Abstract
The recent suggestion of phosphine in Venus’s atmosphere has regenerated interest in the idea of life in clouds. However, such analyses usually neglect the role of water activity, which is a measure of the relative availability of water, in habitability. Here we compute the water activity within the clouds of Venus and other Solar System planets from observations of temperature and water-vapour abundance. We find water-activity values of sulfuric acid droplets, which constitute the bulk of Venus’s clouds, of ≤0.004, two orders of magnitude below the 0.585 limit for known extremophiles. Considering other planets, ice formation on Mars imposes a water activity of ≤0.537, slightly below the habitable range, whereas conditions are biologically permissive (>0.585) at Jupiter’s clouds (although other factors such as their composition may play a role in limiting their habitability). By way of comparison, Earth’s troposphere conditions are, in general, biologically permissive, whereas the atmosphere becomes too dry for active life above the middle stratosphere. The approach used in the current study can also be applied to extrasolar planets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
We confirm that all relevant data are included in the paper and/or its Supplementary Information files. Source data are provided with this paper.
References
DeLeon-Rodriguez, N. et al. Microbiome of the upper troposphere. Proc. Natl Acad. Sci. USA 110, 2575–2580 (2013).
Kabir, E. et al. Recent advances in monitoring, sampling, and sensing techniques for bioaerosols in the atmosphere. ACS Sens. 5, 1254–1267 (2020).
Stevenson, A. et al. Is there a common water-activity limit for the three domains of life? ISME J. 9, 1333–1351 (2015).
Rummel, J. D. et al. A new analysis of Mars “special regions”: findings of the Second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14, 887–968 (2014).
Greaves, J. S. et al. Phosphine gas in the cloud decks of Venus. Nat. Astron. https://doi.org/10.1038/s41550-020-1174-4 (2020).
Mogul, R., Limaye, S. S., Way, M. J. & Cordova, J. A. Venus’ mass spectra show signs of disequilibria in the middle clouds. Geophys. Res. Lett. 48, e2020GL091327 (2021).
Morowitz, H. & Sagan, C. Life in the clouds of Venus? Nature 215, 1259–1260 (1967).
Limaye, S. S. et al. Venus’ spectral signatures and the potential for life in the clouds. Astrobiology 18, 1181–1198 (2018).
Izenberg, N. R. et al. The Venus life equation. Astrobiology https://doi.org/10.1089/ast.2020.2326 (2021).
Sagan, C. The planet Venus. Science 133, 849–858 (1961).
Cockell, C. S. Life on Venus. Planet. Space Sci. 47, 1487–1501 (1999).
Bains, W. et al. Phosphine on Venus cannot be explained by conventional processes. Preprint at http://arxiv.org/abs/2009.06499 (2020).
Seager, S. et al. The Venusian lower atmosphere haze as a depot for desiccated microbial life: a proposed life cycle for persistence of the Venusian aerial biosphere. Astrobiology https://doi.org/10.1089/ast.2020.2244 (2020).
Stevenson, A. et al. Aspergillus penicillioides differentiation and cell division at 0.585 water activity. Environ. Microbiol. 19, 687–697 (2017).
Meisner, A. et al. Soil microbial legacies differ following drying–rewetting and freezing–thawing cycles. ISME J. 15, 1207–1221 (2021).
Stevenson, A. et al. Multiplication of microbes below 0.690 water activity: implications for terrestrial and extraterrestrial life. Envrion. Microbiol. 17, 257–277 (2015).
Benner, S. A., Ricardo, A. & Carrigan, M. A. Is there a common chemical model for life in the Universe? Curr. Opin. Chem. Biol. 8, 672–689 (2004).
Schleper, C. et al. Picrophilus gen. nov., fam. nov.: a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J. Bacteriol. Res. 177, 7050–7059 (1995).
Clegg, S. L., Brimblecombe, P. & Wexler, A. S. Thermodynamic model of the system H+–NH4+–SO42−–NO3−–H2O at tropospheric temperatures. J. Phys. Chem. A 102, 2137–2154 (1998).
