Abstract
Polycyclic aromatic hydrocarbons (PAHs) are abundant in many regions of the Universe, representing a major reservoir for cosmic carbon. However, their formation pathways in cold regions of space remain elusive. Recent astronomical detections show that current astrochemical models drastically underestimate the abundance of aromatic molecules and suggest that additional formation pathways such as ion–molecule reactions need to be considered. Here we reveal efficient low-temperature formation pathways towards nitrogen-containing PAHs via exothermic pyridine+ and acetylene ion–molecule reactions. The experimental approach combines kinetics with spectroscopic probing and unambiguously identifies key reaction intermediates and the final nitrogen-containing PAH product quinolizinium+, a structure that is thought to contribute to the 6.2 μm interstellar emission feature. This study not only provides competing formation pathways relevant in the chemistry of the interstellar medium and Titan’s atmosphere, but also delivers information to verify in-silico potential energy surfaces, astrochemical models and infrared observations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. These source data and the log files of the quantum-chemical calculations are available at: https://doi.org/10.34973/m9mn-d172. Additional data are available from the corresponding author upon request.
Code availability
The code used for data reduction in this work can be found here: https://github.com/aravindhnivas/FELion_GUI3.
References
Allamandola, L. J., Tielens, G. G. M. & Barker, J. R. Interstellar polycyclic aromatic hydrocarbons—the infrared emission bands, the excitation/emission mechanism, and the astrophysical implications. Astrophys. J. Suppl. Ser. 71, 733–775 (1989).
Li, A. Spitzer’s perspective of polycyclic aromatic hydrocarbons in galaxies. Nat. Astron. 4, 339–351 (2020).
Cernicharo, J. et al. Pure hydrocarbon cycles in TMC-1: Discovery of ethynyl cyclopropenylidene, cyclopentadiene, and indene. Astron. Astrophys. 649, L15 (2021).
McGuire, B. A. et al. Detection of two interstellar polycyclic aromatic hydrocarbons via spectral matched filtering. Science 371, 1265–1269 (2021).
Vuitton, V., Yelle, R. V. & McEwan, M. J. Ion chemistry and N-containing molecules in Titan’s upper atmosphere. Icarus 191, 722–742 (2007).
Ali, A., Sittler, E. C., Chornay, D., Rowe, B. R. & Puzzarini, C. Organic chemistry in Titan’s upper atmosphere and its astrobiological consequences: I. Views towards Cassini plasma spectrometer (CAPS) and ion neutral mass spectrometer (INMS) experiments in space. Planet. Space Sci. 109–110, 46–63 (2015).
Zhen, J., Castellanos, P., Paardekooper, D. M., Linnartz, H. & Tielens, A. G. G. M. Laboratory formation of fullerenes from PAHs: top-down interstellar chemistry. Astrophys. J. Lett. 797, 30 (2014).
Berne, O. & Tielens, A. G. G. M. Formation of buckminsterfullerene (C60) in interstellar space. Proc. Natl Acad. Sci. USA 109, 401–406 (2012).
Parker, D. S. N. et al. Low temperature formation of nitrogen-substituted polycyclic aromatic hydrocarbons (PANHs)—barrierless routes to dihydro(iso)quinolines. Astrophys. J. 815, 115 (2015).
Lemmens, A. K., Rap, D. B., Thunnissen, J. M. M., Willemsen, B. & Rijs, A. M. Polycyclic aromatic hydrocarbon formation chemistry in a plasma jet revealed by IR-UV action spectroscopy. Nat. Commun. 11, 269 (2020).
Lee, K. L. K., McGuire, B. A. & McCarthy, M. C. Gas-phase synthetic pathways to benzene and benzonitrile: a combined microwave and thermochemical investigation. Phys. Chem. Chem. Phys. 21, 2946–2956 (2019).
Mccabe, M. N., Hemberger, P., Reusch, E., Bodi, A. & Bouwman, J. Off the beaten path: almost clean formation of indene from the ortho-benzyne + allyl reaction. J. Phys. Chem. Lett. 11, 2859–2863 (2020).
