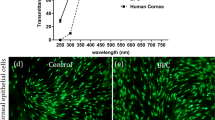
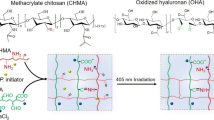
Abstract
Degradation-induced swelling in implanted hydrogels can cause severe adverse reactions in surrounding tissues. Here, we report a new class of hydrogel with extremely low swelling pressure, and demonstrate its use as an artificial vitreous body. The hydrogel has ultralow polymer content (4.0 g l−1), low cytotoxicity, and forms in situ in 10 minutes via the crosslinking of clusters of highly branched polymers of tetra-armed poly(ethylene glycol) prepolymers. After injection and gelation in the eyes of rabbits, the hydrogel functioned as an artificial vitreous body for over a year without adverse effects, and proved effective for the treatment of retinal detachment. The properties of the hydrogel make it a promising candidate as an infill biomaterial for a range of biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Li, Y. L., Rodrigues, J. & Tomas, H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 41, 2193–2221 (2012).
Anseth, K. S. In situ forming degradable networks and their application in tissue engineering and drug delivery. J. Control. Release 78, 199–209 (2002).
Ranga, A., Lutolf, M. P., Hilborn H. & Ossipov, D. A. Hyaluronic acid hydrogels formed in situ by transglutaminase-catalyzed reaction. Biomacromolecules 17, 1553–1560 (2016).
Pritchard, C. D . An infectable thiol-acrylate poly(ethylene glycol) hydrogel for sustained release of methylprednisolone sodium succinate. Biomaterials 32, 587–597 (2011).
Kamata, H., Chung, U., Shibayama, M. & Sakai, T. Anomalous volume phase transition in a polymer gel with alternative hydrophilic-amphiphilic sequence. Soft Matter 8, 6876–6879 (2012).
Li, X., Kondo, S., Chung, U.-i. & Sakai, T. Degradation behavior of polymer gels caused by nonspecific cleavages of network strands. Chem. Mater. 26, 5352–5357 (2014).
Kamata, H., Li, X., Chung, U.-i. & Sakai, T. Design of hydrogels for biomedical applications. Adv. Healthcare Mater. 4, 2360–2374 (2015).
Kamata, H., Akagi, Y., Kayasuga-Kariya, Y., Chung, U.-i. & Sakai, T. “Nonswellable” hydrogel without mechanical hysteresis. Science 343, 873–875 (2014).
Ho, P. C., Chan, I. M., Refojo, M. F. & Tolentino, F. I. The MAI hydrophilic implant for scleral buckling: a review. Ophthalmic Surg. 15, 511–515 (1984).
Tolentino, F. I., Roldan, M., Nassif, J. & Refojo, M. F. Hydrogel implant for scleral buckling. Long-term observations. Retina 5, 38–41 (1985).
Marin, J. F., Tolentino, F. I., Refojo, M. F. & Schepens, C. L. Long-term complications of the MAI hydrogel intrascleral buckling implant. Arch. Ophthalmol. 110, 86–88 (1992).
Roldan-Pallares, M., Hernandez-Montero, J., Llanes, F., Fernandez-Rubio, J. E. & Ortega, F. MIRAgel: hydrolytic degradation and long-term observations. Arch. Ophthalmol. 125, 511–514 (2007).
Flory, P. J. & Rehner, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 11, 521–526 (1943).
Gennes, P.-G. Scaling Concepts in Polymer Physics (Cornell Univ. Press, 1979).
Sakai, T. et al. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 41, 5379–5384 (2008).
Sakai, T. Experimental verification of homogeneity in polymer gels. Polym. J. 46, 517–523 (2014).
Mours, M. & Winter, H. H. Relaxation patterns of nearly critical gels. Macromolecules 29, 7221–7229 (1996).
Winter, H. H. & Mours, M. Rheology of polymers near liquid-solid transitions. Adv. Polym. Sci. 134, 165–234 (1997).
