc. Natl Acad. Sci. USA 113, 656–661 (2016).
Wei, K. et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485 (2015).
Huang, G. N. et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science 338, 1599–1603 (2012).
Ramjee, V. et al. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J. Clin. Invest. 127, 899–911 (2017).
Hatzistergos, K. E. & Vedenko, A. Cardiac cell therapy 3.0: the beginning of the end or the end of the beginning? Circ. Res. 121, 95–97 (2017).
Fernandes, S. et al. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Rep. 5, 753–762 (2015).
Gao, L. et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ. Res. 120, 1318–1325 (2017).
Ebert, A. D., Diecke, S., Chen, I. Y. & Wu, J. C. Reprogramming and transdifferentiation for cardiovascular development and regenerative medicine: where do we stand? EMBO Mol. Med. 7, 1090–1103 (2015).
Ebrahimi, B. In vivo reprogramming for heart regeneration: a glance at efficiency, environmental impacts, challenges and future directions. J. Mol. Cell. Cardiol. 108, 61–72 (2017).
Nam, Y. J. et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl Acad. Sci. USA 110, 5588–5593 (2013).
Cao, N. et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 352, 1216–1220 (2016).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Miyamoto, K. et al. Direct in vivo reprogramming with Sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell 22, 91–103.e5 (2018).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Chen, J. X. et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 111, 50–55 (2012).
Lalit, P. A. et al. Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 18, 354–367 (2016).
Zhang, Y. et al. Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 18, 368–381 (2016).
Lee, S. et al. Direct reprogramming of human dermal fibroblasts into endothelial cells using ER71/ETV2. Circ. Res. 120, 848–861 (2017).
Sayed, N. et al. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation 131, 300–309 (2015).
Shadrin, I. Y. et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8, 1825 (2017).
Jackman, C. P. et al. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159, 48–58 (2018).
Weinberger, F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 8, 363ra148 (2016).
Abilez, O. J. & Wu, J. C. Stem cell reprogramming: a 3D boost. Nat. Mater. 15, 259–261 (2016).
Abilez, O. J. et al. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells 36, 265–277 (2017).
Ruvinov, E. & Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv. Drug Deliv. Rev. 96, 54–76 (2016).
Orr, S. et al. TGF-β affinity-bound to a macroporous alginate scaffold generates local and peripheral immunotolerant responses and improves allocell transplantation. Acta Biomater. 45, 196–209 (2016).
Zhang, D. et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813–5820 (2013).
Menasche, P. et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 71, 429–438 (2018).
Johnson, T. D. & Christman, K. L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 10, 59–72 (2013).
Liu, C. et al. Modeling human disease with induced pluripotent stem cells: from 2D to 3D and beyond development. Development 145, 156–166 (2018).
Ong, C. S. et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 7, 4566 (2017).
Adamiak, M. et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 122, 296–309 (2018).
Shi, Y., Inoue, H., Wu, J. C. & Yamanaka, S. Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 16, 115–130 (2017).
Nguyen, P. K., Neofytou, E., Rhee, J. W. & Wu, J. C. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol. 1, 953–962 (2016).
Qin, X. et al. Photoacoustic imaging of embryonic stem cell-derived cardiomyocytes in living hearts with ultrasensitive semiconducting polymer nanoparticles. Adv. Funct. Mater. 28, 1704939 (2018).
Zhao, X. et al. Comparison of non-human primate versus human induced pluripotent stem cell-derived cardiomyocytes for treatment of myocardial infarction. Stem Cell Rep. 10, 422–435 (2018).
Acknowledgements
This work was supported in part by research grants from the National Institutes of Health R01 HL126527, R01 HL133272 and R24 HL117756, American Heart Association 17MERIT33610009 (to J.C.W.), and iHeart Research Dorothy Dee & Marjorie Helene Boring Trust Award (to A.Z.).
Author information
Authors and Affiliations
Contributions
H.C. and J.C.W. conceptualized the outline and contents of the article. H.C. and A.Z. participated in the researching and writing for the article, and J.C.W. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, A. & Wu, J.C. Harnessing cell pluripotency for cardiovascular regenerative medicine. Nat Biomed Eng 2, 392–398 (2018). https://doi.org/10.1038/s41551-018-0244-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0244-8
This article is cited by
-
Current Review of Regenerative Medicine Therapies for Spine-Related Pain
Current Pain and Headache Reports (2023)
-
Cellular and molecular landscape of mammalian sinoatrial node revealed by single-cell RNA sequencing
Nature Communications (2021)
-
Intensive care for human hearts in pluripotent stem cell models
npj Regenerative Medicine (2020)