Abstract
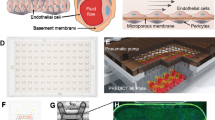
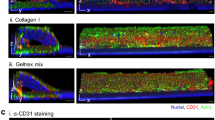
Alterations in the mechanical properties of erythrocytes occurring in inflammatory and haematological disorders such as sickle-cell disease (SCD) and malaria often lead to increased endothelial permeability, haemolysis and microvascular obstruction. However, the associations among these pathological phenomena remain unknown. Here, we show that a perfusable, endothelialized microvasculature-on-a-chip featuring an interpenetrating-polymer-network hydrogel that recapitulates the stiffness of blood vessel intima, basement membrane self-deposition and self-healing endothelial barrier function for longer than one month enables the real-time visualization, with high spatiotemporal resolution, of microvascular obstruction and endothelial permeability under physiological flow conditions. We found that extracellular haem—a haemolytic by-product—induces delayed yet reversible endothelial permeability in a dose-dependent manner, and demonstrate that endothelial interactions with SCD or malaria-infected erythrocytes cause reversible microchannel occlusion and increased in situ endothelial permeability. The microvasculature-on-a-chip enables mechanistic insight into the endothelial barrier dysfunction associated with SCD, malaria and other inflammatory and haematological diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Deanfield, J. E., Halcox, J. P. & Rabelink, T. J. Endothelial function and dysfunction—testing and clinical relevance. Circulation 115, 1285–1295 (2007).
Mehta, D. & Malik, A. B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86, 279–367 (2006).
Buffet, P. A. et al. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117, 381–392 (2011).
Faille, D. et al. Platelet–endothelial cell interactions in cerebral malaria: the end of a cordial understanding. Thromb. Haemost. 102, 1093–1102 (2009).
Ghosh, S., Tan, F. & Ofori-Acquah, S. F. Spatiotemporal dysfunction of the vascular permeability barrier in transgenic mice with sickle cell disease. Anemia 2012, 582018 (2012).
Hebbel, R. P., Osarogiagbon, R. & Kaul, D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation 11, 129–151 (2004).
Frevert, U. & Nacer, A. Fatal cerebral malaria: a venous efflux problem. Front. Cell. Infect. Microbiol. 4, 155 (2014).
Ghosh, S. et al. Nonhematopoietic Nrf2 dominantly impedes adult progression of sickle cell anemia in mice. JCI Insight 1, e81090 (2016).
Manwani, D. & Frenette, P. S. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 122, 3892–3898 (2013).
Storm, J. & Craig, A. G. Pathogenesis of cerebral malaria—inflammation and cytoadherence. Front. Cell. Infect. Microbiol. 4, 100 (2014).
Vercellotti, G. M. & Belcher, J. D. Not simply misshapen red cells: multimolecular and cellular events in sickle vaso-occlusion. J. Clin. Invest. 124, 1462–1465 (2014).
Zhang, B. Y. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 15, 669–678 2016).
Johnston, I. D., McCluskey, D. K., Tan, C. K. L. & Tracey, M. C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 24, 035017 (2014).
Tsai, M. et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Invest. 122, 408–418 (2012).
Huynh, J. et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 3, 112ra122 (2011).
Kohn, J. C., Lampi, M. C. & Reinhart-King, C. A. Age-related vascular stiffening: causes and consequences. Front. Genet. 6, 112 (2015).
Carrion, B. et al. Recreating the perivascular niche ex vivo using a microfluidic approach. Biotechnol. Bioeng. 107, 1020–1028 (2010).
Chen, M. B., Lamar, J. M., Li, R., Hynes, R. O. & Kamm, R. D. Elucidation of the roles of tumor integrin beta1 in the extravasation stage of the metastasis cascade. Cancer Res. 76, 2513–2524 (2016).
Wang, X. L. et al. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16, 282–290 (2016).
Whisler, J. A., Chen, M. B. & Kamm, R. D. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng. C Methods 20, 543–552 (2014).
Price, G. M. et al. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31, 6182–6189 (2010).