Gmitro, J. I. & Vermeulen, T. Vapor-liquid equilibria for aqueous sulfuric acid. AIChE J. 10, 740–746 (1964).
Gmitro, J. I. & Vermeulen, T. Vapor-Liquid Equilibria for Aqueous Sulfuric Acid Technical Report No. UCRL-10886; TID-4500 (Ernest Orlando, Lawrence Berkeley National Laboratory, 1963); https://doi.org/10.2172/876220
Wilson, R. E. Humidity control by means of sulfuric acid solutions, with critical compilation of vapor pressure data. J. Indust. Eng. Chem. 13, 326–331 (1921).
Seiff, A. et al. Models of the structure of the atmosphere of Venus from the surface to 100 kilometers altitude. Adv. Space Res. 5, 3–58 (1985).
Ignatiev, N. I. et al. Water vapour in the lower atmosphere of Venus: a new analysis of optical spectra measured by entry probes. Planet. Space Sci. 45, 427–438 (1997).
Bertaux, J. L. et al. SPICAV on Venus Express: three spectrometers to study the global structure and composition of the Venus atmosphere. Planet. Space Sci. 55, 1673–1700 (2007).
Gao, P., Zhang, X., Crisp, D., Bardeen, C. G. & Yung, Y. L. Bimodal distribution of sulfuric acid aerosols in the upper haze of Venus. Icarus 231, 83–98 (2014).
Carslaw, K. S., Peter, T. & Clegg, S. L. Modeling the composition of liquid stratospheric aerosols. Rev. Geophys. 35, 125–154 (1997).
Martin, S. T. Phase transitions of aqueous atmospheric particles. Chem. Rev. 100, 3403–3454 (2000).
Zhang, X., Liang, M. C., Mills, F. P., Belyaev, D. A. & Yung, Y. L. Sulfur chemistry in the middle atmosphere of Venus. Icarus 217, 714–739 (2012).
Arney, G. et al. Spatially resolved measurements of H2O, HCl, CO, OCS, SO2, cloud opacity, and acid concentration in the Venus near‐infrared spectral windows. J. Geophys. Res. Planets 119, 1860–1891 (2014).
Krasnopolsky, V. A. Vertical profiles of H2O, H2SO4, and sulfuric acid concentration at 45–75 km on Venus. Icarus 252, 327–333 (2015).
Hallsworth, J. E. Wooden owl that redefines Earth’s biosphere may yet catapult a fungus into space. Environ. Microbiol. 21, 2202–2211 (2019).
Koop, T., Luo, B., Biermann, U. M., Crutzen, P. J. & Peter, T. Freezing of HNO3/H2SO4/H2O solutions at stratospheric temperatures: nucleation statistics and experiments. J. Phys. Chem. A 101, 1117–1133 (1997).
Ball, P. Water is an active matrix of life for cell and molecular biology. Proc. Natl Acad. Sci. USA 114, 13327–13335 (2017).
Brown, A. D. Microbial Water Stress Physiology (John Wiley, 1990).
Chin, J. P. et al. Solutes determine the temperature windows for microbial survival and growth. Proc. Natl Acad. Sci. USA 107, 7835–7840 (2010).
Hallsworth, J. E., Heim, S. & Timmis, K. N. Chaotropic solutes cause water stress in Pseudomonas putida. Environ. Microbiol. 5, 1270–1280 (2003).
de Lima Alves, F. et al. Concomitant osmotic and chaotropicity-induced stresses in Aspergillus wentii: compatible solutes determine the biotic window. Curr. Genet. 61, 457–477 (2015).
Santos, R., de Carvalho, C. C. C. R., Stevenson, A., Grant, I. R. & Hallsworth, J. E. Extraordinary stress-tolerance of mycobacteria. Environ. Microbiol. Rep. 7, 746–764 (2015).
