Abstract
Climate change and its related side effects are generating a demand for innovative ways to enhance desalination performance by adopting cost-effective and energy-efficient membrane materials. Molybdenum disulphide (MoS2), a two-dimensional (2D) material, holds the potential to address the deficiency of the current polymeric reverse osmosis (RO) membrane by maximizing the water-energy nexus. The nanoscale thickness of the MoS2 membrane promises better water permeability benefiting from the small diffusion length of the transport of the molecules while maintaining good chemical and mechanical robustness. Although many advantages have been projected, the experimental realization of such near-atomic thickness has not been fully explored because of the technological difficulties associated with the production. This review first highlights the remarkable combination of the ion’s rejection and permeability properties of the MoS2 membrane by discussing two distinct reported approaches for using MoS2 as a membrane for water desalination. Subsequently, the engineering challenges of the MoS2 membrane scalability for water desalination are discussed. Lastly, the possible opportunities for a well-controlled fabrication process critical to achieving and advancing MoS2 membranes from research laboratories to the industrial-scale application are outlined. We aim to provide a collective understanding of the realization of a high permi-selective MoS2 membrane for water desalination.
Similar content being viewed by others
Introduction
Climate change and its associated side effects are putting severe stress on the current freshwater supplies1,2, which will be continuously worsened by population growth, accelerated industrialization, and increased energy demands3,4,5. Water and energy, which are crucial to societal growth, are under increasing stress6,7. This has resulted in the water-energy-food nexus being at the centre of policymaking, development, and research8,9,10.
Despite the fact that water covers approximately 75% of the earth’s surface4,5, 97.5% of this world’s water is saline3,11, with only about 2.5% available for human consumption, industrial, and agricultural purposes. As a result, there has been an increased global initiative to develop affordable and effective solutions to clean, decontaminate, desalinate, and improve water quality and protection for the environment and public health. In particular osmotic pressure-driven membranes have received significant attention in desalination and water purification practices12. Desalination offers an exciting opportunity to augment the existing natural hydrological cycle by introducing water from seas and brackish reservoirs. Overall, it accounts for a modest proportion of the world’s drinkable water13. It may seem hypothetically easy to extract salt from water; in practice, however, it is an expensive and energy-demanding process11. The commonly used desalination technologies are either electricity-based membrane technology or thermal processes involving electricity and heat14. The thermal-based processes are multi-effect distillation (MED), vapour compression (VC), multistage flash (MSF), and the membrane-based technologies are reverse osmosis (RO) and electrodialysis (ED)15. The less-energy-intensive methods of MSF, MED, and RO are gaining more market shares16,17,18. The RO desalination method applies pressure on the saline water and forces the freshwater through a permeable membrane to reject ions and other impurities18,19,20. RO membranes are costly due to energy usage and membrane fouling1, and their total energy consumption is approximately 71% of the overall energy demand of the desalination process21. RO system performance is tied to material quality, durability, fouling resistance, water permeability, salt rejection, etc. RO water costs from $2/m3 in 1998 to $0.5/m3 in 200418. A 2019 World Bank report22 estimated cost reduction of (0.6–1.0) $/m3 within 5 years and (0.3–0.5) $/m3 in 20 years; still, this is high compared to conventional sources. Therefore, more efficient and economical approaches are crucial for water desalination. In practical application, increasing permeability and selectivity will reduce the operating and capital costs of RO processes.
Aromatic polyamide (PA) and polymeric thin-film composite (TFC) membranes are commonly utilized but have high energy consumption, low flux and salt rejection, and poor fouling resistance23,24,25,26,27; thus, the water-energy nexus will be maximized through novel membrane materials with greater performance and reduced energy costs28. Alternatively, 2D materials with extremely small thickness (few angstroms) have potentials for higher water permeability, benefiting from the small diffusion length of these materials for molecular transport. The mechanical flexibilities of 2D materials such as MoS2 also offer membrane integration advantage17.
