Abstract
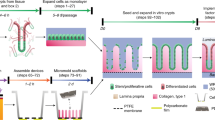
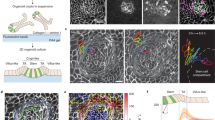
The natural ability of stem cells to self-organize into functional tissue has been harnessed for the production of functional human intestinal organoids. Although dynamic mechanical forces play a central role in intestinal development and morphogenesis, conventional methods for the generation of intestinal organoids have relied solely on biological factors. Here, we show that the incorporation of uniaxial strain, using compressed nitinol springs, in human intestinal organoids transplanted into the mesentery of mice induces growth and maturation of the organoids. Assessment of morphometric parameters, transcriptome profiling and functional assays of the strain-exposed tissue revealed higher similarities to native human intestine, with regard to tissue size and complexity, and muscle tone. Our findings suggest that the incorporation of physiologically relevant mechanical cues during the development of human intestinal tissue enhances its maturation and enterogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wells, J. M. & Spence, J. R. How to make an intestine. Development 141, 752–760 (2014).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
McCracken, K. W., Howell, J. C., Wells, J. M. & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 (2011).
Watson, C. L. et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 20, 1310–1314 (2014).
Workman, M. J. et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 23, 49–59 (2017).
Finkbeiner, S. R. et al. Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivoStem Cell Reports 4, 1140–1155 (2015).
Dedhia, P. H., Bertaux-Skeirik, N., Zavros, Y. & Spence, J. R. Organoid models of human gastrointestinal development and disease. Gastroenterology 150, 1098–1112 (2016).
Yu, H. et al. The contributions of human mini-intestines to the study of intestinal physiology and pathophysiology. Annu. Rev. Physiol. 79, 291–312 (2017).
Nelson, C. M. On buckling morphogenesis. J. Biomech. Eng. 138, 021005 (2016).
Kurpios, N. A. et al. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc. Natl Acad. Sci. USA 105, 8499–8506 (2008).
Shyer, A. E. et al. Villification: how the gut gets its villi. Science 342, 212–218 (2013).
Shyer, A. E., Huycke, T. R., Lee, C., Mahadevan, L. & Tabin, C. J. Bending gradients: how the intestinal stem cell gets its home. Cell 161, 569–580 (2015).
Savin, T. et al. On the growth and form of the gut. Nature 476, 57–62 (2011).
Stark, R., Panduranga, M., Carman, G. & Dunn, J. C. Development of an endoluminal intestinal lengthening capsule. J. Pediatr. Surg. 47, 136–141 (2012).
Rouch, J. D. et al. Scalability of an endoluminal spring for distraction enterogenesis. J. Pediatr. Surg. 51, 1988–1992 (2016).
Demehri, F. R. et al. Development of an endoluminal intestinal attachment for a clinically applicable distraction enterogenesis device. J. Pediatr. Surg. 51, 101–106 (2016).
Demehri, F. R., Freeman, J. J., Fukatsu, Y., Luntz, J. & Teitelbaum, D. H. Development of an endoluminal intestinal lengthening device using a geometric intestinal attachment approach. Surgery 158, 802–811 (2015).
Luntz, J., Brei, D., Teitelbaum, D. & Spencer, A. Mechanical extension implants for short-bowel syndrome. Proc. SPIE Int. Soc. Opt. Eng. 6173, 617309 (2006).
Allard, J. et al. Immunohistochemical toolkit for tracking and quantifying xenotransplanted human stem cells. Regen. Med. 9, 437–452 (2014).
Shekherdimian, S., Panduranga, M. K., Carman, G. P. & Dunn, J. C. The feasibility of using an endoluminal device for intestinal lengthening. J. Pediatr. Surg. 45, 1575–1580 (2010).
Marsh, M. N. & Swift, J. A. A study of the small intestinal mucosa using the scanning electron microscope. Gut 10, 940–949 (1969).
Hooton, D., Lentle, R., Monro, J., Wickham, M. & Simpson, R. The secretion and action of brush border enzymes in the mammalian small intestine. Rev. Physiol. Biochem. Pharmacol. 168, 59–118 (2015).
Clarke, L. L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1151–G1166 (2009).
Gomez-Pinilla, P. J. et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370–G1381 (2009).
Mammoto, A., Mammoto, T. & Ingber, D. E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 125, 3061–3073 (2012).
Burn, S. F. & Hill, R. E. Left–right asymmetry in gut development: what happens next? Bioessays 31, 1026–1037 (2009).
Sherwood, R. I., Chen, T. Y. & Melton, D. A. Transcriptional dynamics of endodermal organ formation. Dev. Dyn. 238, 29–42 (2009).
Zorn, A. M. & Wells, J. M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 (2009).
Spence, J. R., Lauf, R. & Shroyer, N. F. Vertebrate intestinal endoderm development. Dev. Dyn. 240, 501–520 (2011).
Shahbazi, M. N. et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 18, 700–708 (2016).
Soffers, J. H. M., Hikspoors, J. P. J. M., Mekonen, H. K., Koehler, S. E. & Lamers, W. H.The growth pattern of the human intestine and its mesentery. BMC Dev. Biol. 15, 31 (2015).