Doddipatla, S. et al. Low-temperature gas-phase formation of indene in the interstellar medium. Sci. Adv. 7, eabd4044 (2021).
Parker, D. S. N. & Kaiser, R. I. On the formation of nitrogen-substituted polycyclic aromatic hydrocarbons (NPAHs) in circumstellar and interstellar environments. Chem. Soc. Rev. 46, 452–463 (2017).
Parker, D. S. N. et al. Low temperature formation of naphthalene and its role in the synthesis of PAHs (polycyclic aromatic hydrocarbons) in the interstellar medium. Proc. Natl Acad. Sci. USA 109, 53–58 (2012).
Parker, D. S. N. et al. Gas phase synthesis of (iso)quinoline and its role in the formation of nucleobases in the interstellar medium. Astrophys. J. 803, 53 (2015).
Larsson, M., Geppert, W. D. & Nyman, G. Ion chemistry in space. Rep. Prog. Phys. 75, 066901 (2012).
Huntress, W. T. Jr. Laboratory studies of bimolecular reactions of positive ions in interstellar clouds, in comets, and in planetary atmospheres of reducing composition. Astrophys. J. Suppl. Ser. 33, 495–514 (1977).
Snow, T. P., Le Page, V., Keheyan, Y. & Bierbaum, V. M. The interstellar chemistry of PAH cations. Nature 391, 259–260 (1998).
Gerlich, D. Experimental investigations of ion–molecule reactions relevant to interstellar chemistry. J. Chem. Soc. Faraday Trans. 89, 2199–2208 (1993).
Herbst, E., Adams, N. G. & Smith, D. Laboratory measurements of ion–molecule reactions pertaining to interstellar hydrocarbon synthesis. Astrophys. J. 269, 329–333 (1983).
Asvany, O., Schlemmer, S. & Gerlich, D. Deuteration of CHn+ (n = 3–5) in collisions with HD measured in a low-temperature ion trap. Astrophys. J. 617, 685–692 (2004).
Shiels, O., Kelly, P., Blanksby, S., da Silva, G. & Trevitt, A. J. Barrierless reactions of three benzonitrile radical cations with ethylene. Aust. J. Chem. 73, 705–713 (2020).
Shiels, O. J. et al. Reactivity trends in the gas-phase addition of acetylene to the N-protonated aryl radical cations of pyridine, aniline, and benzonitrile. J. Am. Soc. Mass. Spectrom. 32, 537–547 (2021).
Soliman, A. R., Hamid, A. M., Attah, I., Momoh, P. & El-Shall, M. S. Formation of nitrogen-containing polycyclic cations by gas-phase and intracluster reactions of acetylene with the pyridinium and pyrimidinium ions. J. Am. Chem. Soc. 135, 155–166 (2013).
Jusko, P. et al. The FELion cryogenic ion trap beam line at the FELIX free-electron laser laboratory: infrared signatures of primary alcohol cations. Faraday Discuss. 217, 172–202 (2019).
Rap, D. B., Marimuthu, A. N., Redlich, B. & Brünken, S. Stable isomeric structures of the pyridine cation (C5H5N•+) and protonated pyridine (C5H5NH+) elucidated by cold ion infrared spectroscopy. J. Mol. Spectrosc. 373, 111357 (2020).
Dunbar, R. C. Polyatomic ion–molecule radiative association: theoretical framework and predictions: observations of NO+ + C6H5CN as an example. Int. J. Mass Spectrom. Ion Process. 100, 423–443 (1990).
Oepts, D., van der Meer, A. F. G. & van Amersfoort, P. W. The free-electron-laser user facility FELIX. Infrared Phys. Technol. 36, 297–308 (1995).
Kislov, V. V., Islamova, N. I., Kolker, A. M., Lin, S. H. & Mebel, A. M. Hydrogen abstraction acetylene addition and Diels–Alder mechanisms of PAH formation: a detailed study using first principles calculations. J. Chem. Theory Comput. 1, 908–924 (2005).