Oleinikova, A., Smolin, N., Brovchenko, I., Geiger, A. & Winter, R. Formation of spanning water networks on protein surfaces via 2D percolation transition. J. Phys. Chem. B 109, 1988–1998 (2005).
Arcangeli, C., Bizzarri, A. R. & Cannistraro, S. Role of interfacial water in the molecular dynamics-stimulated dynamical transition of plastocyanin. Chem. Phys. Lett. 291, 7–14 (1998).
Roos-Serote, M. et al. Proximate humid and dry regions in Jupiter’s atmosphere indicate complex local meteorology. Nature 405, 158–160 (2000).
Seiff, A. et al. Thermal structure of Jupiter’s atmosphere near the edge of a 5‐μm hot spot in the north equatorial belt. J. Geophys. Res. Planets 103, 22857–22889 (1998).
Wong, M. H., Mahaffy, P. R., Atreya, S. K., Niemann, H. B. & Owen, T. C. Updated Galileo probe mass spectrometer measurements of carbon, oxygen, nitrogen, and sulfur on Jupiter. Icarus 171, 153–170 (2004).
Li, C. et al. The water abundance in Jupiter’s equatorial zone. Nat. Astron. 4, 609–616 (2020).
Roos-Serote, M., Atreya, S. K., Wong, M. K. & Drossart, P. On the water abundance in the atmosphere of Jupiter. Planet. Space Sci. 52, 397–414 (2004).
Schuerger, A. C., Richards, J. T., Newcombe, D. A. & Venkateswaran, K. Rapid inactivation of seven Bacillus spp. under simulated Mars UV irradiation. Icarus 181, 52–62 (2006).
Withers, P. & Smith, M. D. Atmospheric entry profiles from the Mars exploration rovers Spirit and Opportunity. Icarus 185, 133–142 (2006).
Fedorova, A. A. et al. Stormy water on Mars: The distribution and saturation of atmospheric water during the dusty season. Science 367, 297–300 (2020).
Murphy, D. M. & Koop, T. Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q. J. R. Meteorol. Soc. 131, 1539–1565 (2005).
Kreidberg, L. et al. A precise water abundance measurement for the hot Jupiter WASP-43b. Astrophys. J. Lett. 793, L27 (2014).
Villanueva, G. et al. No phosphine in the atmosphere of Venus. Preprint at https://arxiv.org/abs/2010.14305 (2020).
Snellen, I. A. G., Guzman-Ramirez, L., Hogerheijde, M. R., Hygate, A. P. S. & van der Tak, F. F. S. Re-analysis of the 267 GHz ALMA observations of Venus—no statistically significant detection of phosphine. Astron. Astrophys. 644, L2 (2020).
Witze, A. ‘Life on Venus’ claim faces strongest challenge yet. Nature 590, 19–20 (2021).
Greene, T. P. et al. Characterizing transiting exoplanet atmospheres with JWST. Astrophys. J. 817, 17 (2016).
Hoffmann, E. H. et al. An advanced modeling study on the impacts and atmospheric implications of multiphase dimethyl sulfide chemistry. Proc. Natl Acad. Sci. USA 113, 11776–11781 (2016).
Attard, E. et al. Effects of atmospheric conditions on ice nucleation activity of Pseudomonas. Atmos. Chem. Phys. 12, 10667–10677 (2012).
Wagner, W. & Pruss, A. International equations for the saturation properties of ordinary water substance. Revised according to the International Temperature Scale of 1990. Addendum J. Phys. Chem. Ref. Data 16, 893 (1987). J. Phys. Chem. Ref. Data 22, 783–787 (1993).
Wexler, A. S. & Clegg, S. L. Atmospheric aerosol models for systems including the ions H+, NH4+, Na+, SO42−, NO3−, Cl−, Br−, and H2O. J. Geophys. Res. 107, ACH14-1–ACH14-14 (2002).
