Abstract
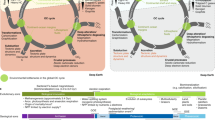
Understanding the chemical evolution of newly formed terrestrial planets involves uncertainties in atmospheric chemical composition and assessing the plausibility of biomolecule synthesis. In this study, an original scenario for the origin of methane on Mars and terrestrial planets is suggested. Carbon dioxide in Martian and other planetary atmospheres can be abiotically converted into a mixture of methane and carbon monoxide by ‘methanogenesis’ on porous mineral photoactive surfaces under soft ultraviolet irradiation. On young planets exposed to heavy bombardment by interplanetary matter, this process can be followed by biomolecule synthesis through the reprocessing of reactive reducing atmospheres by impact-induced shock waves. The proposed mechanism of methanogenesis may help to answer the question concerning the formation of methane and carbon monoxide by photochemical processes, the formation of biomolecules on early Earth and other terrestrial planets, and the source and seasonal variation of methane concentrations on Mars.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kasting, J. F. Earth’s early Atmosphere. Science 259, 920–926 (1993).
Delano, J. W. Redox history of the Earth’s interior since approximately 3900 Ma: implications for prebiotic molecules. Orig. Life Evol. Biosph. 4–5, 311–341 (2001).
Yang, X., Gaillard, F. & Scaillet, B. A relatively reduced Hadean continental crust and implications for the early atmosphere and crustal rheology. Earth Planet. Sci. Lett. 393, 210–219 (2014).
Marty, B. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313, 56–66 (2012).
Lammer, H. et al. Origin and loss of nebula-captured hydrogen envelopes from ‘sub’- to ‘super-Earths’ in the habitable zone of Sun-like stars. Mon. Not. R. Astron. Soc. 439, 3225–3238 (2014).
de Niem, D., Kuehrt, E., Morbidelli, A. & Motschmann, U. Atmospheric erosion and replenishment induced by impacts upon the Earth and Mars during a heavy bombardment. Icarus 221, 495–507 (2012).
Sekine, Y. et al. An experimental study on Fischer-Tropsch catalysis: implications for impact phenomena and nebular chemistry. Meteorit. Planet. Sci. 41, 715–729 (2006).
Hashimoto, G. L., Abe, Y. & Sugita, S. The chemical composition of the early terrestrial atmosphere: formation of a reducing atmosphere from CI-like material. J. Geophys. Res. 112, E05010 (2007).
Schaefer, L. & Fegley, B. Jr Outgassing of ordinary chondritic material and some of its implications for the chemistry of asteroids, planets, and satellites. Icarus 186, 462–483 (2007).
Webster, C. R. et al. Mars methane detection and variability at Gale crater. Science 347, 415–417 (2015).
Sullivan, W. T. III & Baross, J. (eds) Planets and Life: The Emerging Science of Astrobiology 1st edn (Cambridge Univ. Press, New York, 2007).
Chyba, C. & Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules — an inventory for the origin of life. Nature 355, 125–132 (1992).
Koeberl, C. Impact processes on the early Earth. Elements 2, 211–216 (2006).
Ferus, M. et al. High-energy chemistry of formamide: a simpler way for nucleobase formation. J. Phys. Chem. 118, 719–736 (2014).
Ferus, M. et al. High-energy chemistry of formamide: a unified mechanism of nucleobase formation. Proc. Natl Acad. Sci. USA 112, 657–662 (2015).
Nair, H., Allen, M., Anbar, A. D., Yung, Y. L. & Clancy, R. T. A photochemical model of the Martian atmosphere. Icarus 111, 124–150 (1994).
Hu, R., Bloom, A. A., Gao, P., Miller, C. E. & Yung, Y. L. Hypotheses for near-surface exchange of methane on Mars. Astrobiology 16, 539–550 (2016).
Levin, G. V. & Straat, P. A. The case for extant life on Mars and its possible detection by the Viking labeled release experiment. Astrobiology 16, 798–810 (2016).
Shkrob, I. A., Chemerisov, S. D. & Marin, T. W. Photocatalytic decomposition of carboxylated molecules on light-exposed Martian regolith and its relation to methane production on Mars. Astrobiology 10, 425–436 (2010).
