Abstract
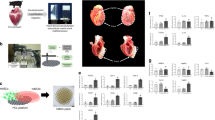
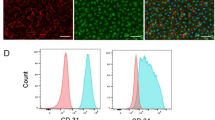
Stem cell transplantation, as used clinically, suffers from low retention and engraftment of the transplanted cells. Inspired by the ability of platelets to recruit stem cells to sites of injury on blood vessels, we hypothesized that platelets might enhance the vascular delivery of cardiac stem cells (CSCs) to sites of myocardial infarction injury. Here, we show that CSCs with platelet nanovesicles fused onto their surface membranes express platelet surface markers that are associated with platelet adhesion to injury sites. We also find that the modified CSCs selectively bind collagen-coated surfaces and endothelium-denuded rat aortas, and that in rat and porcine models of acute myocardial infarction the modified CSCs increase retention in the heart and reduce infarct size. Platelet-nanovesicle-fused CSCs thus possess the natural targeting and repairing ability of their parental cell types. This stem cell manipulation approach is fast, straightforward and safe, does not require genetic alteration of the cells, and should be generalizable to multiple cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mozaffarian, D. et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131, 29–322 (2015).
Madonna, R. et al. Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 37, 1789–1798 (2016).
Sepantafar, M. et al. Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol. Adv. 34, 362–379 (2016).
Weissman, I. L. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 287, 1442–1446 (2000).
Cheng, K. et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun. 5, 4880 (2014).
Tongers, J. et al. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur. Heart J. 32, 1197–1206 (2011).
van der Spoel, T. I. et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc. Res. 91, 649–658 (2011).
Lippi, G. et al. Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol. 8, 502–512 (2011).
Stellos, K. et al. Circulating platelet-progenitor cell coaggregate formation is increased in patients with acute coronary syndromes and augments recruitment of CD34+ cells in the ischaemic microcirculation. Eur. Heart J. 34, 2548–2556 (2013).
Li, T. S. et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 59, 942–953 (2012).
Smith, R. R. et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908 (2007).
Cheng, K. et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ. Res. 106, 1570–1581 (2010).
Lee, S. T. et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J. Am. Coll. Cardiol. 57, 455–465 (2011).
Cheng, K. et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail. 2, 49–61 (2014).
Malliaras, K. et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (cardiosphere-derived autologous stem cells to reverse ventricular dysfunction). J. Am. Coll. Cardiol. 63, 110–22 (2014).
Makkar, R. R. et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012).
Cheng, K. et al. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 21, 1121–1135 (2012).
Kanazawa, H. et al. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ. Heart Fail. 8, 322–332 (2015).
Nasiri, S. Infusible platelet membrane as a platelet substitute for transfusion: an overview. Blood Transfus. 11, 337–342 (2013).
Hu, C. M. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Cheng, K. et al. Brief report: mechanism of extravasation of infused stem cells. Stem Cells 30, 2835–2842 (2012).
Allen, T. A. et al. Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells 35, 170–180 (2017).
Gawaz, M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 61, 498–511 (2004).
Xu, Y. et al. Activated platelets contribute importantly to myocardial reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 290, H692–699 (2006).
Anselmo, A. C. et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8, 11243–11253 (2014).
Vandergriff, A. C. et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 35, 8528–8539 (2014).
Li, T. S. et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 28, 2088–2098 (2010).
Li, C. et al. Allogenic dendritic cell and tumor cell fused vaccine for targeted imaging and enhanced immunotherapeutic efficacy of gastric cancer. Biomaterials 54, 177–187 (2015).
Kawada, M. Vaccination of fusion cells of rat dendritic and carcinoma cells prevents tumor growth in vivo. Int. J. Cancer 105, 520–526 (2003).
Lentz, B. R. Polymer-induced membrane fusion: potential mechanism and relation to cell fusion events. Chem. Phys. Lipids 73, 91–106 (1994).
Huang, Y. et al. Fusions of tumor-derived endothelial cells with densritic cells induces antitumor immunity. Sci. Rep. 7, 46544 (2017).
Brown, A. C. et al. Molecular interference of fibrin’s divalent polymerization mechanism enables modulation of multiscale material properties. Biomaterials 49, 27–36 (2015).
Brown, A. C. et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat. Mater. 13, 1108–1114 (2014).
Tang, J. et al. Heart repair using nanogel-encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano 11, 9738–9749 (2017).
Tang, J. et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat. Commun. 8, 13724 (2017).
Acknowledgements
This work was supported by US National Institute of Health (HL123920 and HL137093), NC State University Chancellor’s Faculty Excellence Program, NC State Chancellor’s Innovation Fund, University of North Carolina General Assembly Research Opportunities Initiative grant and the National Natural Science Foundation of China (81370216, 81570274).
Author information
Authors and Affiliations
Contributions
J.T., T.S., K.H., P.-U.D. and K.C. designed the research, performed biochemical, cellular and animal experiments, analysed the data and drafted the paper. P.-U.D., Z.W., A.V., M.T.H., T.A., J.C., T.L., E.S., E.M., L.L., L.R., A.L., A.B., T.G.C., D.S., Z.G. and G.A.S. performed cellular and in vitro experiments, and/or provided comments to improve the paper. K.C. and J.Z. provided financial support. K.C. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and table.
Videos
Supplementary Video 1
Angiogram showing blood flow before ischaemia.
Supplementary Video 2
Angiogram showing the location of balloon inflation and blood flow during ischaemia.
Supplementary Video 3
Angiogram showing blood flow after ischaemia.
Rights and permissions
About this article
Cite this article
Tang, J., Su, T., Huang, K. et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng 2, 17–26 (2018). https://doi.org/10.1038/s41551-017-0182-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-017-0182-x
This article is cited by
-
Engineering of Cell Derived-Nanovesicle as an Alternative to Exosome Therapy
Tissue Engineering and Regenerative Medicine (2024)
-
Thylakoid engineered M2 macrophage for sonodynamic effect promoted cell therapy of early atherosclerosis
Nano Research (2024)
-
Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery
Journal of Biomedical Science (2023)
-
Macrophage-driven cardiac inflammation and healing: insights from homeostasis and myocardial infarction
Cellular & Molecular Biology Letters (2023)
-
Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges
Military Medical Research (2023)