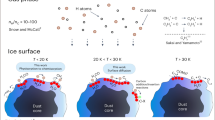
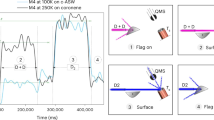
Abstract
Astrochemical reactions on the surfaces of dust grains, for instance, are thought to be responsible for the formation of complex organic molecules, which are of potential importance for the origin of life. In the situation where the chemical composition of dust surfaces is not precisely known, knowledge of the fundamental reaction properties gains significance. Here we describe an experimental technique that can be used to measure the energy released in reactions involving of a single pair of reactants. These data can be directly compared with the results of quantum chemical computations leading to unequivocal conclusions regarding the reaction pathways and the presence of energy barriers. It allows the prediction of the outcomes of astrochemical surface reactions with higher accuracy compared with that achieved based on gas-phase studies. However, for the highest accuracy, some understanding of the catalytic influence of specific surfaces on the reactions is required. The method was applied to study the reactions of C atoms with H2, O2 and C2H2. The formation of HCH, CO + O and triplet cyclic-C3H2 products has been revealed, correspondingly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Carruthers, G. R. Atomic and molecular hydrogen in interstellar space. Space Sci. Rev. 10, 459–482 (1970).
Herbst, E. & van Dishoeck, E. F. Complex organic interstellar molecules. Annu. Rev. Astron. Astrophys. 47, 427–480 (2009).
Ehrenfreund, P. & Charnley, S. B. Organic molecules in the interstellar medium, comets, and meteorites: a voyage from dark clouds to the early Earth. Annu. Rev. Astron. Astrophys. 38, 427–483 (2000).
Fray, N. et al. High-molecular-weight organic matter in the particles of comet 67P/Churyumov–Gerasimenko. Nature 538, 72–74 (2016).
Elsila, J. E. et al. Meteoritic amino acids: diversity in compositions reflects parent body histories. ACS Cent. Sci. 2, 370–379 (2016).
Pearce, B. K. D., Pudritz, R. E., Semenov, D. A. & Henning, T. K. Origin of the RNA world: the fate of nucleobases in warm little ponds. Proc. Natl Acad. Sci. USA 114, 11327–11332 (2017).
OrÓ, J. Synthesis of organic compounds by high-energy electrons. Nature 197, 971–974 (1963).
Wakelam, V. et al. A kinetic database for astrochemistry (KIDA). Astrophys. J. Suppl. Ser. 199, 21 (2012).
Krasnokutski, S. A. et al. Ultra-low-temperature reactions of carbon atoms with hydrogen molecules. Astrophys. J. Lett. 818, L31 (2016).
Lee, Y. T. Molecular-beam studies of elementary chemical processes. Science 236, 793–798 (1987).
Lee, Y. T. Molecular-beam studies of elementary chemical processes (Nobel lecture). Angew. Chem. Int. Ed. 26, 939–951 (1987).
Schauermann, S., Silbaugh, T. L. & Campbell, C. T. Single-crystal adsorption calorimetry on well-defined surfaces: from single crystals to supported nanoparticles. Chem. Rec. 14, 759–774 (2014).
Toennies, J. P. & Vilesov, A. F. Superfluid helium droplets: a uniquely cold nanomatrix for molecules and molecular complexes. Angew. Chem. Int. Ed. 43, 2622–2648 (2004).
Schollkopf, W. & Toennies, J. P. Nondestructive mass selection of small van-der-Waals clusters. Science 266, 1345–1348 (1994).
Goyal, S., Schutt, D. L. & Scoles, G. Vibrational spectroscopy of sulfur-hexafluoride attached to helium clusters. Phys. Rev. Lett. 69, 933–936 (1992).
Mozhayskiy, V., Slipchenko, M. N., Adamchuk, V. K. & Vilesov, A. F. Use of helium nanodroplets for assembly, transport, and surface deposition of large molecular and atomic clusters. J. Chem. Phys. 127, 094701–094706 (2007).
Krasnokutski, S. A. & Huisken, F. Oxidative reactions of silicon atoms and clusters at ultralow temperature in helium droplets. J. Phys. Chem. A 114, 13045–13049 (2010).
Krasnokutski, S. A. & Huisken, F. Low-temperature chemistry in helium droplets: reactions of aluminum atoms with O2 and H2O. J. Phys. Chem. A 115, 7120–7126 (2011).
Krasnokutski, S. A., Huisken, F., Jäger, C. & Henning, T. Growth and destruction of PAH molecules in reactions with carbon atoms. Astrophys. J. 836, 32 (2017).
Krasnokutski, S. A. & Huisken, F. Ultra-low-temperature reactions of C(3 P 0) atoms with benzene molecules in helium droplets. J. Chem. Phys. 141, 214306 (2014).
Krasnokutski, S. A. et al. Formation of silicon oxide grains at low temperature. Astrophys. J. 782, 15 (2014).
Lewis, W. K., Harruff-Miller, B. A., Leatherman, P., Gord, M. A. & Bunker, C. E. Helium droplet calorimetry of strongly bound species: carbon clusters from C2 to C12. Rev. Sci. Instrum. 85, 094102 (2014).