2D-layered materials with atomic thickness have opened new ways of customizing membrane composition, the mechanical strength, membrane structure, surface hydrophilicity, load density, surface load, and surface roughness of the membrane layer29 Due to their excellent permeability and selectivity, MoS2 and graphene have stood out in the literature for water cleaning11,13,30,31,32. Because of its strength and light weight, chemical and biofouling resistance, hydrophilicity, mechanical and thermal characteristics, and numerous uses, graphene has received more attention1,2,13,33,34,35. It has the potential as a more efficient material to replace the conventional membrane in the RO process because this single atom thick membrane could reduce the resistance encountered by water molecules during transport, thus reducing applied pressure for water desalination31,36,37,38,39. Notwithstanding, the graphene-based membrane has some limitations, as highlighted by Sapkota et al.40: (1) graphene membrane swelling compromises the selectivity; (2) frictional interaction between the graphene surface functional group and water lowers the water transportation (3) shortened lifespan as a result of effectiveness loss and mechanical failure caused by swelling. MoS2 membrane, on the other hand, has been found to perform better than graphene membrane in water desalination32,41,42,43. This was attributed to several factors which will be discussed in this review, such as the structure and properties, the MoS2 desalination performance, and the scalability of the MoS2 membrane for water desalination.
MoS2 structure and properties
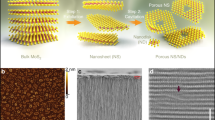
MoS2 crystals forming vertically stacked layers with molybdenum (Mo) and sulphur (S) atoms are held together by weakly interacting van der Waals interactions44. Such structure has striking optical, electronic, and mechanic properties and has found applications in electronics44,45,46, catalysis47,48, biomedical49,50 and energy51,52,53. From Fig. 1a, the MoS2 crystal structure comprises two sulphur atoms with Mo atoms sandwiched between them. The interlayer spacing is 0.62 nm, and the free space spacing is 0.30 nm. This compound occurs in various phases distinguished by different coordinating structures (trigonal, prismatic, and octahedral) and stacking orders, such as 1 T, 2H, and 3R-phases. All these have different electronics properties and have different applications, and can be transformed from one to another54,55,56. Figure 1b, c show the 2H and 1 T structures of the MoS2. The 2H-MoS2 nanosheets find applications in electronics, energy harvesting, and other environmental applications like disinfection and photocatalytic degradation, while the 1T-shows improved electrocatalytic activity for hydrogen evolution reaction55. Details of the different applications are in reference55. The monolayer MoS2 experimental in-plane Young modulus is 270 ± 100 GPa57,58. It also has band gaps of 1.2‒1.8 eV, and this makes MoS2 be promising 2D material59,60,61,62. Although MoS2 has a high out-of-plane stiffness that limits its applicability as flexible electronics, this property allows MoS2 to be an appropriate 2D building block to make separation membranes with relatively fixed nanochannels important for long-standing performance55.
a Is the 3D illustration of the MoS2 structure with interlayer and free spacing (b) is the 2H phase with trigonal prismatic coordination (c) is the 1 T octahedral coordination phase. Reproduced with permission from Environ. Sci. Technol. 51, 15 (2017). Copyright 2017 American Chemical Society55.
Important considerations of MoS2 for water desalination
MoS2 has great promise as a 2D building block material for new membranes with outstanding separation ability, enabling antifouling, multifunctional properties high thermal and mechanical stability, easy synthesis process, and other outstanding properties such as the photocatalytic and anti-bacterial characteristics55. Experimentally, some reports have indicated that nanosheets of MoS2 can be used in membrane-based separation, such as nanofiltration (NF), RO, forward osmosis (FO), pervaporation, and gas separation63.
Boretti et al.1 discussed several important considerations in membrane development, including selectivity, permeability, concentration polarization, fouling, chemical and physical stability, economic and environmental cost, and the overall cost-to-benefit ratio. Many simulations32,36,43 and experiments64,65,66,67 have been done on the transition of MoS2 material from research laboratories to industrial-scale applications. For instance, molecular dynamics (MD) simulations were used to predict the design of these membranes to establish the chemistries allowing objective separation with minimized energy and chemical input68. Besides, MD provided the transportation details of small molecules through nanopores and allowed researchers to collect statistical data such as the permeation of ions and water molecules through nanopores and to calculate the energetics of the ions and water permeation69. Different models could result in different flux rates because different numbers of site, flexibility, the partial charges and Lennard Jones (LJ) parameters can significantly change the observed flow70. Several software environments are also used in modeling the integration of MoS2 in the RO system. The most common in literature is the LAMMPS (Large scale Atomic/Molecular Massively Parallel Simulator) MD simulator software package developed by the Sandia laboratory71, which supports a wide range of classical MD force field methods.