Civiš, S. et al. Photocatalytic transformation of CO2 to CH4 and CO on acidic surface of TiO2 anatase. Opt. Mater. (Amst) 80–83 (2016).
Shkrob, I. A., Marin, T. W., He, H. & Zapol, P. Photoredox reactions and the catalytic cycle for carbon dioxide fixation and methanogenesis on metal oxides. J. Phys. Chem. C 116, 9450–9460 (2012).
Raulin, F., McKay, C., Lunine, J. & Owen, T. in Titan from Cassini-Huygens (eds Brown, R. H., Lebreton, J. P. & Waite, J.) 215–233 (Springer, 2009).
Bell, J. F. (ed.) The Martian Surface: Composition, Mineralogy and Physical Properties (Cambridge Univ. Press, New York, 2008).
Clark, B. C. III et al. Evidence for montmorillonite or its compositional equivalent in Columbia Hills, Mars. J. Geophys. Res. 112, E06S01 (2007).
Ehlmann, B. L. & Edwards, C. S. Mineralogy of the Martian surface. Ann. Rev. Earth Planet. Sci. 42, 291–315 (2014).
Hazen, R. M. et al. Mineral evolution. Am. Mineral. 93, 1693–1720 (2008).
Catling, D. C. et al. Atmospheric origins of perchlorate on Mars and in the Atacama. J. Geophys. Res. Planets 115, E00E11 (2010).
Villanueva, G. L. et al. A sensitive search for organics (CH4, CH3OH, H2CO, C2H6, C2H2, C2H4), hydroperoxyl(HO2), nitrogen compounds (N2O, NH3, HCN) and chlorine species (HCl, CH3Cl) on Mars using ground-based high-resolution infrared spectroscopy. Icarus. 223, 11–27 (2013).
Ming, D. W. et al. Science. 343, 1245267 (2014).
Gordon, P. R. & Sephton, M. A. Organic matter detection on Mars by pyrolysis-FTIR: an analysis of sensitivity and mineral matrix effects. Astrobiology 16, 831–845 (2016).
Hecht, M. H. et al. Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix lander site. Science 325, 64–67 (2009).
Habisreutinger, S. N., Schmidt-Mende, L. & Stolarczyk, J. K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 52, 7372–7408 (2013).
Ronto, G. et al. Solar UV irradiation conditions on the surface of Mars. Photochem. Photobiol. 77, 34–40 (2003).
Schuerger, A. C., Mancinelli, R. L., Kern, R. G., Rothschild, L. J. & McKay, C. P. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated Martian environments: implications for the forward contamination of Mars. Icarus 165, 253–276 (2003).
Chun, S. F. S., Pang, K. D., Cutts, J. A. & Ajello, J. M. Photocatalytic oxidation of organic compounds on Mars. Nature 274, 875–876 (1978).
Quinn, R. C. & Zent, A. P. Peroxide-modified titanium dioxide: a chemical analog of putative Martian soil oxidants. Orig. Life Evol. Biosph. 29, 59–72 (1999).
Wong, A. S., Atreya, S. K. & Encrenaz, T. Chemical markers of possible hot spots on Mars. J. Geophys. Res. 108, 5026 (2003).
Krasnopolsky, V. A., Maillard, J. P. & Owen, T. C. Detection of methane in the martian atmosphere: Evidence for life? Icarus 172, 537–547 (2004).
Mumma, M. J. et al. Strong release of methane on Mars in northern summer 2003. Science 323, 1041–1045 (2009).
Webster, G., Brown, D. & Cantillo, L. Second cycle of Martian seasons completing for Curiosity rover. NASA www.nasa.gov/feature/jpl/second-cycle-of-martian-seasons-completing-for-curiosity-rover (11 May 2016).
Encrenaz, T. et al. Seasonal variations of the Martian CO over Hellas as observed by OMEGA/Mars Express. Astron. Astrophys. 459, 265–270 (2006).
