Abstract
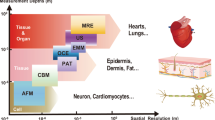
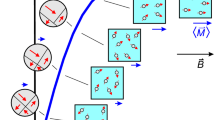
The function of miniature wireless medical devices, such as capsule endoscopes, biosensors and drug-delivery systems, depends critically on their location inside the body. However, existing electromagnetic, acoustic and imaging-based methods for localizing and communicating with such devices suffer from limitations arising from physical tissue properties or from the performance of the imaging modality. Here, we embody the principles of nuclear magnetic resonance in a silicon integrated-circuit approach for microscale device localization. Analogous to the behaviour of nuclear spins, the engineered miniaturized radio frequency transmitters encode their location in space by shifting their output frequency in proportion to the local magnetic field; applied field gradients thus allow each device to be located precisely from its signal’s frequency. The devices are integrated in circuits smaller than 0.7 mm3 and manufactured through a standard complementary-metal-oxide-semiconductor process, and are capable of sub-millimetre localization in vitro and in vivo. The technology is inherently robust to tissue properties, scalable to multiple devices, and suitable for the development of microscale devices to monitor and treat disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sitti, M. et al. Biomedical applications of untethered mobile milli/microrobots. Proc. IEEE103, 205–224 (2015).
Bergeles, C. & Yang, G. Z. From passive tool holders to microsurgeons: safer, smaller, smarter surgical robots. IEEE Trans. Biomed. Eng.61, 1565–1576 (2014).
Ciuti, G., Menciassi, A. & Dario, P. Capsule endoscopy: from current achievements to open challenges. IEEE Rev. Biomed. Eng.4, 59–72 (2011).
Yim, S., Gultepe, E., Gracias, D. H. & Sitti, M. Biopsy using a magnetic capsule endoscope carrying, releasing, and retrieving untethered microgrippers. IEEE Trans. Biomed. Eng.61, 513–521 (2014).
Ciuti, G. et al. Frontiers of robotic endoscopic capsules: a review. J. Micro-Bio Robot.11, 1–18 (2016).
Alivisatos, A. P. et al. Nanotools for neuroscience and brain activity mapping. ACS Nano7, 1850–1866 (2013).
Seo, D. et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust neuron neuroresource wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron91, 529–539 (2016).
Williams, B. J., Anand, S. V., Rajagopalan, J. & Saif, M. T. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun.5, 3081 (2014).
Nelson, B. J., Kaliakatsos, I. K. & Abbott, J. J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng.12, 55–85 (2010).
Liu, L., Towfighian, S. & Hila, A. A review of locomotion systems for capsule endoscopy. IEEE Rev. Biomed. Eng.8, 138–151 (2015).
Than, T. D., Alici, G., Zhou, H. & Li, W. A review of localization systems for robotic endoscopic capsules. IEEE Trans. Biomed. Eng.59, 2387–2399 (2012).
Pourhomayoun, M., Jin, Z. & Fowler, M. L. Accurate localization of in-body medical implants based on spatial sparsity. IEEE Trans. Biomed. Eng.61, 590–597 (2014).
Ye, Y., Pahlavan, K., Bao, G., Swar, P. & Ghaboosi, K. Comparative performance evaluation of RF localization for wireless capsule endoscopy applications. Int. J. Wirel. Inf. Networks21, 208–222 (2014).
Chandra, R., Johansson, A. J., Gustafsson, M. & Tufvesson, F. A microwave imaging-based technique to localize an in-body RF source for biomedical applications. IEEE Trans. Biomed. Eng.62, 1231–1241 (2015).
Bao, G., Pahlavan, K. & Mi, L. Hybrid localization of microrobotic endoscopic capsule inside small intestine by data fusion of vision and RF sensors. IEEE Sens. J.15, 2669–2678 (2015).
Hu, C. et al. A cubic 3-axis magnetic sensor array for wirelessly tracking magnet position and orientation. Sensors J. IEEE10, 903–913 (2010).
Schlageter, V., Besse, P. A., Popovic, R. S. & Kucera, P. Tracking system with five degrees of freedom using a 2D-array of Hall sensors and a permanent magnet. Sensors Actuat. A Phys.92, 37–42 (2001).
Schlageter, V., Drljaca, P., Popovic, R. S. & Kucera, P. A magnetic tracking system based on highly sensitive integrated Hall sensors. JSME Int. J. Ser. C45, 967–973 (2002).
Wu, X. et al. Wearable magnetic locating and tracking system for MEMS medical capsule. Sensors Actuat. A Phys.141, 432–439 (2008).
Nagaoka, T. & Uchiyama, A. Development of a small wireless position sensor for medical capsule devices. Conf. Proc. IEEE Eng. Med. Biol. Soc.3, 2137–2140 (2004).
Guo, X., Yan, G. & He, W. A novel method of three-dimensional localization based on a neural network algorithm. J. Med. Eng. Technol.33, 192–198 (2009).
Hashi, S., Yabukami, S., Kanetaka, H., Ishiyama, K. & Arai, K. I. Numerical study on the improvement of detection accuracy for a wireless motion capture system. IEEE Trans. Magnet.45, 2736–2739 (2009).
Hashi, S., Yabukami, S., Kanetaka, H., Ishiyama, K. & Arai, K. I. Wireless magnetic position-sensing system using optimized pickup coils for higher accuracy. IEEE Trans. Magnet.47, 3542–3545 (2011).