When the energy efficiency among some 2D materials was compared in terms of water permeability and selectivity, single-layer MoS2 outperformed graphene, phosphorene, boron nitride, and MoSe2, as shown in Fig. 2. The water structure and dynamics of the membrane surface, the energy barrier, the water packing, and the velocity at the nanopore were all attributed to the performance41. MoS2 nanosheets have other advantages compared to graphene-based nanosheets, which include better selectivity- MoS2 nanopores are intrinsically charged because of the electron redistribution between the Mo and S atoms; this can increase the ions selectivity through the repulsive membrane ions interactions40. In addition, MoS2 has better stability in aqueous solution (graphene swells in water), antifouling, and photocatalytic function55. MoS2 membranes may remain relatively unaffected in water over a wide pH range in a long period72. Two distinct ways reported in using 2D materials for water desalination are: (a) using the inherent nanochannels in the stacked 2D channels and (b) creating nanopores in plane73.
a Applied pressure effect (b) water molecules filtered (c) Ions rejection (potassium and chlorine) as function of the applied pressure. Reproduced with permission from ACS Energy Lett. 5, 7 (2017). Copyright 2020 American Chemical Society41.
Lamellar/Stacked MoS2 membrane
It is suggested that the nanochannels of the lamellar MoS2 membrane can efficiently reject solutes through the sieving mechanism, as shown in Fig. 3b, where the water can pass through the nanochannels free spacing (i.e., the vacant space between neighbouring sheets). The nanosheet produces a more stable lamellar structure than graphene oxide (GO)65,74. The nanosheets in MoS2 have greater molecular interactions compared to GO due to the large Hamaker constant75. On the other hand, GO is said to have poor selectivity between the membrane interlayer; this was attributed to the lamellar structure swelling in the aqueous phased, primarily due to the numerous hydrophilic groups present in GO nanosheets. The swelling resulted in increased interlayer spacing between the nanochannels and compromised the water-salt selectivity76.
a Nanoporous MoS2 membrane (b) Stacked MoS2 membrane. Reproduced with permission from Homaeigohar, S. et al. NPG Asia Mater 9, e427, 2017; licensed under a Creative Commons Attribution (CC BY) license2.
Most of the stacked MoS2 membranes mentioned in the literatures have been successful in the rejection of comparatively large dye molecules, the size of which is much greater than diameters of hydrated salt ions65,74,77. This important gap between theory and experiment of 2D membrane selectivity has yet to be investigated experimentally. The interlayer spacing height in the lamellar MoS2 is 0.3 nm; this is lesser than the diameter of ions, Cl− (0.664 nm), and Na+ (0.716 nm), which is slightly bigger than that of water molecules (0.276 nm)23. This distance can only allow one layer of water molecules, invariably affecting the water permeation72,78. Compaction of MoS2 membranes results in a more neatly packed nanostructure with fewer voids or looseness, which leads to steady water flow and separation performance65. However, extensive research of mass transportation using layered 2D films is considered essential. Such findings will aid in developing layered 2D membranes in the future. The typical spacing recorded from literature is 0.6–0.65 nm;17,23,40,55 however, from the experiments, an interlayer spacing of ~1.01 nm was observed from vertically aligned side. In addition based experimental measurement by Wang et al. calculated the interlayer spacing of MoS2 membrane to be 1.2 ± 0.1 nm65. When the thickness (0.3 nm) is deducted from the interlayer spacing, this brings to 0.9 ± 0.1 nm, which is capable of removing multivalent ions and other organic molecules while allowing free water movement. Applying these membranes for monovalent ion removal would be achievable if the space were tuned below the sizes of the hydrated ions1,79,80. Monovalent ion separation using a membrane-based approach requires materials having pores/channels less than 1 nm79. Exfoliated nanosheets, through covalent functionalization, can effectively control the interlayer spacing of MoS2, thus enhancing the sieving performance of the nanolaminate MoS2 membrane81. As a result, interlayer spacing provides opportunities to tune the membranes’ selectivity while also improving their stability81. To cross-link and bind neighbouring layers together, certain materials and applications necessitate the use of an external component inserted between the interlayers-intercalation.