Jia, S. Technical note: Comparison and interconversion of pH based on different standard states for aerosol acidity characterization. Atmos. Chem. Phys. 18, 11125–11133 (2018).
International Union of Pure and Applied Chemistry IUPAC Compendium of Chemical Terminology 2nd edn (complied by McNaught, A. D. & Wilkinson, A.) (Blackwell Scientific Publications, 1997); https://doi.org/10.1351/goldbook
Lamb, D. & Verlinde, J. Physics and Chemistry of Clouds (Cambridge Univ. Press, 2011).
Camuffo, D. Physics of drop formation and micropore condensation. In: Microclimate for Cultural Heritage Conservation, Restoration, and Maintenance of Indoor and Outdoor Monuments (2ed. Camuffo, D.) 165–201 (Elsevier, 2014).
Wix, A., Brachert, L., Sinanis, S. & Schaber, K. A simulation tool for aerosol formation during sulphuric acid absorption in a gas cleaning process. J. Aerosol Sci. 41, 1066–1079 (2010).
Gardner, J. A. et al. Measurement of the mass accommodation coefficient of SO2 (g) on water droplets. J. Geophys. Res. 92, 10887–10895 (1987).
Schulze-Makuch, D., Grinspoon, D. H., Abbas, O., Irwin, L. N. & Bullock, M. A. A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 4, 11–18 (2004).
Dartnell, L. R. et al. Constraints on a potential aerial biosphere on Venus: I. Cosmic rays. Icarus 257, 396–405 (2015).
Winston, P. W. & Bates, D. H. Saturated solutions for the control of humidity in biological research. Ecology 41, 232–237 (1960).
Lee, C. J. D. et al. NaCl-saturated brines are thermodynamically moderate, rather than extreme, microbial habitats. FEMS Microbiol. Rev. 42, 672–693 (2018).
Harrison, J. P. et al. Aerobically respiring prokaryotic strains exhibit a broader temperature–pH–salinity space for cell division than anaerobically respiring and fermentative strains. J. R. Soc. Interface 12, 20150658 (2015).
Benison, K. C., O’Neill, W. K., Blain, D. & Hallsworth, J. E. Water activities of acid brine lakes approach the limit for life. Astrobiology https://doi.org/10.1089/ast.2020.2334 (2021).
Moger-Reischer, R. Z. & Lennon, J. T. Microbial ageing and longevity. Nat. Rev. Microbiol. 17, 679–690 (2019).
Khaleque, H. N., Kaksonen, A. H., Boxall, N. J. & Watkin, E. L. J. Chloride ion tolerance and pyrite bioleaching capabilities of pure and mixed halotolerant, acidophilic iron- and sulfur-oxidizing cultures. Miner. Eng. 120, 87–93 (2018).
Khaleque, H. N. et al. Genome-based classification of two halotolerant extreme acidophiles, Acidihalobacter prosperus V6 (=DSM 14174 =JCM 32253) and ‘Acidihalobacter ferrooxidans’ V8 (=DSM 14175 =JCM 32254) as two new species, Acidihalobacter aeolianus sp. nov. and Acidihalobacter ferrooxydans sp. nov., respectively. Int. J. Syst. Evol. Microbiol. 69, 1557–1565 (2019).
Hallsworth, J. E. et al. Limits of life in MgCl2‐containing environments: chaotropicity defines the window. Environ. Microbiol. 9, 801–813 (2007).
Stevenson, A., Hamill, P. G., Dijksterhuis, J. & Hallsworth, J. E. Water‐, pH‐ and temperature relations of germination for the extreme xerophiles Xeromyces bisporus (FRR 0025), Aspergillus penicillioides (JH06THJ) and Eurotium halophilicum (FRR 2471). Microb. Biotechnol. 10, 330–340 (2017).
Rosso, L., Lobry, J. R., Bajard, S. & Flandrois, J. P. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl. Environ. Microbiol. 61, 610–616 (1995).
Beale, E. Confidence regions in non-linear estimation. J. R. Stat. Soc. Ser. B 22, 41–88 (1960).