Frenklach, M., Clary, D. W., Gardiner, W. C. & Stein, S. E. Detailed kinetic modeling of soot formation in shock-tube pyrolysis of acetylene. Symp. Combust. 20, 887–901 (1985).
Yim, M. K. & Choe, J. C. Formation of C4H4•+ from the pyridine radical cation: a theoretical mechanistic and kinetic study. J. Phys. Chem. A 115, 3087–3094 (2011).
Feng, J. Y., Lee, Y. P., Witek, H. A. & Ebata, T. Vacuum ultraviolet photoionization induced proton migration and formation of a new C–N bond in pyridine clusters revealed by infrared spectroscopy and mass spectrometry. J. Phys. Chem. Lett. 12, 4936–4943 (2021).
Bohme, D. K. Proton transport in the catalyzed gas-phase isomerization of protonated molecules. Int. J. Mass Spectrom. Ion Process. 115, 95–110 (1992).
Tu, Y. P. & Holmes, J. L. The chemistry of solvated distonic ions: preparation, isomerization, and fragmentation. J. Am. Chem. Soc. 122, 5597–5602 (2000).
Ibrahim, Y., Mabrouki, R., Meot-Ner, M. & El-Shall, M. S. Hydrogen bonding interactions of pyridine•+ with water: stepwise solvation of distonic cations. J. Phys. Chem. A 111, 1006–1014 (2007).
Anicich, V. G., Milligan, D. B., Fairley, D. A. & McEwan, M. J. Termolecular ion–molecule reactions in Titan’s atmosphere. I: principal ions with principal neutrals. Icarus 146, 118–124 (2000).
Anicich, V. G. & McEwan, M. J. Ion–molecule chemistry in Titan’s ionosphere. Planet. Space Sci. 45, 897–921 (1997).
Milligan, D. B. et al. Termolecular ion–molecule reactions in Titan’s atmosphere. II: the structure of the association adducts of HCNH+ with C2H2 and C2H4. J. Am. Soc. Mass. Spectrom. 12, 557–564 (2001).
Eichelberger, B. R., Snow, T. P. & Bierbaum, V. M. Collision rate constants for polarizable ions. J. Am. Soc. Mass. Spectrom. 14, 501–505 (2003).
Waite, J. H. et al. Planetary science: the process of tholin formation in Titan’s upper atmosphere. Science 316, 870–875 (2007).
Vinatier, S. et al. Vertical abundance profiles of hydrocarbons in Titan’s atmosphere at 15° S and 80° N retrieved from Cassini/CIRS spectra. Icarus 188, 120–138 (2007).
Sagan, C. & Khare, B. N. Tholins: organic chemistry of interstellar grains and gas. Nature 277, 102–107 (1979).
Crary, F. J. et al. Heavy ions, temperatures and winds in Titan’s ionosphere: combined Cassini CAPS and INMS observations. Planet. Space Sci. 57, 1847–1856 (2009).
Hudgins, D. M., Bauschlicher, C. W. Jr. & Allamandola, L. J. Variations in the peak position of the 6.2 μm interstellar emission feature: a tracer of N in the interstellar polycyclic aromatic hydrocarbon population. Astrophys. J. 632, 316–332 (2005).
Canelo, C. M., Friaça, A. C. S., Sales, D. A., Pastoriza, M. G. & Ruschel-Dutra, D. Variations in the 6.2 μm emission profile in starburst-dominated galaxies: a signature of polycyclic aromatic nitrogen heterocycles (PANHs)? Mon. Not. R. Astron. Soc. 475, 3746–3763 (2018).
Hudgins, D. M. & Allamandola, L. J. The spacing of the interstellar 6.2 and 7.7 micron emission features as an indicator of polycyclic aromatic hydrocarbon size. Astrophys. J. 513, L69–L73 (1999).
Yang, Y. et al. Laboratory formation and photochemistry of covalently bonded polycyclic aromatic nitrogen heterocycle (PANH) clusters in the gas phase. Mon. Not. R. Astron. Soc. 498, 1–11 (2020).
Jiao, C. Q., Boatz, J. A., DeJoseph, C. A. & Garscadden, A. Condensation reaction of C4H4+ with pyridine. Int. J. Mass Spectrom. 288, 22–35 (2009).