Civis, S., Ferus, M., Zukalova, M., Kavan, L. & Zelinger, Z. The application of high-resolution IR spectroscopy and isotope labeling for detailed investigation of TiO2/gas interface reactions. Opt. Mater. (Amst) 36, 159–162 (2013).
Cockell, C. S. Biological effects of high ultraviolet radiation on early Earth—a theoretical evaluation. J. Theor. Biol. 193, 717–729 (1998).
Ferris, J. P., Hill, A. R. J., Liu, R. & Orgel, L. E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381, 59–61 (1996).
Ferus, M. et al. Formation of nucleobases in a Miller-Urey reducing atmosphere. Proc. Natl Acad. Sci. USA 114, 4306–4311 (2017).
Ferus, M. et al. On the road from formamide ices to nucleobases: IR-spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J. Am. Chem. Soc. 134, 20788–20796 (2012).
Civis, M. et al. Spectroscopic investigations of high-density-energy plasma transformations in a simulated early reducing atmosphere containing methane, nitrogen and water. Phys. Chem. Chem. Phys. 18, 27317–27325 (2016).
Civis, S., Ferus, M., Kubelik, P., Chernov, V. E. & Zanozina, E. M. Li I spectra in the 4.65-8.33 micron range: high-L states and oscillator strengths. Astron. Astrophys. 545, A61 (2012).
Ferus, M. et al. High energy radical chemistry formation of HCN-rich atmospheres on early Earth. Sci. Rep. 7, 6275 (2017).
Civiš, S., Ferus, M., Kubat, P., Zukalova, M. & Kavan, L. Oxygen-isotope exchange between CO2 and solid Ti18O2. J. Phys. Chem. C 115, 11156–11162 (2011).
Ferus, M. et al. Spontaneous and photoinduced conversion of CO2 on TiO2 anatase (001)/(101) surfaces. J. Phys. Chem. C 118, 26845–26850 (2014).
Civiš, S. et al. Oxygen atom exchange between gaseous CO2 and TiO2 nanoclusters. J. Phys. Chem. C 119, 3605–3612 (2015).
Civiš, S. et al. Spontaneous oxygen isotope exchange between carbon dioxide and oxygen-containing minerals: Do the minerals ‘breathe’ CO2? J. Phys. Chem. C 120, 508–516 (2016).
Kavan, L. et al. Oxygen-isotope labeled titania: Ti18O2. Phys. Chem. Chem. Phys. 13, 11583–11586 (2011).
Harris, F. J. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc. IEEE 66, 51–83 (1978).
OPUS Spectroscopy Software, Version 6 (Bruker Optic, 2006); www.brukeroptics.com.
Acknowledgements
This work is a part of a research series funded by the Czech Science Foundation (grants no. 17-05076S and 13-07724S), by the programme of Regional Cooperation between the Regions and the Institutes of the Czech Academy of Sciences in 2017 (project numbers: R200401721 and R200401521) and by the STS Missions programme within the COST Actions CM1401 and TD1308. The authors also thank the PALS facility staff for supporting the experiments, particularly L. Juha, J. Ullschmied, J. Skála, J. Dostál, P. Prchal and J. Mareš. We also thank the Ministry of Education, Youth and Sports of the Czech Republic for supporting the PALS infrastructure operation and research by grants LM2015083 and LG15013.
Author information
Authors and Affiliations
Contributions
S.C. and M.F. came up with the idea; A.K., M.F. and S.C. conducted the experiments; O.I. conducted the GC-MS analysis; A.K. and P.K. performed evaluation of the data; L.K. and M.Z. prepared the TiO2 samples; S.C., M.F., A.K., L.K. and M.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Text, Supplementary Figures 1–15, Supplementary Tables 1–3, Supplementary References
Rights and permissions
About this article
Cite this article
Civiš, S., Knížek, A., Ivanek, O. et al. The origin of methane and biomolecules from a CO2 cycle on terrestrial planets. Nat Astron 1, 721–726 (2017). https://doi.org/10.1038/s41550-017-0260-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41550-017-0260-8
This article is cited by
-
Ariel – a window to the origin of life on early earth?
Experimental Astronomy (2022)