Tran, K., Brun, R. & Kuo, B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Ther. Adv. Gastroenterol. 5, 249–260 (2012).
Engmann, J. & Burbidge, A. S. Fluid mechanics of eating, swallowing and digestion—overview and perspectives. Food Funct. 4, 443–447 (2013).
Wieck, M. M. et al. Prolonged absence of mechanoluminal stimulation in human intestine alters the transcriptome and intestinal stem cell niche. Cell Mol. Gastroenterol. Hepatol. 3, 367.e1–388.e1 (2017).
Terry, B. S., Lyle, A. B., Schoen, J. A. & Rentschler, M. E. Preliminary mechanical characterization of the small bowel for in vivo robotic mobility. J. Biomech. Eng. 133, 091010 (2011).
Gregersen, H., Kassab, G. S. & Fung, Y. C. The zero-stress state of the gastrointestinal tract: biomechanical and functional implications. Dig. Dis. Sci. 45, 2271–2281 (2000).
Low, L. A. & Tagle, D. A.Organs-on-chips: progress, challenges, and future directions. Exp. Biol. Med. (Maywood) 242, 1573–1578 (2017).
Christoffersson, J., van Noort, D. V. & Mandenius, C. F.Developing organ-on-a-chip concepts using bio-mechatronic design methodology. Biofabrication 9, 025023 (2017).
Sato, T. & Clevers, H.SnapShot: growing organoids from stem cells. Cell 161, 1700.e1 (2015).
McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404 (2014).
Grapin-Botton, A. Three-dimensional pancreas organogenesis models. Diabetes Obes. Metab. 18, 33–40 (2016).
Mahe, M. M., Brown, N. E., Poling, H. M. & Helmrath, M. A. In vivo model of small intestine. Methods Mol. Biol. 1597, 229–245 (2017).
Giles, D. A. et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 23, 829–838 (2017).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Roberts, A., Pimentel, H., Trapnell, C. & Pachter, L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 27, 2325–2329 (2011).
Roberts, A., Trapnell, C., Donaghey, J., Rinn, J. L. & Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 12, R22 (2011).
Hsu, F. et al. The UCSC known genes. Bioinformatics 22, 1036–1046 (2006).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 15, 550 (2014).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Marini, F. pcaExplorer: Interactive Visualization of RNA-Seq Data Using a Principal Components Approach R package version 2.3.0. (2017).
Acknowledgements
The authors thank G. L. Keller and Veterinary Services staff for support in completing the animal work, J. M. Kofron for kind assistance with the IVIS Spectrum microCT imaging and B. Donnelly for assistance with the protein work. This work was funded in part by NIH grants P30 DK078392 (DHC Pilot and Feasibility Award to M.M.M.; DNA sequencing and iPSCs core facilities) and NIH NIDKK grant 1K99DK110414-02 (to M.M.M.), as well as an Athena Blackburn Research Scholar Award in neuroenteric diseases from the American Gastroenterology Association (to M.M.M.) and a ‘New Team’ grant (BOGUS to M.M.M.) from the Bioregate Regenerative Medicine Cluster, University of Nantes and Pays de la Loire Region. The Dunn, Helmrath and Wells laboratories are members of the Intestinal Stem Cell Consortium, supported by NIDDK and NIAID (U01DK103117 to M.A.H.). This research was also supported in part by the Cincinnati Children’s Research Foundation and the Cincinnati Biobank, as well as the Better Outcomes for Children Biorepository.
Author information
Authors and Affiliations
Contributions
H.M.P. and M.M.M. conceived and designed the study. H.M.P., D.W., T.A.H., M.A.H. and M.M.M. performed the experiments and collected the data. N.B. generated the HIOs. N.H. and J.C.Y.D. manufactured the springs. H.M.P., D.W., S.C. and M.M.M. analysed the data. H.M.P., M.B., S.P.H., J.M.W., M.A.H. and M.M.M. interpreted the experimental findings. H.M.P. and M.M.M. prepared the figures and drafted the manuscript. H.M.P., S.P.H., J.M.W., M.A.H. and M.M.M. made critical revisions to the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures and tables.
Rights and permissions
About this article
Cite this article
Poling, H.M., Wu, D., Brown, N. et al. Mechanically induced development and maturation of human intestinal organoids in vivo. Nat Biomed Eng 2, 429–442 (2018). https://doi.org/10.1038/s41551-018-0243-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0243-9
This article is cited by
-
Middle-out methods for spatiotemporal tissue engineering of organoids
Nature Reviews Bioengineering (2023)
-
Mechanically enhanced biogenesis of gut spheroids with instability-driven morphomechanics
Nature Communications (2023)
-
In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice
Nature Biotechnology (2023)
-
Multi-disciplinary Insights from the First European Forum on Visceral Myopathy 2022 Meeting
Digestive Diseases and Sciences (2023)
-
Mechanical stretching boosts expansion and regeneration of intestinal organoids through fueling stem cell self-renewal
Cell Regeneration (2022)