Abstract
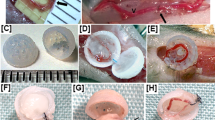
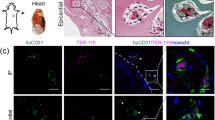
Arterial bypass grafts remain the gold standard for the treatment of end-stage ischaemic disease. Yet patients unable to tolerate the cardiovascular stress of arterial surgery or those with unreconstructable disease would benefit from grafts that are able to induce therapeutic angiogenesis. Here, we introduce an approach whereby implantation of 3D-printed grafts containing endothelial-cell-lined lumens induces spontaneous, geometrically guided generation of collateral circulation in ischaemic settings. In rodent models of hind limb ischaemia and myocardial infarction, we demonstrate that the vascular patches rescue perfusion of distal tissues, preventing capillary loss, muscle atrophy and loss of function. Inhibiting anastomoses between the construct and the host’s local capillary beds, or implanting constructs with unpatterned endothelial cells, abrogates reperfusion. Our 3D-printed grafts constitute an efficient and scalable approach to engineer vascular patches that are able to guide rapid therapeutic angiogenesis and perfusion for the treatment of ischaemic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Underlying Cause of Death 1999–2013 CDC WONDER Online Database (Centers for Disease Control and Prevention, National Center for Health Statistics, accessed 3 February 2015); https://wonder.cdc.gov/ucd-icd10.html
Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death: United States and Each State, 1999–2014 (Centers for Disease Control and Prevention, National Center for Health Statistics, 2015).
Mozaffarian, D. et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131, 29–322 (2015).
Fryar, C. D., Chen, T. C. & Li, X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief 103, 1–8 (2012).
Fowkes, F. G. et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382, 1329–1340 (2013).
Hall, M. J., DeFrances, C. J., Williams, S. N., Golosinskiy, A. & Schwartzman, A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat. Rep. 29, 1–20 (2010).
Conte, M. S. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) and the (hoped for) dawn of evidence-based treatment for advanced limb ischaemia. J. Vasc. Surg. 51, 69S–75S (2010).
Salacinski, H. J. et al. The mechanical behavior of vascular grafts: a review. J. Biomater. Appl. 15, 241–278 (2001).
Slovut, D. P. & Lipsitz, E. C. Surgical technique and peripheral artery disease. Circulation 126, 1127–1138 (2012).
Kakkar, A. M. & Abbott, J. D. Percutaneous versus surgical management of lower extremity peripheral artery disease. Curr. Atheroscler. Rep. 17, 479 (2015).
Kappetein, A. P., Van Mieghem, N. M. & Head, S. J. Revascularization options: coronary artery bypass surgery and percutaneous coronary intervention. Cardiol. Clin. 32, 457–461 (2014).
L’Heureux, N. et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 12, 361–365 (2006).
Dahl, S. L. et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 3, 68ra9 (2011).
Quint, C. et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl Acad. Sci. USA 108, 9214–9219 (2011).
Henry, T. D. et al. The VIVA trial: vascular endothelial growth factor in ischaemia for vascular angiogenesis. Circulation 107, 1359–1365 (2003).
Simons, M. et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 (2002).
Giacca, M. & Zacchigna, S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 19, 622–629 (2012).
Ferrara, N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res. Treat. 36, 127–137 (1995).
Peters, M. C., Polverini, P. J. & Mooney, D. J. Engineering vascular networks in porous polymer matrices. J. Biomed. Mater. Res. 60, 668–678 (2002).
Ehrbar, M. et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 94, 1124–1132 (2004).
Phelps, E. A., Landázuri, N., Thulé, P. M., Taylor, W. R. & García, A. J. Bioartificial matrices for therapeutic vascularization. Proc. Natl Acad. Sci. USA 107, 3323–3328 (2010).
Sadr, N. et al. SAM-based cell transfer to photopatterned hydrogels for microengineering vascular-like structures. Biomaterials 32, 7479–7490 (2011).
Gupta,, R., Tongers, J. & Losordo,, D. W. Human studies of angiogenic gene therapy. Circ. Res. 105, 724–736 (2009).
Koike, N. et al. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138–139 (2004).
Au, P., Tam, J., Fukumura, D. & Jain, R. K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111, 4551–4558 (2008).
Kusuma, S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl Acad. Sci. USA 110, 12601–12606 (2013).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005).
Baranski, J. D. et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl Acad. Sci. USA 110, 7586–7591 (2013).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Hiesinger, W. et al. Computational protein design to reengineer stromal cell-derived factor-1α generates an effective and translatable angiogenic polypeptide analog. Circulation 124, S18–S26 (2011).
MacArthur, J. W. et al. Mathematically engineered stromal cell-derived factor-1α stem cell cytokine analog enhances mechanical properties of infarcted myocardium. J. Thorac. Cardiov. Sur. 145, 278–284 (2013).
