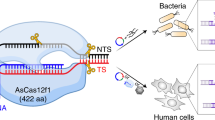
Abstract
Cpf1, a type-V CRISPR–Cas effector endonuclease, exhibits gene-editing activity in human cells through a single RNA-guided approach. Here, we report the design and assessment of an array of 42 types of engineered Acidaminococcus sp. Cpf1 (AsCpf1) CRISPR RNA (crRNA) and 5 types of AsCpf1 mRNA in human cell lines. We show that the top-performing modified crRNA (cr3′5F, containing five 2′-fluoro ribose at the 3′ terminus) and AsCpf1 mRNA (full ψ-modification) improved gene-cutting efficiency by, respectively, 127% and 177%, with respect to unmodified crRNA and plasmid-encoding AsCpf1. We also show that the combination of cr3′5F and ψ-modified AsCpf1 or Lachnospiraceae bacterium Cpf1 (LbCpf1) mRNA augmented gene-cutting efficiency by over 300% with respect to the same control, and discovered that 11 out of 16 crRNAs from Cpf1 orthologues enabled genome editing in the presence of AsCpf1. Engineered CRISPR–Cpf1 systems should facilitate a broad range of genome editing applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bosley, K. S. et al. CRISPR germline engineering—the community speaks. Nat. Biotechnol. 33, 478–486 (2015).
Makarova, K. S. et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 9, 467–477 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Zetsche, B. et al. Cpf1 is a Single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell 163, 759–771 (2015).
Fonfara, I., Richter, H., Bratovic, M., Le Rhun, A. & Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521 (2016).
Yamano, T. et al. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell 165, 949–962 (2016).
Dong, D. et al. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 532, 522–526 (2016).
Hur, J. K. et al. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat. Biotechnol. 34, 807–808 (2016).
Kim, Y. et al. Generation of knockout mice by Cpf1-mediated gene targeting. Nat. Biotechnol. 34, 808–810 (2016).
Kleinstiver, B. P. et al. Genome-wide specificities of CRISPR–Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 34, 869–874 (2016).
Kim, D. et al. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 34, 863–868 (2016).
Zetsche, B. et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 35, 31–34 (2017).
Gao, P., Yang, H., Rajashankar, K. R., Huang, Z. & Patel, D. J. Type V CRISPR–Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 26, 901–913 (2016).
Slaymaker, I.M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Kleinstiver, B. P. et al. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Hendel, A. et al. Chemically modified guide RNAs enhance CRISPR–Cas genome editing in human primary cells. Nat. Biotechnol. 33, 985–989 (2015).
Rahdar, M. et al. Synthetic CRISPR RNA-Cas9-guided genome editing in human cells. Proc. Natl Acad. Sci. USA 112, E7110–E7117 (2015).
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Dang, Y. et al. Optimizing sgRNA structure to improve CRISPR–Cas9 knockout efficiency. Genome Biol. 16, 280 (2015).
Watts, J. K., Deleavey, G. F. & Damha, M. J. Chemically modified siRNA: tools and applications. Drug Discov. Today 13, 842–855 (2008).
Kariko, K., Muramatsu, H., Keller, J. M. & Weissman, D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 20, 948–953 (2012).
Andries, O. et al. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 217, 337–344 (2015).
Li, B., Luo, X. & Dong, Y. Effects of chemically modified messenger RNA on protein expression. Bioconjug. Chem. 27, 849–853 (2016).
Bolukbasi, M. F., Gupta, A. & Wolfe, S. A. Creating and evaluating accurate CRISPR–Cas9 scalpels for genomic surgery. Nat. Methods 13, 41–50 (2016).
Kim, D. et al. Digenome-seq: genome-wide profiling of CRISPR–Cas9 off-target effects in human cells. Nat. Methods 12, 237–243, (2015).
Lin, Y. N. et al. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res. 42, 7473–7485 (2014).
Pinello, L. et al. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat. Biotechnol. 34, 695–697 (2016).
Acknowledgements
We acknowledge F. Zhang and B. Zetsche for providing Cpf1 plasmids and technical assistance. This work was supported by the Early Career Investigator Award from the Bayer Hemophilia Awards Program, Research Awards from the National PKU Alliance, New Investigator Grant from the AAPS Foundation, R01HL136652 from the National Heart, Lung, and Blood Institute, as well as the start-up fund from the College of Pharmacy at The Ohio State University.
Author information
Authors and Affiliations
Contributions
B.L. and Y.D. conceived and designed the experiments; B.L., W.Z., X.L., C.Z. and X.Z. performed the experiments; B.L., W.Z., X.L., X.Z., C.L., C.Z. and Y.D. analysed the data; B.L. and Y.D. wrote the paper with edits and comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures, tables and notes. (PDF 1260 kb)
Rights and permissions
About this article
Cite this article
Li, B., Zhao, W., Luo, X. et al. Engineering CRISPR–Cpf1 crRNAs and mRNAs to maximize genome editing efficiency. Nat Biomed Eng 1, 0066 (2017). https://doi.org/10.1038/s41551-017-0066
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0066
This article is cited by
-
Comparison of DNA targeting CRISPR editors in human cells
Cell & Bioscience (2023)
-
A selective and atom-economic rearrangement of uridine by cascade biocatalysis for production of pseudouridine
Nature Communications (2023)
-
The current situations and limitations of genetic engineering in cyanobacteria: a mini review
Molecular Biology Reports (2023)
-
Engineered Cas12a-Plus nuclease enables gene editing with enhanced activity and specificity
BMC Biology (2022)
-
CRISPR-Cas12a nucleases function with structurally engineered crRNAs: SynThetic trAcrRNA
Scientific Reports (2022)