Chu et al.72 used intercalation to increase the interlayer distance of the 2D MoS2 membrane, and the intercalated MoS2 exhibited higher permeability without sacrificing the salt rejection. Lu et al.75 on the other hand, carried out a detailed selectivity and separation performance of lamellar 2D MoS2 membranes nanosheet stacking behaviour. They reported failure of the separation layer of the 2D nanosheets MoS2 in relation to the water-salt selectivity. Using intercalation with amphiphilic molecules, they tuned the nanochannels size and studied the mass transport in the lamellar structure through Monte Carlo simulation. Figure 4 gives the comparative details of the pristine MoS2. The interlayer spacing is 0.63 nm, and the intercalated MoS2 nanosheet depicted as In-MoS2 has interlayer spacing of 1.46 nm. They found that a small deviation in stacking of the MoS2 nanosheet leads to serious microporous defects, which compromises the lamellar nanostructure selectivity through negating of the sieving mechanism of the interlayer. They concluded that well controlled fabrication process was necessary, such that the lamellar structure could be tuned to achieve a highly selective and defect-free 2D desalination membrane.
a Is the XRD pattern of the MoS2. b pristine MoS2 without intercalation (c) intercalation was denoted as “In-MoS2” with interlayer spacing of 1.46 nm. d is TEM image (e) image of TEM (f) is the SEM cross-sectional images of the pristine MoS2 film that has an interlayer spacing of 0.63 nm, (g) SEM cross-sectional images of the intercalated MoS2 film with interlayer spacing of 1.46 nm. Reproduced with permission from Environ. Sci. Technol. 54, 15 (2020). Copyright 2020 American Chemical Society75.
Wang et al.65 mechanistically correlated MoS2 membrane performance to the size in the various hydration states nanochannels. They found that fully hydrated membranes of the MoS2 held a 1.2 nm interlayer spacing (which is 0.9 nm free spacing), resulting in high water permeability and moderate-high ion and rejection. By contrast, fully dried MoS2 membranes, owing to the permanent nanosheet restacking, had a 0.62 nm interlayer width and were nearly waterproof. Figure 5 shows MoS2 membranes size and the different hydration states of nanochannels.
Reproduced with permission from Nano Lett. 17, 12 (2017). Copyright 2017 American Chemical Society65.
Sapkota et al.40 went further to use a scalable method that combined the advantages of the ultrathin nanoporous single layer and layer-stacked membranes. The two-step preparation process of the porous MoS2 nanosheets (NSs), the nanodisks and the interlayer spacing of the stacked nanosheets are shown in Fig. 6. The approach involved producing membranes from controlled size nanosheets, nanodics, and peptides, leading to engineered porosity where the interlayer spacing, the pore size, and the surface charge can be tuned. The study showed good selectivity and high-water permeability of the membrane due to the presence of the pores in nanosheets, the interlayer spacing, and the interspaced nanodisc (the nanodisc increases the number of sub-nanometer channels).
a Shows the two-step process representation of the high permeability sub-nanometre nanoporous composite MoS2 membranes. b The as-prepared topographic atomic force micrographs of the porous NSs. c The cross-section SEM image of the laminate composite of NS/ND membrane (d) Shows the high-resolution HAADF STEM image of a laminate cross-section of 6.2 Å interlayer spacing and showing defects in the interlayer voids/defects as shown with the arrow. Reproduced with permission from Sapkota, B. et al. Nat. Commun. 11, 2747, 2020; licensed under a Creative Commons Attribution (CC BY) license40.
Other findings shed light on how the framework and other variables such as nanomaterials areal packing density and membrane thickness influence separation behaviour and membrane desalination efficiency82.
To derive the full benefits of the lamellar MoS2, a well-controlled process of fabrication wherein the lamellar structure could be tuned to achieve a highly selective defect-free 2D desalination membrane and high permeability is necessary.
Nanoporous MoS2 membrane
Another way of filtering water is to create membrane with high density of subnanometer pores with high out-of-plane mechanical strength in the material through isolated defects or by a chemical etching process and ion irradiation30,75 during the growth process. Transport of the water molecules (~0.28 nm van der Waals diameter) through nanoporous membrane pores is essentially determined by pore size83. Nanopores with diameters starting from a number of angstroms to several nanometers can be created to form nanochannels32,84,85. Figure 7 is a schematic representation of an RO system with a nanoporous membrane.