MacArthur, J. W. et al. Sustained release of engineered stromal cell-derived factor 1-α from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation 128, S79–S86 (2013).
Shudo, Y. et al. A tissue-engineered chondrocyte cell sheet induces extracellular matrix modification to enhance ventricular biomechanics and attenuate myocardial stiffness in ischaemic cardiomyopathy. Tissue Eng. Part A 19–20, 2515–2525 (2015).
Rodell, C. B. et al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 25, 636–644 (2015).
Murray, C. D. The physiological principle of minimum work: I. The vascular system and the cost of blood volume. Proc. Natl Acad. Sci. USA 12, 207–214 (1926).
Kassab, G. S. Scaling laws of vascular trees: of form and function. Am. J. Physiol. Heart C 290, H894–H903 (2006).
Kang, H.-W., Atala, A. & Yoo, J. J. in Essentials of 3D Biofabrication and Translation (eds Atala, A . & Yoo, J. J .) Ch. 10 (Elsevier, 2015).
Visconti, R. P. et al. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin. Biol. Ther. 10, 409–420 (2010).
Sydney Gladman, A., Matsumoto, E. A., Nuzzo, R. G., Mahadevan, L. & Lewis, J. A. Biomimetic 4D printing. Nat. Mater. 15, 413–418 (2016).
Moroni, F. & Mirabella, T. Decellularized matrices for cardiovascular tissue engineering. Am. J. Stem Cells 13, 1–20 (2014).
Sooppan, R. et al. In vivo anastomosis and perfusion of a three-dimensionally-printed construct containing microchannel networks. Tissue Eng. Part C 22, 1–7 (2016).
Jaipersad, A. S., Lip, G. Y., Silverman, S. & Shantsila, E. The role of monocytes in angiogenesis and atherosclerosis. J. Am. Coll. Cardiol. 63, 1–11 (2014).
Waters, R. E., Terjung, R. L., Peters, K. G. & Annex, B. H. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J. Appl. Physiol. 97, 773–780 (2004).
Hall, M. J. et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat. Report 24, 1–20 (2010).
Norgren, L. et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 45, S5–67 (2007).
Belch, J. J. et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch. Intern. Med. 163, 884–892 (2003).
Al Mahameed, A. Peripheral arterial disease. Cleveland Clinic Center for Continuing Educationhttp://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/peripheral-arterial-disease/ (2009).
Hoefer, I. E. et al. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ. Res. 94, 1179–1185 (2004).
Mirabella, T., Cilli, M., Carlone, S., Cancedda, R. & Gentili, C. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischaemic model. Biomaterials 32, 3689–3699 (2011).
Mirabella, T. et al. Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischaemic fasciocutaneous flap. Stem Cells Dev. 21, 2179–2188 (2012).
National Research Council Guide for the Care and Use of Laboratory Animals 8th edn (National Academies, 2011).
Acknowledgements
We thank J. Eyckmans, R. Chaturvedi and M. Shockley for helpful discussions. This work was supported in part by grants from the National Institutes of Health (NIH; EB00262, EB08396, HL118851), the Biological Design Center of Boston University and the BU-Coulter Foundation Translational Partnership Program. D.C. was supported by the National Science Foundation. C.K.O. was supported by the American Heart Association Grant-in-Aid (16GRNT27090006). Y.J.W. was supported by NIH grant 1R01 (HL089315-01). J.W.M. was supported by the American Heart Association (12POST11620024).
Author information
Authors and Affiliations
Contributions
T.M., J.W.M., D.C., C.K.O., Y.J.W., M.T.Y. and C.S.C. conceived, developed and mentored the project. T.M., J.W.M., D.C. and M.T.Y. performed the experiments. T.M. and J.W.M, analysed the data. T.M. and C.S.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.S.C. is a cofounder of, and owns equity in, Innolign Biomedical, a company that is developing tissue-engineered products.
Supplementary information
Supplementary Information
Supplementary figures (PDF 4779 kb)
Rights and permissions
About this article
Cite this article
Mirabella, T., MacArthur, J., Cheng, D. et al. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat Biomed Eng 1, 0083 (2017). https://doi.org/10.1038/s41551-017-0083
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0083
This article is cited by
-
Phototoxicity-free blue light for enhancing therapeutic angiogenic efficacy of stem cells
Cell Biology and Toxicology (2023)
-
ZnO/glass-based SAW tweezer for dexterous particle patterning and patterned cell culturing
Microfluidics and Nanofluidics (2023)
-
Intelligent Vascularized 3D/4D/5D/6D-Printed Tissue Scaffolds
Nano-Micro Letters (2023)
-
High-efficient serum-free differentiation of endothelial cells from human iPS cells
Stem Cell Research & Therapy (2022)
-
Engineering the multiscale complexity of vascular networks
Nature Reviews Materials (2022)