Abstract
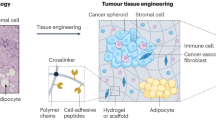
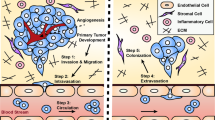
The pre-metastatic niche — the accumulation of aberrant immune cells and extracellular-matrix proteins in target organs — primes the initially healthy organ microenvironment and renders it amenable for subsequent colonization by metastatic cancer cells. By attracting metastatic cells, mimics of the pre-metastatic niche offer both diagnostic and therapeutic potential. However, deconstructing the complexity of the niche by identifying the interactions between cell populations as well as the mediatory roles of the immune system, soluble factors, extracellular-matrix proteins and stromal cells has proved challenging. Experimental models are needed to recapitulate niche-population biology in situ and to mediate in vivo tumour-cell homing, colonization and proliferation. In this Review, we outline the biology of the pre-metastatic niche and discuss advances in the engineering of niche-mimicking biomaterials that regulate the behaviour of tumour cells at an implant site. Such ‘oncomaterials’ offer strategies for the early detection of metastatic events, the inhibition of the formation of the pre-metastatic niche and the attenuation of metastatic progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Maru, Y. The lung metastatic niche. J. Mol. Med. 93, 1185–1192 (2015).
Azizidoost, S. et al. Hepatic metastatic niche: from normal to pre-metastatic and metastatic niche. Tumour Biol. 37, 1493–1503 (2015).
Winkler, F. The brain metastatic niche. J. Mol. Med. 93, 1213–1220 (2015).
Kaplan, R. N., Rafii, S. & Lyden, D. Preparing the “soil”: the premetastatic niche. Cancer Res. 66, 11089–11093 (2006).
Sleeman, J. P. The lymph node pre-metastatic niche. J. Mol. Med. 93, 1173–1184 (2015).
Nguyen, D. X., Bos, P. D. & Massague, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009).
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Ranklin, E. B. & Giaccia, A. J. Hypoxic control of metastasis. Science 352, 175–180 (2016).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Chafe, S. C. et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res. 75, 996–1008 (2015).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Giles, A. J. et al. Activation of hematopoietic stem/progenitor cells promotes immunosuppression within the pre-metastatic niche. Cancer Res. 76, 1335–1347 (2016).
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl Acad. Sci. USA 107, 21248–21255 (2010).
Liu, Y. et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30, 243–256 (2016).
Olkhanud, P. B. et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 69, 5996–6004 (2009).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Sceneay, J. et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 72, 3906–3911 (2012).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Gill, B. J. & West, J. L. Modeling the tumor extracellular matrix: tissue engineering tools repurposed towards new frontiers in cancer biology. J. Biomech. 47, 1969–1978 (2014).
Infanger, D. W., Lynch, M. E. & Fischbach, C. Engineered culture models for studies of tumor-microenvironment interactions. Annu. Rev. Biomed. Eng. 15, 29–53 (2013).
Gu, L. & Mooney, D. J. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat. Rev. Cancer 16, 56–66 (2016).
Ehsan, S. M., Welch-Reardon, K. M., Waterman, M. L., Hughes, C. C. & George, S. C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. 6, 603–610 (2014).
Jeon, J. S. et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl Acad. Sci. USA 112, 214–219 (2015).
Jeon, J. S., Zervantonakis, I. K., Chung, S., Kamm, R. D. & Charest, J. L. In vitro model of tumor cell extravasation. PLoS ONE 8, e56910 (2013).
Ko, C. Y. et al. The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis. Biomaterials 33, 876–885 (2012).
Bersani, F. et al. Bioengineered implantable scaffolds as a tool to study stromal-derived factors in metastatic cancer models. Cancer Res. 74, 7229–7238 (2014).
Barkan, D. et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008).
de la Fuente, A. et al. M-Trap: exosome-based capture of tumor cells as a new technology in peritoneal metastasis. J. Natl Cancer Inst. 107, djv184 (2015).