Fu, W. & Zhang, X. Global phosphorus dynamics in terms of phosphine. npj Clim. Atmos. Sci. 3, 51 (2020).
Rozenberg, M., Loewenschuss, A. & Nielsen, C. J. Hydrogen bonding in the sulfuric acid–methanol–water system: A matrix isolation and computational study. J. Phys. Chem. A 119, 2271–2280 (2015).
Rozenberg, M., Loewenschuss, A. & Nielsen, C. J. H-bonding of sulfuric acid with its decomposition products: An infrared matrix isolation and computational study of the H2SO4·H2O·SO3 complex. J. Phys. Chem. A 120, 3450–3455 (2016).
Stevenson, A. & Hallsworth, J. E. Water and temperature relations of Actinobacteria. Environ. Microbiol. Rep. 6, 744–755 (2014).
Ball, P. & Hallsworth, J. E. Water structure and chaotropicity: their uses, abuses, and biological implications. Phys. Chem. Chem. Phys. 17, 8297–8305 (2015).
Cray, J. A. Chaotropicity: a key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 33, 228–259 (2015).
Roy, C. et al. Microbiome and ecology of hot spring-microbialite system on the Trans-Himalayan plateau. Sci. Rep. 10, 5917 (2020).
Stevenson, A. et al. Glycerol enhances fungal germination at the water-activity limit for life. Environ. Microbiol. 19, 947–967 (2017).
Williams, J. P. & Hallsworth, J. E. Limits of life in hostile environments: no barriers to biosphere function? Environ. Microbiol. 11, 3292–3308 (2009).
Wakisaka, A. & Matsuura, K. Microheterogeneity of ethanol-water binary mixtures observed at the cluster level. J. Mol. Liq. 129, 25–32 (2006).
Zhao, H., Zhanga, Q. & Du, L. Hydrogen bonding in cyclic complexes of carboxylic acid–sulfuric acid and their atmospheric implications. RSC Adv. 6, 71733–71743 (2016).
Fleming, E. L., Chandra, S., Shoeberl, M. R. & Barnett, J. J. Monthly Mean Global Climatology of Temperature, Wind, Geopotential Height, and Pressure for 0–120 km NASA Technical Memorandum TM-100697 (NASA, 1988).
Warneck, P. & Williams, J. The Atmospheric Chemist’s Companion (Springer, 2012).
Bohren, C. F. & Clothiaux, E. Fundamentals of Atmospheric Radiation (Wiley, 2006).
National Oceanic and Atmospheric Administration U.S. Standard Atmosphere 1976 (Governmental Printing Office, 1976).
Acknowledgements
We are grateful to S. L. Clegg (University of East Anglia, England, UK) for helpful discussions on the use of the E-AIM at low water activity and the provision of some code; C. S. Cockell (University of Edinburgh, Scotland, UK), D. Y. Sorokin (Winogradsky Institute of Microbiology, Russia) and A. Ventosa (University of Seville, Spain) for providing information about thermotolerance of halophiles; M. S. Marley (NASA Ames Research Center, CA, USA) for information on Jupiter and exoplanets; A. Méndez (University of Puerto Rico, Puerto Rico) for inputs relating to analysis of Earth’s atmosphere; J. R. Lobry (University of Lyons, France) who helped with use of the cardinal pH model; N. J. Tosca (University of Cambridge, England, UK) for discussions about thermodynamic properties of aqueous sulfuric acid solutions; and E. L. J. Watkin (Curtin University, Australia) who provided information about stress tolerance of Acidihalobacter. J.E.H. was funded by the Biotechnology and Biological Sciences Research Council (BBSRC, United Kingdom) project BBF003471; M.-P.Z. was supported by projects PID2019-104205GB-C21 of Ministry of Science and Innovation and MDM-2017-0737 Unidad de Excelencia ‘María de Maeztu’- Centro de Astrobiología (CSIC-INTA) (Spain); and O.V.G. was supported by the Centre of Environmental Biotechnology Project (grant 810280) funded by the European Regional Development Fund (ERDF) through the Welsh Government.