Carpi, F., Kastelein, N., Talcott, M. & Pappone, C. Magnetically controllable gastrointestinal steering of video capsules. IEEE Trans. Biomed. Eng.58, 231–234 (2011).
Kuth, R., Reinschke, J. & Rockelein, R. Method for determining the position and orientation of an endoscopy capsule guided through an examination object by using a navigating magnetic field generated by means of a navigation device. German patent US20070038063 (2007).
Boese, J., Rahn, N. & Sandkamp, B. Method for determining the position and orientation of an object, especially of a catheter, from two-dimensional X-ray images. German patent US7801342 (2010).
Than, T. D. et al. An effective localization method for robotic endoscopic capsules using multiple positron emission markers. IEEE Trans. Robot.30, 1174–1186 (2014).
Dumoulin, C. L., Souza, S. P. & Darrow, R. D. Real-time position monitoring of invasive devices using magnetic resonance. Magn. Reson. Med.29, 411–415 (1993).
Krieger, A. et al. An MRI-compatible robotic system with hybrid tracking for MRI-guided prostate intervention. IEEE Trans. Biomed. Eng.58, 3049–3060 (2011).
Zabow, G., Dodd, S., Moreland, J. & Koretsky, A. Micro-engineered local field control for high-sensitivity multispectral MRI. Nature453, 1058–1063 (2008).
Nagy, Z. et al. in Proc. IEEE International Conference on Robotics and Automation 2593–2598 (2009).
Gumprecht, J. D. J., Lueth, T. C. & Khamesee, M. B. Navigation of a robotic capsule endoscope with a novel ultrasound tracking system. Microsyst. Technol.19, 1415–1423 (2013).
Wells, P. Current status and future technical advances of ultrasonic imaging. Eng. Med. Biol. Mag. IEEE19, 14–20 (2000).
Carpi, F. & Shaheed, H. Grand challenges in magnetic capsule endoscopy. Expert Rev. Med. Devices10, 433–436 (2013).
Popovic, R. S. Hall Effect Devices 2nd edn (CRC Press, Boca Raton, FL, 2003).
Saritas, E. U. et al. Magnetic particle imaging (MPI) for NMR and MRI researchers. J. Magn. Reson.229, 116–126 (2013).
Agrawal, D. R. et al. Conformal phased surfaces for wireless powering of bioelectronic microdevices. Nat. Biomed. Eng.1, 0043 (2017).
Agarwal, A. et al. A 4 µW, ADPLL-based implantable amperometric biosensor in 65 µm CMOS. In 2017 Symposia on VLSI Circuits (2017).
Nazari, M. H., Mujeeb-U-Rahman, M. & Scherer, A. An implantable continuous glucose monitoring microsystem in 0.18 µm CMOS. In 2014 Symposium on VLSI Circuits Digest of Technical Papers 1–2 (2014).
Ning, H. et al. Holographic patterning of high-performance on-chip 3D lithium-ion microbatteries. Proc. Natl Acad. Sci. USA112, 6573–6578 (2015).
Liu, T. et al. High-density lithium-ion energy storage utilizing the surface redox reactions in folded graphene films. Chem. Mater.27, 3291–3298 (2015).
Lai, W. et al. Ultrahigh-energy-density microbatteries enabled by new electrode architecture and micropackaging design. Adv. Mater.22, e139–e144 (2010).
Biederman, W. et al. A 4.78 mm2 fully-integrated neuromodulation SoC combining 64 acquisition channels with digital compression and simultaneous dual stimulation. IEEE J. Solid-State Circ.50, 1038–1047 (2015).
Monge, M. et al. A fully intraocular high-density self-calibrating epiretinal prosthesis. IEEE Trans. Biomed. Circuits Syst.7, 747–760 (2013).
Ritchie, C. J. et al. Minimum scan speeds for suppression of motion artifacts in CT. Radiology185, 37–42 (1992).
Acknowledgements
The authors thank A. Agarwal for insightful discussions and assistance with the chip design, and A. Shapero for assistance with chip encapsulation. We thank K.-C. Chen, M. Raj, B. Abiri, A. Safaripur, F. Bohn, H. Davis, P. Ramesh and G. Lu for helpful and constructive discussions. We appreciate the help and assistance of the Caltech High-speed Integrated Circuits group. This research was supported by the Heritage Medical Research Institute (M.G.S. and A.E.), the Burroughs Wellcome Fund (M.G.S.) and the Caltech Rosen Bioengineering Center graduate scholarship (M.M.).
Author information
Authors and Affiliations
Contributions
M.M., M.G.S. and A.E. conceived and planned the research. M.M. designed the integrated circuit and all printed circuit boards, and developed the code to program the FPGA. M.M. performed characterization and in vitro experiments. M.M. and A.L.-G. performed in vivo experiments. M.M. analysed data. M.M., M.G.S. and A.E. wrote the manuscript with input from all other authors. M.G.S. and A.E. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary discussion, figures and references.
Rights and permissions
About this article
Cite this article
Monge, M., Lee-Gosselin, A., Shapiro, M.G. et al. Localization of microscale devices in vivo using addressable transmitters operated as magnetic spins. Nat Biomed Eng 1, 736–744 (2017). https://doi.org/10.1038/s41551-017-0129-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-017-0129-2
This article is cited by
-
Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics
Nature Electronics (2023)
-
Mucosa-interfacing electronics
Nature Reviews Materials (2022)
-
Wireless on-demand drug delivery
Nature Electronics (2021)
-
Metasurfaces for bioelectronics and healthcare
Nature Electronics (2021)
-
Transmitting location
Nature Biomedical Engineering (2017)