Rao, S. S. et al. Enhanced survival with implantable scaffolds that capture metastatic breast cancer cells in vivo. Cancer Res. 76, 5209–5218 (2016).
Azarin, S. M. et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun. 6, 8094 (2015).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Krebs, M. G. et al. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat. Rev. Clin. Oncol. 11, 129–144 (2014).
Holzapfel, B. M. et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Delivery Rev. 65, 581–603 (2013).
Thibaudeau, L. et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis. Model. Mech. 7, 299–309 (2014).
Moreau, J. E. et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 67, 10304–10308 (2007).
Aguado, B. A. et al. Secretome identification of immune cell factors mediating metastatic cell homing. Sci. Rep. 5, 17566 (2015).
Yang, L., Edwards, C. M. & Mundy, G. R. Gr-1+CD11b+ myeloid-derived suppressor cells: formidable partners in tumor metastasis. J. Bone Miner. Res. 25, 1701–1706 (2010).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Sharma, S. K. et al. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J. Immunol. 194, 5529–5538 (2015).
Monteiro, A. C. et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 8, e68171 (2013).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Cox, T. R. et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015).
Peinado, H., Lavotshkin, S. & Lyden, D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 21, 139–146 (2011).
Smith, H. A. & Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 91, 411–429 (2013).
Headley, M. B. et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531, 513–517 (2016).
Boehler, R. M., Graham, J. G. & Shea, L. D. Tissue engineering tools for modulation of the immune response. Biotechniques 51, 239–254 (2011).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Franz, S., Rammelt, S., Scharnweber, D. & Simon, J. C. Immune responses to implants — a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692–6709 (2011).
Mikos, A. G., McIntire, L. V., Anderson, J. M. & Babensee, J. E. Host response to tissue engineered devices. Adv. Drug Delivery Rev. 33, 111–139 (1998).
Liu, Y. & Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 30, 668–681 (2016).
Rutkowski, M. R. et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015).
Hiratsuka, S. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Tomita, T., Sakurai, Y., Ishibashi, S. & Maru, Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene 30, 3429–3439 (2011).
Rafii, S. & Lyden, D. S100 chemokines mediate bookmarking of premetastatic niches. Nat. Cell Biol. 8, 1321–1323 (2006).
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010).
Krishnan, V., Vogler, E. A., Sosnoski, D. M. & Mastro, A. M. In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. J. Cell Physiol. 229, 453–462 (2014).
Pathi, S. P., Lin, D. D., Dorvee, J. R., Estroff, L. A. & Fischbach, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 32, 5112–5122 (2011).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Gower, R. M. et al. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials 35, 2024–2031 (2014).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Kwon, H. et al. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. J. Tissue Eng. Regen. Med. 4, 590–599 (2010).
Katsuno, Y. et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27, 6322–6333 (2008).
Feeley, B. T. et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone 38, 154–166 (2006).
Andersen, C. B. et al. Structure of the haptoglobin–haemoglobin complex. Nature 489, 456–459 (2012).
Fujita, K. et al. Serum fucosylated haptoglobin as a novel prognostic biomarker predicting high-Gleason prostate cancer. Prostate 74, 1052–1058 (2014).
Pompach, P. et al. Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Mol. Cell. Proteomics 12, 1281–1293 (2013).
Sun, L. et al. Combination of haptoglobin and osteopontin could predict colorectal cancer hepatic metastasis. Ann. Surg. Oncol. 19, 2411–2419 (2012).
Takeda, Y. et al. Fucosylated haptoglobin is a novel type of cancer biomarker linked to the prognosis after an operation in colorectal cancer. Cancer 118, 3036–3043 (2012).
Azmi, A. S., Bao, B. & Sarkar, F. H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 32, 623–642 (2013).
Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769 (2014).
Abels, E. R. & Breakefield, X. O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 36, 301–312 (2016).