Wong, K. H. K., Truslow, J. G. & Tien, J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31, 4706–4714 (2010).
Nichol, J. W. et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010).
Nguyen, D. H. T. et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl Acad. Sci. USA 110, 6712–6717 (2013).
Heintz, K. A. et al. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Adv. Healthc. Mater. 5, 2153–2160 (2016).
Brandenberg, N. & Lutolf, M. P. In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv. Mater. 28, 7450–7456 (2016).
Zheng, Y. et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl Acad. Sci. USA 109, 9342–9347 (2012).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Linville, R. M., Boland, N. F., Covarrubias, G., Price, G. M. & Tien, J. Physical and chemical signals that promote vascularization of capillary-scale channels. Cell Mol. Bioeng. 9, 73–84 (2016).
Chrobak, K. M., Potter, D. R. & Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 71, 185–196 (2006).
Kuijpers, A. J. et al. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 11, 225–243 (2000).
Yu, Q., Zhou, J. & Fung, Y. C. Neutral axis location in bending and Young’s modulus of different layers of arterial wall. Am. J. Physiol. 265, H52–H60 (1993).
Handorf, A. M., Zhou, Y. X., Halanski, M. A. & Li, W. J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11, 1–15 (2015).
Jain, R. K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 46, 149–168 (2001).
Warkentin, T. E., Moore, J. C., Anand, S. S., Lonn, E. M. & Morgan, D. G. Gastrointestinal bleeding, angiodysplasia, cardiovascular disease, and acquired von Willebrand syndrome. Transfus. Med. Rev. 17, 272–286 (2003).
Giannotta, M., Trani, M. & Dejana, E. VE–cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Yuan, W., Lv, Y., Zeng, M. & Fu, B. M. Non-invasive measurement of solute permeability in cerebral microvessels of the rat. Microvasc. Res. 77, 166–173 (2009).
Zhang, D. C., Xu, C. L., Manwani, D. & Frenette, P. S. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood 127, 801–809 (2016).
Gimenez, F., Barraud de Lagerie, S., Fernandez, C., Pino, P. & Mazier, D. Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell Mol. Life Sci. 60, 1623–1635 (2003).
Chang, J. et al. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood 116, 1779–1786 (2010).
Yao, L. et al. Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood 94, 3820–3828 (1999).
Martinelli, R. et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J. Cell Biol. 201, 449–465 (2013).
Schaer, D. J., Buehler, P. W., Alayash, A. I., Belcher, J. D. & Vercellotti, G. M. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121, 1276–1284 (2013).
Belcher, J. D. et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123, 377–390 (2014).
Ghosh, S. et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J. Clin. Invest. 123, 4809–4820 (2013).
Asakura, T., Asakura, K., Obata, K., Mattiello, J. & Ballas, S. K. Blood samples collected under venous oxygen pressure from patients with sickle cell disease contain a significant number of a new type of reversibly sickled cells: constancy of the percentage of sickled cells in individual patients during steady state. Am. J. Hematol. 80, 249–256 (2005).
Byun, H. et al. Optical measurement of biomechanical properties of individual erythrocytes from a sickle cell patient. Acta Biomater. 8, 4130–4138 (2012).
Lu, X. et al. The measurement of shear modulus and membrane surface viscosity of RBC membrane with Ektacytometry: a new technique. Math. Biosci. 209, 190–204 (2007).
Bernabeu, M. et al. Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc. Natl Acad. Sci. USA 113, E3270–E3279 (2016).
Turner, L. et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498, 502–505 2013).
Yeh, Y. T. et al. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS ONE 7, e46889 (2012).
Sack, K. D., Teran, M. & Nugent, M. A. Extracellular matrix stiffness controls VEGF signaling and processing in endothelial cells. J. Cell. Physiol. 231, 2026–2039 (2016).
Hayashi, A. & Kanzaki, T. Swelling of agarose gel and its related changes. Food Hydrocoll. 1, 317–325 (1987).