Reproduced with permission from ACS Nano 10, 2 (2016). Copyright 2016 American Chemical Society9.
Simulation results showed that nanoscale pores could differentiate between salt, water, and a range of other ions types.
Apart from the water permeability which is affected by the pore size, the rejection defined as \(\left( {R = 1 - C_{permeate}/C_{feed}} \right)\) Where R is the rejection, Cpermeate and Cfeed are the concentrations of the permeate and feed solutions, is also greatly influenced by the pore size distribution33. The rejection of solutes that are present in the feed water can be approximated by an empirical function which is the ratio of radius of the solute to the radius of the pore \(a/r_p\). To achieve total solute rejection (Cpermeate = 0) in the feed, the entire pore distribution would be smaller than the targeted solute. Total rejection is realized when rp ≤ a1,33
Producing nanoporous membranes on a wide scale while maintaining separation performance might be difficult, the reason why the nanoporous experimental realization of this material for desalination is still scarce. However, the transport properties in these nanopores have been explained by MD simulation, particularly the spontaneous filling and the fast water transport, ions rejection, and the ions selection69. To better leverage the capability of the 2D membrane and resolve the permi-selective trade-off, the size of the pore as highlighted above and the area density should be optimized and regulated independently86.
Transport of solutes can be described using size and steric effects since they can be major energy barriers. For solutes to pass through rigid enclosed pore diameters smaller than their own bare size, there would be need for the reorientation of the bonds between molecules and atoms rearrangement in a membrane material87. Water transport through the smallest pores of graphene, graphyne, and MoS2 can accommodate only a single water molecule through their cross-section hence exhibiting a single-file water molecules movement; however, when the pores diameter is ~1.5 nm, the water molecules assume certain configurations when the water passes through the pores83. Water shell increases ions size, which invariable hinders transport through membrane of similar pores dimensions; however, the water shell is usually rearranged or removed when the ions enter pores smaller than the hydrated ions. When the nanopore diameter reaches the hydrated ions size, different ions types are rejected by the nanoporous membrane offering effective water desalination.
Steric exclusion (sieving), Donnan (charge) interactions, and solute–membrane affinity (i.e., hydrophobic attraction, hydrogen bonding, and dielectric effects) are the three main solute–membrane interactions88,89,90. It is generally accepted that size-based diffusion selectivity, resulting from steric resistance to solute, is the most common method for achieving selectivity in traditional polymeric membranes82. However, the dielectric exclusion mechanism is also one of the main mechanisms for the polyamide desalination membranes91. Dielectric exclusion is caused by the interactions of ions with the binding electric charges created by ions at interfaces between mediums with varying dielectric constants90,92,93. Thus, in the filtering analysis, dielectric exclusion cannot be overlooked93,94. Dielectric exclusion has significant pore geometry dependency and non-monotonic nature as a function of solvent dielectric constant. In establishing the rejection process linked to the dielectric effects, it is believed that the difference between the dielectric constant of the aqueous solution in the pores and the dielectric constant of the membrane material would play a major role91. Because the polarization charges have the same sign as the ions in aqueous solutions, interactions always induce repulsion of each ion, regardless of its sign. Thus the dielectric exclusion effect is considered as an additional rejection mechanism produced by the difference in dielectric constants between the membrane material and the aqueous solution. The dielectric exclusion in addition to other rejection mechanisms such as the steric effect should be well considered in the rejection mechanism95. When examining water and ion transport over nanoporous MoS2 films, it is important to understand this process. A deeper knowledge of dielectric exclusion can aid in the creation of basic structure-property-performance relationships. Such frameworks could result in innovative membrane design and enhanced nanofluidic transport modeling.