Dutra, M. & Hinde, R. Accurate simulations of helium pick-up experiments using a rejection-free Monte Carlo method. AIP Adv. 8, 045203 (2018).
Loginov, E. et al. Photoabsorption of AgN (N ~ 6–6000) nanoclusters formed in helium droplets: transition from compact to multicenter aggregation. Phys. Rev. Lett. 106, 233401–233404 (2011).
Eyler, E. E. & Melikechi, N. Near-threshold continuum structure and the dissociation-energies of H2, HD, and D2. Phys. Rev. A 48, R18–R21 (1993).
Csaszar, A. G., Leininger, M. L. & Szalay, V. The standard enthalpy of formation of CH2. J. Chem. Phys. 118, 10631–10642 (2003).
Harding, L. B., Guadagnini, R. & Schatz, G. C. Theoretical-studies of the reactions H + CH → C + H2 and C + H2 → CH2 using an abinitio global ground-state potential surface for CH2. J. Phys. Chem. 97, 5472–5481 (1993).
Takahashi, J. & Yamashita, K. Ab initio studies on the interstellar molecules C3H2 and C3H and the mechanism for the neutral–neutral reaction C(3 P) + C2H2. J. Chem. Phys. 104, 6613–6627 (1996).
Kaiser, R. I., Ochsenfeld, C., Head-Gordon, M., Lee, Y. T. & Suits, A. G. Crossed-beam reaction of carbon atoms with hydrocarbon molecules. III: Chemical dynamics of propynylidyne (l-C3H;X2Πj) and cyclopropynylidyne (c-C3H;X2 B 2) formation from reaction of C(3 P j) with acetylene, C2H2(X1Σ+ g). J. Chem. Phys. 106, 1729–1741 (1997).
Walker, A. V. & King, D. A. Reaction of gaseous oxygen with adsorbed carbon on Pt{110}(1×2). J. Chem. Phys. 112, 1937–1945 (2000).
Ruscic, B., Feller, D. & Peterson, K. A. Active thermochemical tables: dissociation energies of several homonuclear first-row diatomics and related thermochemical values. Theor. Chem. Acc. 133, 1415 (2013).
Minissale, M., Congiu, E., Manico, G., Pirronello, V. & Dulieu, F. CO2 formation on interstellar dust grains: a detailed study of the barrier of the CO plus O channel. Astron. Astrophys. 559, A49 (2013).
Spielfiedel, A. et al. Bent valence excited states of CO2. J. Chem. Phys. 97, 8382–8388 (1992).
Lu, Z., Chang, Y. C., Yin, Q. Z., Ng, C. Y. & Jackson, W. M. Evidence for direct molecular oxygen production in CO2 photodissociation. Science 346, 61–64 (2014).
Kobayashi, H. et al. Hydrogenation and deuteration of C2H2 and C2H4 on cold grains: a clue to the formation mechanism of C2H6 with astronomical interest. Astrophys. J. 837, 155 (2017).
Lamberts, T. & Kastner, J. Influence of surface and bulk water ice on the reactivity of a water-forming reaction. Astrophys. J. 846, 43 (2017).
Rimola, A., Taquet, V., Ugliengo, P., Balucani, N. & Ceccarelli, C. Combined quantum chemical and modeling study of CO hydrogenation on water ice. Astron. Astrophys. 572, A70 (2014).
Harvey, L., Paule, S., Martin, C. & Maryvonne, G. The abundance of C3H2 and other small hydrocarbons in the diffuse interstellar medium. Astrophys. J. Lett. 753, L28 (2012).
Henning, T. & Salama, F. Carbon in the Universe. Science 282, 2204–2210 (1998).
Beuther, H. et al. Carbon in different phases ([CII], [CI], and CO) in infrared dark clouds: cloud formation signatures and carbon gas fractions. Astron. Astrophys. 571, A53 (2014).
Krasnokutski, S. A. & Huisken, F. Resonant two-photon ionization spectroscopy of Al atoms and dimers solvated in helium nanodroplets. J. Chem. Phys. 142, 084311 (2015).
Krasnokutski, S. A. & Huisken, F. A simple and clean source of low-energy atomic carbon. Appl. Phys. Lett. 105, 113506 (2014).
Acknowledgements
The authors are grateful for the support by the Max Planck Society and the DFG (contract No. KR 3995/3-1).
Author information
Authors and Affiliations
Contributions
T.K.H. and S.A.K. designed the research and wrote the paper; S.A.K. performed research and analysed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–4
Rights and permissions
About this article
Cite this article
Henning, T.K., Krasnokutski, S.A. Experimental characterization of the energetics of low-temperature surface reactions. Nat Astron 3, 568–573 (2019). https://doi.org/10.1038/s41550-019-0729-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41550-019-0729-8
This article is cited by
-
A pathway to peptides in space through the condensation of atomic carbon
Nature Astronomy (2022)
-
Photoelectron Spectroscopy of Coronene Molecules Embedded in Helium Nanodroplets
Journal of Low Temperature Physics (2021)