Zheng, Y. C., Li, S. P., Weng, Z. L. & Gao, C. Hyperbranched polymers: advances from synthesis to applications. Chem. Soc. Rev. 44, 4091–4130 (2015).
Kainthan, R. K., Muliawan, E. B., Hatzikiriakos, S. G. & Brooks, D. E. Synthesis, characterization, and viscoelastic properties of high molecular weight hyperbranched polyglycerols. Macromolecules 39, 7708–7717 (2006).
Sakai, T., Katashima, T., Matsushita, T. & Chung, U.-i. Sol-gel transition behavior near critical concentration and connectivity. Polymer J. 48, 629–634 (2016).
Wang, Q. et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 463, 339–343 (2010).
Denlinger, J. L. & Balazs, E. A. Replacement of the liquid vitreous with sodium hyaluronate in monkeys. I. Short-term evaluation. Exp. Eye Res. 31, 81–99 (1980).
Swindle-Reilly, K. E. et al. Rabbit study of an in situ forming hydrogel vitreous substitute. Invest. Ophth. Vis. Sci. 50, 4840–4846 (2009).
Pritchard, C. D. et al. Evaluation of viscoelastic poly(ethylene glycol) sols as vitreous substitutes in an experimental vitrectomy model in rabbits. Acta Biomater. 7, 936–943 (2011).
Tao, Y. et al. Evaluation of an in situ chemically crosslinked hydrogel as a long-term vitreous substitute material. Acta Biomater. 9, 5022–5030 (2013).
Crafoord, S., Andreasson, S. & Ghosh, F. Experimental vitreous tamponade using polyalkylimide hydrogel. Graef. Arch. Clin. Exp. 249, 1167–1174 (2011).
Hoshi, S. et al. In vivo and in vitro feasibility studies of intraocular use of polyethylene glycol-based synthetic sealant to close retinal breaks in porcine and rabbit eyes. Invest. Ophthalmol. Vis. Sci. 56, 4705–4711 (2015).
Vink, H. Precision measurements of osmotic pressure in concentrated polymer solutions. Eur. Polym. J. 7, 1411–1419 (1971).
Horkay, F., Tasaki, I. & Basser, P. J. Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 1, 84–91 (2000).
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) through Grants-in-Aid for the Graduate Program for Leaders in Life Innovation (GPLLI), by the International Core Research Center for Nanobio, Core-to-Core Program A. Advanced Research Networks, and Grants-in-Aid for Young Scientists (A) grant number 23700555 to T.S., Scientific Research (S) grant number 16H06312 to U.C., and Scientific Research (C) grant number 26462631 to F.O. This work was also supported by the Japan Science and Technology Agency (JST) through the S-innovation program and Center of Innovation program (to U.C.) and PREST (to T.S.).
Author information
Authors and Affiliations
Contributions
T.S. planned and supervised the project. K.H., F.O., S.H., T.K., D.C.Z., X.L., M.S., E.G. and S.O. designed and performed the experiments. U.C. and T.O. contributed to discussions throughout the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures and video captions. (PDF 1513 kb)
Supplementary Video 1
Oligo-TetraPEG hydrogel in a glass vial. (MOV 25225 kb)
Supplementary Video 2
Surgical procedures in the left eye of a normal Dutch pigmented rabbit model. (MOV 64484 kb)
Rights and permissions
About this article
Cite this article
Hayashi, K., Okamoto, F., Hoshi, S. et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat Biomed Eng 1, 0044 (2017). https://doi.org/10.1038/s41551-017-0044
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0044
This article is cited by
-
In vivo evaluation of a Nano-enabled therapeutic vitreous substitute for the precise delivery of triamcinolone to the posterior segment of the eye
Drug Delivery and Translational Research (2024)
-
Statistical analysis of organelle movement using state-space models
Plant Methods (2023)
-
Percolation-induced gel–gel phase separation in a dilute polymer network
Nature Materials (2023)
-
Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy
Nature Communications (2022)
-
A benchmark for gel structures: bond percolation enables the fabrication of extremely homogeneous gels
Polymer Journal (2021)