Gaussian 16 (Gaussian Inc., 2016).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Barone, V., Cimino, P. & Stendardo, E. Development and validation of the B3LYP/N07D computational model for structural parameter and magnetic tensors of large free radicals. J. Chem. Theory Comput. 4, 751–764 (2008).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 134105 (2010).
Panchagnula, S. et al. Structural investigation of doubly-dehydrogenated pyrene cations. Phys. Chem. Chem. Phys. 22, 21651–21663 (2020).
Lemmens, A. K. et al. Anharmonicity in the mid-infrared spectra of polycyclic aromatic hydrocarbons: molecular beam spectroscopy and calculations. Astron. Astrophys. 628, A130 (2019).
Hohenstein, E. G., Chill, S. T. & Sherrill, C. D. Assessment of the performance of the M05#2X and M06#2X exchange correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 4, 1996–2000 (2008).
Kozuch, S. & Martin, J. M. L. DSD-PBEP86: in search of the best double-hybrid DFT with spin-component scaled MP2 and dispersion corrections. Phys. Chem. Chem. Phys. 13, 20104–20107 (2011).
Kozuch, S. & Martin, J. M. L. Spin-component-scaled double hybrids: an extensive search for the best fifth-rung functionals blending DFT and perturbation theory. J. Comput. Chem. 34, 2327–2344 (2013).
Sloan, G. C., Kraemer, K. E., Price, S. D. & Shipman, R. F. A uniform database of 2.4–45.4 micron spectra from the Infrared Space Observatory Short Wavelength Spectrometer. Astrophys. J. Suppl. Ser. 147, 379–401 (2003).
Oomens, J., Sartakov, B. G., Tielens, A. G. G. M., Meijer, G. & von Helden, G. Gas-phase infrared spectrum of the coronene cation. Astrophys. J. 560, L99–L103 (2001).
Oomens, J., Tielens, A. G. G. M., Sartakov, B. G., von Helden, G. & Meijer, G. Laboratory infrared spectroscopy of cationic polycyclic aromatic hydrocarbon molecules. Astrophys. J. 591, 968–985 (2003).
Acknowledgements
We gratefully acknowledge the support of Radboud University and of the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO), for providing the required beam time at the FELIX laboratory and the skilful assistance of the FELIX staff. This work was sponsored by NWO Exact and Natural Sciences as part of the research programme ROSAA (NWO START-UP grant 740.018.010; S.B., A.N.M.) and through the use of supercomputer facilities at SURFsara in Amsterdam (NWO Rekentijd grant 2021.055). We thank the Cologne Laboratory Astrophysics group for providing the FELion ion trap instrument for the current experiments and the Cologne Center for Terahertz Spectroscopy funded by the Deutsche Forschungsgemeinschaft (grant SCHL 341/15-1) for supporting its operation. We thank J. Oomens for helpful discussion.
Author information
Authors and Affiliations
Contributions
D.B.R. contributed to the conception and design of the work, data collection, data analysis and interpretation, performing quantum-chemical calculations and drafting and editing of the article. J.G.M.S. contributed to the data collection, performing quantum-chemical calculations and editing of the article. A.N.M. developed data analysis and interpretation tools. B.R. contributed to data interpretation and editing of the article. S.B. contributed to the conception and design of the work, data collection, data analysis and interpretation and drafting and editing of the article. All authors discussed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Astronomy thanks Adam Trevitt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
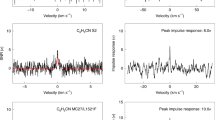
Extended Data Fig. 1 First order reaction rate constants obtained from the kinetic profiles of (a) m/z 104, (b) m/z 105, (c) m/z 106 and (d) m/z 130 plotted against the acetylene number density.
Second order reaction rate coefficients are obtained using a linear fit to obtain (a) k2, (b) kRA, (c) kproto and (d) k2,C7. When using large acetylene number densities, required for the measurements on m/z 106 and 130, the reaction towards m/z 104 and 105 has almost proceeded completely during the helium pulse phase (which is not included in the fit) and yields a larger fit error for the latter. The error bars indicate the 1σ errors and the grey shaded bands define the 2σ uncertainty regions of the fits.
