Abstract
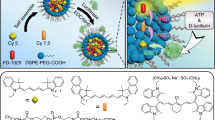
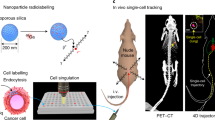
The identification and molecular profiling of early metastases remains a major challenge in cancer diagnostics and therapy. Most in vivo imaging methods fail to detect small cancerous lesions, a problem that is compounded by the distinct physical and biological barriers associated with different metastatic niches. Here, we show that intravenously injected rare-earth-doped albumin-encapsulated nanoparticles emitting short-wave infrared light (SWIR) can detect targeted metastatic lesions in vivo, allowing for the longitudinal tracking of multi-organ metastases. In a murine model of human breast cancer, the nanoprobes enabled whole-body SWIR detection of adrenal-gland microlesions and bone lesions that were undetectable via contrast-enhanced magnetic resonance imaging as early as three and five weeks post-inoculation, respectively. Whole-body SWIR imaging of nanoprobes functionalized to differentially target distinct metastatic sites and administered to a biomimetic murine model of human breast cancer resolved multi-organ metastases that showed varied molecular profiles in the lungs, adrenal glands and bones. Real-time surveillance of lesions in multiple organs should facilitate pre- and post-therapy monitoring in preclinical settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol.28, 3271–3277 (2010).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science331, 1559–1564 (2011).
Dawood, S., Broglio, K., Ensor, J., Hortobagyi, G. N. & Giordano, S. H. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann. Oncol.21, 2169–2174 (2010).
Dawood, S. et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer117, 1819–1826 (2011).
Manders, K. et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer6, 179 (2006).
Plunkett, T. A., Smith, P. & Rubens, R. D. Risk of complications from bone metastases in breast cancer. Implications for management. Eur. J. Cancer36, 476–482 (2000).
Ganapathy, V. et al. Luminal breast cancer metastasis is dependent on estrogen signaling. Clin. Exp. Metastasis29, 493–509 (2012).
Ganapathy, V. et al. Targeting the transforming growth factor-β pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer9, 122 (2010).
Setyawati, M. I., Tay, C. Y., Docter, D., Stauber, R. H. & Leong, D. T. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev.44, 8174–8199 (2015).
Cleaver, O. & Melton, D. A. Endothelial signaling during development. Nat. Med.9, 661–668 (2003).
Barua, S. & Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today9, 223–243 (2014).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol.33, 941–951 (2015).
Smith, A. M., Mancini, M. C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotech.4, 710–711 (2009).
Sordillo, L. A., Pu, Y., Pratavieira, S., Budansky, Y. & Alfano, R. R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt.19, 056004 (2014).
Naczynski, D. J. et al. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun.4, 2199 (2013).
van Saders, B., Al-Baroudi, L., Tan, M. C. & Riman, R. E. Rare-earth doped particles with tunable infrared emissions for biomedical imaging. Opt. Mater. Express3, 566–573 (2013).
Tan, M. C., Connolly, J. & Riman, R. E. Optical efficiency of short wave infrared emitting phosphors. J. Phys. Chem. C115, 17952–17957 (2011).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell3, 537–549 (2003).
Zevon, M. et al. CXCR-4 targeted, short wave infrared (SWIR) emitting nanoprobes for enhanced deep tissue imaging and micrometastatic cancer lesion detection. Small11, 6347–6357 (2015).
Zhao, X., He, S. & Tan, M. C. Design of infrared-emitting rare earth doped nanoparticles and nanostructured composites. J. Mater. Chem. C4, 8349–8372 (2016).
Sheng, Y., De Liao, L., Thakor, N. V. & Tan, M. C. Nanoparticles for molecular imaging. J. Biomed. Nanotechnol.10, 2641–2676 (2014).
Naczynski, D. J. et al. Albumin nanoshell encapsulation of near-infrared-excitable rare-earth nanoparticles enhances biocompatibility and enables targeted cell imaging. Small6, 1631–1640 (2010).
Hendrix, C. W. et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob. Agents Ch.44, 1667–1673 (2000).
De Clercq, E. The bicyclam AMD3100 story. Nat. Rev. Drug Discov.2, 581–587 (2003).
Baglioni, M. et al. Binding of the doxorubicin-lactosaminated human albumin conjugate to HCC cells is mediated by the drug moieties. Dig. Liver Dis.40, 963–964 (2008).
Kratz, F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Control Release132, 171–183 (2008).