Wendler, F. et al. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene 36, 877–884 (2017).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Brinton, L. T., Sloane, H. S., Kester, M. & Kelly, K. A. Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 72, 659–671 (2015).
Barkan, D., Green, J. E. & Chambers, A. F. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 46, 1181–1188 (2010).
Oskarsson, T. Extracellular matrix components in breast cancer progression and metastasis. Breast 22 (Suppl. 2), S66–S72 (2013).
Desgrosellier, J. S. & Cheresh, D. A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 (2010).
Barney, L. E. et al. A cell-ECM screening method to predict breast cancer metastasis. Integr. Biol. 7, 198–212 (2015).
Aguado, B. A. et al. Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 33, 13–24 (2016).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Lu, W. D. et al. Development of an acellular tumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PLoS ONE 9, e103672 (2014).
Mishra, D. K. et al. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann. Thorac. Surg. 93, 1075–1081 (2012).
Villasante, A., Marturano-Kruik, A. & Vunjak-Novakovic, G. Bioengineered human tumor within a bone niche. Biomaterials 35, 5785–5794 (2014).
Kubala, L. et al. The potentiation of myeloperoxidase activity by the glycosaminoglycan-dependent binding of myeloperoxidase to proteins of the extracellular matrix. Biochim. Biophys. Acta 1830, 4524–4536 (2013).
van der Veen, B. S., de Winther, M. P. & Heeringa, P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signal. 11, 2899–2937 (2009).
Taubenberger, A. V., Quent, V. M., Thibaudeau, L., Clements, J. A. & Hutmacher, D. W. Delineating breast cancer cell interactions with engineered bone microenvironments. J. Bone Miner. Res. 28, 1399–1411 (2013).
Lee, J. et al. Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc. Natl Acad. Sci. USA 109, 19638–19643 (2012).
Vaiselbuh, S. R., Edelman, M., Lipton, J. M. & Liu, J. M. Ectopic human mesenchymal stem cell-coated scaffolds in NOD/SCID mice: an in vivo model of the leukemia niche. Tissue Eng. Part C Methods 16, 1523–1531 (2010).
Seib, F. P., Berry, J. E., Shiozawa, Y., Taichman, R. S. & Kaplan, D. L. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 51, 313–319 (2015).
Holzapfel, B. M. et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35, 4108–4115 (2014).
Lee, E. et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat. Commun. 5, 4715 (2014).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
De Boeck, A. et al. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics 13, 379–388 (2013).
De Vlieghere, E. et al. Tumor-environment biomimetics delay peritoneal metastasis formation by deceiving and redirecting disseminated cancer cells. Biomaterials 54, 148–157 (2015).
Yoneda, T., Williams, P. J., Hiraga, T., Niewolna, M. & Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Miner. Res. 16, 1486–1495 (2001).
Martine, L. C. et al. Engineering a humanized bone organ model in mice to study bone metastases. Nat. Protoc. 12, 639–663 (2017).
Hugo, H. et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J. Cell. Physiol. 213, 374–383 (2007).
Ell, B. & Kang, Y. Transcriptional control of cancer metastasis. Trends Cell Biol. 23, 603–611 (2013).
Ocana, O. H. et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Holmgren, L., O’Reilly, M. S. & Folkman, J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1, 149–153 (1995).
Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Naumov, G. N. et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 62, 2162–2168 (2002).
Giancotti, F. G. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency. Am. J. Pathol. 153, 865–873 (1998).
Weiss, L. Metastatic inefficiency. Adv. Cancer Res. 54, 159–211 (1990).
Weiss, L. Metastatic inefficiency: intravascular and intraperitoneal implantation of cancer cells. Cancer Treat. Res. 82, 1–11 (1996).
Arrigoni, C., Bersini, S., Gilardi, M. & Moretti, M. In vitro co-culture models of breast cancer metastatic progression towards bone. Int. J. Mol. Sci. 17, 1405 (2016).
Bersini, S. et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35, 2454–2461 (2014).