Extended Data Fig. 2 Exemplary kinetic profile of the reaction of pyridine+ with acetylene in a continuous high-pressure regime at 150 K.
An ion pulse length of 5 ms and acetylene number densities of 1.8 x 109 to 3.1 x 109 have been used. Measurements are shown as points with 1σ error bars from multiple iterations on each datapoint. The solid lines display the results of the fitted reaction equation model. The grey area is not included in the fit, as the ions are still being thermalized after entering the trap. The value for k2 that describes the bimolecular reaction to m/z 104 is fixed to 4.9 x 10−10 cm3 molecule−1 s−1 as determined from low pressure regime scans.
Extended Data Fig. 3 Experimental IRMPD infrared spectrum of m/z 105 (grey) and comparison with scaled harmonic infrared spectra of different C7H7N•+ isomers (coloured sticks).
The vibrational frequencies have been calculated for (a) two conformers of 2-vinyl-β-distonic-H-pyridine+ (Ca-3, blue sticks), (b) two conformers of 2-acetylene-H-pyridine+ (Ca-2 and Cb-2, pink sticks), (c) pyridine-C2H2+ complex (C-1, blue-green sticks) and 2-vinyl-pyridine+ (Cb-3, green sticks) and (d) N-acetylene-pyridine+ (N-1, purple sticks) and N-vinyl-α-distonic-pyridine+ (N-2, dark-purple sticks) at the B3LYP/N07D-GD3 level of theory. The assigned structures for m/z 105 are shown in panel (a).
Extended Data Fig. 4 Comparison between the experimental IRMPD spectrum of m/z 130 produced in the reaction in the ion trap (grey) and the IRPD spectrum of m/z 130 formed inside the ion storage source (red).
To evaluate the B3LYP/N07D level of theory, the calculated anharmonic infrared spectra of quinolizinium+ (N-5) and H-quinoline+ (Ca-6) are plotted as orange and green sticks, respectively.
Extended Data Fig. 5 Calculated potential energy surface of the first part of the C formation pathway (black) as shown in Fig. 5 and an alternative route via N-acetylene-pyridine+ (N-1).
The energies are zero-point energy corrected and calculated with respect to the entrance energy of pyridine+ and acetylene at the B3LYP/6-311++G(d,p) level of theory. Energies obtained from additional calculations at the DSD-PBEP86-GD3BJ/aug-cc-pVQZ//M06-2X/6-31++G(2df,p) level of theory for the transition states TS-NC-2 and TS-Cb-1 and the product Cb-2 are shown next to it.
Extended Data Fig. 6 Experimental IRMPD infrared spectrum of m/z 104 (grey) and comparison with scaled harmonic infrared spectra of different C7H6N+ isomers (coloured sticks).
The vibrational frequencies have been calculated for (a) 2-ethynyl-H-pyridine+ (Ca-7, red sticks), (b) N-ethynyl-pyridine+ (N-6, blue sticks) and (c) 3 and 4-ethynyl-H-pyridine+ (green and orange sticks, respectively) at the B3LYP/N07D-GD3 level of theory. The assigned structure for m/z 104 is shown in panel (a).
Supplementary information
Supplementary Information
Ordinary differential equations model, Supplementary Table 1 and Figs. 1 and 2.
Source data
Source Data Fig. 2
Mass spectra and kinetic plots.
Source Data Fig. 3
Experimental and calculated infrared spectra.
Source Data Fig. 5
Experimental and calculated infrared spectra.
Rights and permissions
About this article
Cite this article
Rap, D.B., Schrauwen, J.G.M., Marimuthu, A.N. et al. Low-temperature nitrogen-bearing polycyclic aromatic hydrocarbon formation routes validated by infrared spectroscopy. Nat Astron 6, 1059–1067 (2022). https://doi.org/10.1038/s41550-022-01713-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41550-022-01713-z