Author information
Authors and Affiliations
Contributions
J.E.H., P.B. and M.-P.Z. conceived the study; J.E.H., C.P.M., T.K. and M.-P.Z. designed the approach; all authors obtained and analysed the data (T.K., M.-P.Z., J.E.H. and J.B. for water activity of H2SO4–H2O mixtures; C.P.M. for the Martian and Jovian atmospheres and relative humidity of the Venusian atmosphere; T.K., C.P.M. and J.E.H. for the Earth case study; T.K., C.P.M., J.E.H., M.-P.Z. and J.B. for quantification of sulfuric acid concentration and water activity of the droplets in Venusian clouds; J.E.H., T.D.D. and O.V.G. for acidity and water-activity limits of life on Earth; M.K.D., P.B. and J.E.H. for activities of sulfuric acid on the cellular system; and J.E.H., T.D.D., C.P.M., M.K.D., M.-P.Z., J.M.-T., T.K., J.B. and P.B. for determination of habitability for Venus’s acid clouds); T.K., C.P.M., J.E.H., T.D.D., M.K.D. and M.-P.Z. constructed the displays; J.E.H. produced an initial draft of the manuscript; all authors contributed to writing the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Astronomy thanks Abel Méndez, Dirk Schulze-Makuch and Nicholas Tosca for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, text on biophysical limits of terrestrial microbes, text relating to the equilibration of droplets, text for Fig. 3, text relating to validation of water activity for H2SO4–H2O mixtures, text for Fig. 6b, and references.
Supplementary Data 1
Supplementary Table 1a: water-vapour-pressure data (in torr) as a function of temperature and sulfuric acid concentrations in liquid H2SO4–H2O mixtures. Supplementary Table 1b: water-activity data as a function of temperature and sulfuric acid concentrations in liquid H2SO4–H2O mixtures. Supplementary Table 2a: water-activity data as a function of temperature and sulfuric acid concentrations in liquid H2SO4–H2O mixtures. Supplementary Table 2b: water-activity data as a function of temperature and sulfuric acid concentrations in liquid H2SO4–H2O mixtures from Wilson22. Supplementary Table 3: properties and typical conditions for major cloud types in Earth’s atmosphere.
Source data
Source Data Fig. 1
Source Data for Fig. 1 (water activity of liquid H2SO4–H2O mixtures as a function of temperature and sulfuric acid concentration).
Source Data Fig. 2
Source Data for Fig. 2 (water activity of the Venusian atmosphere).
Source Data Fig. 4
Source Data for Fig. 4 (water activity in the Jovian atmosphere).
Source Data Fig. 5
Source Data for Fig. 5 (average water-vapour mixing ratio profiles in Earth’s atmosphere).
Source Data Fig. 6
Source Data for Fig. 6a (relative humidity and water-activity ranges at different altitudes in Earth’s atmosphere).
Source Data Fig. 7
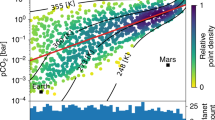
Source Data for Fig. 7 (generalized exoplanet water-activity analysis).
Rights and permissions
About this article
Cite this article
Hallsworth, J.E., Koop, T., Dallas, T.D. et al. Water activity in Venus’s uninhabitable clouds and other planetary atmospheres. Nat Astron 5, 665–675 (2021). https://doi.org/10.1038/s41550-021-01391-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41550-021-01391-3
This article is cited by
-
Venus, the Planet: Introduction to the Evolution of Earth’s Sister Planet
Space Science Reviews (2023)
-
The Habitability of Venus
Space Science Reviews (2023)
-
Proposed energy-metabolisms cannot explain the atmospheric chemistry of Venus
Nature Communications (2022)
-
Habitable potentials
Nature Astronomy (2021)