Ulmert, D., Solnes, L. & Thorek, D. Contemporary approaches for imaging skeletal metastasis. Bone Res.3, 15024 (2015).
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature436, 518–524 (2005).
Minn, A. J. et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Investig.115, 44–55 (2005).
Toy, R., Peiris, P. M., Ghaghada, K. B. & Karathanasis, E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine9, 121–134 (2014).
Berman, A. T., Thukral, A. D., Hwang, W. T., Solin, L. J. & Vapiwala, N. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin. Breast Cancer13, 88–94 (2013).
Sethi, N. & Kang, Y. Notch signalling in cancer progression and bone metastasis. Br. J. Cancer105, 1805–1810 (2011).
Hazan, R. B., Phillips, G. R., Qiao, R. F., Norton, L. & Aaronson, S. A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol.148, 779–790 (2000).
Gligorijevic, B. et al. Intravital imaging and photoswitching in tumor invasion and intravasation microenvironments. Micros Today18, 34–37 (2010).
Hulit, J. et al. The use of fluorescent proteins for intravital imaging of cancer cell invasion. Methods Mol. Biol.872, 15–30 (2012).
Kedrin, D. et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods5, 1019–1021 (2008).
Fein, M. R. & Egeblad, M. Caught in the act: revealing the metastatic process by live imaging. Dis. Model. Mech.6, 580–593 (2013).
Lunov, O. et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano5, 1657–1669 (2011).
Suva, L. J., Griffin, R. J. & Makhoul, I. Mechanisms of bone metastases of breast cancer. Endocr. Relat. Cancer16, 703–713 (2009).
Murugan, K. et al. Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomed.10, 2191–2206 (2015).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev.66, 2–25 (2014).
Kettiger, H., Schipanski, A., Wick, P. & Huwyler, J. Engineered nanomaterial uptake and tissue distribution: from cell to organism. Int. J. Nanomed.8, 3255–3269 (2013).
Kang, Y. & Pantel, K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell23, 573–581 (2013).
Acknowledgements
We are grateful for access to the Rutgers Molecular Imaging Core (D. Adler), Analytical Core at the Environmental and Occupational Health Sciences Institute, Rutgers University (B. Buckley and E. McCandish), and for funding from the National Institutes of Health National Institute of Biomedical Imaging and Bioengineering (EB018378-01 and EB015169-02), Singapore University of Technology and Design-Massachusetts Institute of Technology International Design Centre (project number IDG31400106), and the Singapore Ministry of Education (project number MOE2014-T2-2-145). We also thank Y. Kang of Princeton University for the SCP28, SCP2 and 4175-TR cells. We acknowledge Malvern Instruments for providing the equipment used for the DLS measurements.
Author information
Authors and Affiliations
Contributions
H.K., M.Z., V.G., S.G., C.M.R. and P.V.M. conceived the study and designed the experiments. H.K., V.G., M.Z., W.B-P., M.J.D. and S.R.B performed the animal experiments. H.K., M.Z. and M.J.D. performed in vitro experiments. M-C.T., X.Z., Y.S. and R.E.R. designed and fabricated the rare-earth nanoparticles. H.K., M.Z., L.H.M., L.M.H., V.G. and M.C.P. analysed the data. H.K., M.Z., M-C.T., C.M.R., M.C.P., V.G. and P.V.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and video captions.
Supplementary Video 1
Real-time short-wave-infrared-light imaging of athymic nude mice in supine position intravenously injected with 200 μl of rare-earth albumin nanocomposites.
Rights and permissions
About this article
Cite this article
Kantamneni, H., Zevon, M., Donzanti, M.J. et al. Surveillance nanotechnology for multi-organ cancer metastases. Nat Biomed Eng 1, 993–1003 (2017). https://doi.org/10.1038/s41551-017-0167-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-017-0167-9
This article is cited by
-
Advances in magnetic nanoparticle-based magnetic resonance imaging contrast agents
Nano Research (2023)
-
In vivo bioorthogonal labeling of rare-earth doped nanoparticles for improved NIR-II tumor imaging by extracellular vesicle-mediated targeting
Nano Research (2023)
-
Nanotechnology-aided advancement in the combating of cancer metastasis
Cancer and Metastasis Reviews (2022)
-
Integrating the second near-infrared fluorescence imaging with clinical techniques for multimodal cancer imaging by neodymium-doped gadolinium tungstate nanoparticles
Nano Research (2021)
-
Shortwave infrared emitting multicolored nanoprobes for biomarker-specific cancer imaging in vivo
BMC Cancer (2020)