Qiu, Y. et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl Acad. Sci. USA 111, 14430–14435 (2014).
Wang, C., Lu, H. & Schwartz, M. A. A novel in vitro flow system for changing flow direction on endothelial cells. J. Biomech. 45, 1212–1218 (2012).
Price, G. M. & Tien, J. Methods for forming human microvascular tubes in vitro and measuring their macromolecular permeability. Methods Mol. Biol. 671, 281–293 (2011).
Yuan, F., Leunig, M., Berk, D. A. & Jain, R. K. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc. Res. 45, 269–289 (1993).
Yuan, Y., Chilian, W. M., Granger, H. J. & Zawieja, D. C. Permeability to albumin in isolated coronary venules. Am. J. Physiol. 265, H543–H552 (1993).
Acknowledgements
This work was performed in part at the Georgia Tech Institute for Electronics and Nanotechnology (a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542174)). We acknowledge the clinical research personnel at Emory University and the Children’s Healthcare of Atlanta who helped to obtain samples, and the patients for donating blood. We acknowledge D. Archer and L. A. Brown for valuable discussions. We acknowledge the rest of the Lam Lab for technical support and suggestions. Financial support was provided by National Science Foundation CAREER Award 1150235 (to W.A.L.), National Institutes of Health grants U01HL117721 (to S.F.O.-A., C.H.J. and W.A.L.), U54HL112309 (to W.A.L.) and R01HL121264 (to W.A.L.), and the National Institute for Neurological Disorders and Strokes grant R21NS085382 (to T.J.L.).
Author information
Authors and Affiliations
Contributions
Y.Q. and W.A.L. designed the device. Y.Q., W.A.L., S.F.O.-A., C.H.J. and T.J.L. conceived and designed the project. Y.Q., B.A., Y.S., C.E.H., R.T., P.N.M., R.G.M. and J.C.C. performed the experimental work. Y.Q., W.A.L., S.F.O.-A., C.H.J. and T.J.L. analysed the data. Y.Q., W.A.L., S.F.O.-A., C.H.J., T.J.L., P.N.M. and J.C.C. wrote the manuscript. All authors discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and video captions.
Supplementary Video 1
3D rendering of confocal microscopy immunostaining images of the adherens-junction protein VE-cadherin in the IPN-hydrogel-based endothelialized microfluidic system.
Supplementary Video 2
Diffusion of BSA-AF594 from acellular (non-endothelialized) microchannels can be used as a positive control to measure permeability.
Supplementary Video 3
Perfused BSA-AF594 was maintained in the ‘vascular’ space of the endothelialized microchannels during the permeability assay.
Supplementary Video 4
Perfusion of RBCs isolated from the sickle-cell disease patients with lower percentages of ISCs (~2.5%) into the engineered microvasculature (4-hour perfusion).
Supplementary Video 5
Perfusion of RBCs isolated from the sickle-cell disease patients with higher percentages of ISCs into the engineered microvasculature (4-hour perfusion).
Supplementary Video 6
Perfusion of malaria-infected RBCs into the engineered microvasculature (4-hour perfusion).
Rights and permissions
About this article
Cite this article
Qiu, Y., Ahn, B., Sakurai, Y. et al. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat Biomed Eng 2, 453–463 (2018). https://doi.org/10.1038/s41551-018-0224-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0224-z
This article is cited by
-
Vascularized organoid-on-a-chip: design, imaging, and analysis
Angiogenesis (2024)
-
Addressing Cardiovascular Toxicity Risk of Electronic Nicotine Delivery Systems in the Twenty-First Century: “What Are the Tools Needed for the Job?” and “Do We Have Them?”
Cardiovascular Toxicology (2024)
-
Modeling human HSV infection via a vascularized immune-competent skin-on-chip platform
Nature Communications (2022)
-
Microvascularized tumor organoids-on-chips: advancing preclinical drug screening with pathophysiological relevance
Nano Convergence (2021)
-
Composable microfluidic spinning platforms for facile production of biomimetic perfusable hydrogel microtubes
Nature Protocols (2021)