Liu, X. Q., Kiefl, R., Roskopf, C., Tian, F. & Huber, R. M. Interactions among lung cancer cells, fibroblasts, and macrophages in 3D co-cultures and the impact on MMP-1 and VEGF expression. PLoS ONE 11, e0156268 (2016).
Talukdar, S. & Kundu, S. C. Engineered 3D silk-based metastasis models: interactions between human breast adenocarcinoma, mesenchymal stem cells and osteoblast-like cells. Adv. Funct. Mater. 23, 5249–5260 (2013).
Sieh, S. et al. Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone 63, 121–131 (2014).
Weigelt, B., Peterse, J. L. & van‘t Veer, L. J. Breast cancer metastasis: markers and models. Nat. Rev. Cancer 5, 591–602 (2005).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Esmaeilsabzali, H., Beischlag, T. V., Cox, M. E., Parameswaran, A. M. & Park, E. J. Detection and isolation of circulating tumor cells: principles and methods. Biotechnol. Adv. 31, 1063–1084 (2013).
Yoon, H. J., Kozminsky, M. & Nagrath, S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano 8, 1995–2017 (2014).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Bichsel, C. A. et al. Diagnostic microchip to assay 3D colony-growth potential of captured circulating tumor cells. Lab Chip 12, 2313–2316 (2012).
Lee, J., Kohl, N., Shanbhang, S. & Parekkadan, B. Scaffold-integrated microchips for end-to-end in vitro tumor cell attachment and xenograft formation. Technology 3, 179–188 (2015).
Yu, M. et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Bednarz-Knoll, N., Alix-Panabières, C. & Pantel, K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res. 13, 228 (2011).
Yi, J. & Backman, V. Imaging a full set of optical scattering properties of biological tissue by inverse spectroscopic optical coherence tomography. Opt. Lett. 37, 4443–4445 (2012).
Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest. 126, 1208–1215 (2016).
Tauro, B. J. et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteomics 12, 587–598 (2013).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Frangioni, J. V. New technologies for human cancer imaging. J. Clin. Oncol. 26, 4012–4021 (2008).
Nemeth, J. A. et al. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 59, 1987–1993 (1999).
Xia, T. S. et al. Bone metastasis in a novel breast cancer mouse model containing human breast and human bone. Breast Cancer Res. Treat. 132, 471–486 (2012).
Kwon, H. et al. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. J. Tissue Eng. Regen. Med. 4, 590–599 (2010).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Acknowledgements
We thank K. Aguado for figure illustrations. B.A.A. and G.G.B. acknowledge the support of a National Science Foundation Graduate Research Fellowship. Financial support for this work was provided by the National Institutes of Health and the National Cancer Institute (R01 CA173745).
Author information
Authors and Affiliations
Contributions
B.A.A., G.G.B. and L.D.S. wrote and edited the manuscript. B.A.A. prepared the figures. B.A.A. and G.G.B. prepared the tables. S.S.R. and J.S.J. edited and advised on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary tables (PDF 163 kb)
Rights and permissions
About this article
Cite this article
Aguado, B., Bushnell, G., Rao, S. et al. Engineering the pre-metastatic niche. Nat Biomed Eng 1, 0077 (2017). https://doi.org/10.1038/s41551-017-0077
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0077
This article is cited by
-
Aging microenvironment and antitumor immunity for geriatric oncology: the landscape and future implications
Journal of Hematology & Oncology (2023)
-
A synthetic metastatic niche reveals antitumor neutrophils drive breast cancer metastatic dormancy in the lungs
Nature Communications (2023)
-
Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis
Nature Communications (2023)
-
KISS1 metastasis suppressor in tumor dormancy: a potential therapeutic target for metastatic cancers?
Cancer and Metastasis Reviews (2023)
-
Single-cell RNA-sequencing identifies anti-cancer immune phenotypes in the early lung metastatic niche during breast cancer
Clinical & Experimental Metastasis (2022)