Carbon nanotubes and graphene have been widely studied for water transport and desalination among nanoporous materials. It has been reported that GO acts as a molecular sieve in aqueous solution, blocking all hydrated solutes with radii greater than 4.5 Å; this is inadequate for desalination applications because hydrated sodium ion is smaller than this96. Similar to this, there is a need for study to get the optimum radii of the pore for MoS2. Based on simulations results, the optimal size of the graphene-based membrane for achieving the maximum water permeability for the hydrogenated pore is 23.1 Å, while for the hydroxylated pores is 16.3 Å. Meanwhile, atomic simulation suggests that for absolute salt rejection for GO membrane, the pore diameter should be <5.5 Å97. Water molecules are blocked from passing through MoS2 nanopores with a diameter of 0.23 nm or smaller55. According to Heiranian et al.32, over 88% of ions were rejected for pore areas in the range of 20–60 Å2 for the MoS2 membrane that is about 5.05–8.74 Å. In fact, theory forecasted that nanopore sizes of ~4.4−10.5 Å would provide an optimal performance of both high water-permeation flux and salt-rejection rate for 2D MoS2 layers17,43,55.
Operating conditions (e.g., driving force) and the composition of the solution as well as the interactions with other solutes and the hosts medium (i.e., pore walls and water) were proved to affect the energy barriers in solute transportation, which could provide the most important mechanism in induce solute-solute selectivity87.
Heireran et al.32 studied nanoporous MoS2 membrane with three types of pore edges by using MD to study water permeation: the first was mixed with Mo and S atoms, the second was Mo atoms, and the third was the third S only atoms. Water flux (Q) is given as a function of the density (ρ) within the pore, the velocity (V) of the water that passes via the pore and pore area (A),
Increasing the pore area affects the capacity of the pore to reject salt in water desalination. As the area of the pore increases, the efficiency of the rejection reduces, leaving ρ V as the control parameters to maximize the flow of water via the pore. They concluded that pores with Mo only and the mixed pores performed better than the S-only pores. This was attributed to the fact that regions with only Mo had greater local water density due to the hourly glass shape. Mo terminated pore mimics the conical shape of aquaporin biological channels, which reduced flow resistance at the pore entrance and the pore exit. Figure 8 shows different Mo and S-only pore architecture.
a The velocity profile of the water molecules at the Mo only edges of the nanopores (b) Water molecules velocity profile at the edges of the nanopores of the S only atoms. c The schematic representation of the different pore architectures for the Mo only and S only compared to graphene nanopore. d The performance comparison of the various membranes ion rejection and the water permeation rate32. Reproduced with permission from Heiranian et al. Nat. Commun. 6, 8616, 2015; licensed under a Creative Commons Attribution (CC BY) license32.
MoS2 membrane have high permeability of (30–355.3 L m−2h−1bar−1)9,64,65,98 whereas the state of the RO membrane has permeability of about (2.3 L m−2h−1bar−1)73,99.
Similar to the suggestion by Mi97, a promising method for desalination is to create stacked nanoporous MoS2 (SNM) membrane. In doing so, the properties and advantages of both the lamellar and nanoporous MoS2 can be harnessed to optimize the permi-selectivity properties. Water will pass through not just the in-plane nanopores only but will also permeate via the stacked interlayer spacing, thus combining both the scalability and selectivity properties derived from both systems. It will have better selectivity because of the multiple rejection mechanisms such as the nanopore’s gating effects (charge repulsion and size exclusion) and the gating effect due to the interlayer spacing.
Selectivity
There has recently been increased research interest in new materials such as two-dimensional (2D) nanosheets. The discovery of extraordinary transport properties that might result in membranes with ultra-high-water permeability spurred such interest in new materials.
Increasing permeability and selectivity will reduce the operating and capital costs of RO processes. Higher membrane selectivity can lower RO process energy consumption beyond the advantages attainable with increased membrane permeability. These new materials may also have crucial properties for solute–solute selectivity, such as structural and chemical homogeneity, as well as easy tunability100—features that existing membranes lack. A deeper knowledge of the molecular-level processes for solute transport under subnanometre confinement is required in order to build membranes with enhanced solute–solute selectivity87.
According to Wang and Ming55, MoS2 nanosheets absorbents have the following benefits compared to GO. First, the sulphur atom in MoS2 monolayer is a soft Lewis base with a high affinity for soft-acid heavy metal ions, resulting in an extraordinarily high adsorption capacity. Second, MoS2 has considerably better selectivity than GO. This is due to the soft-soft interaction, which makes MoS2 adsorbents extremely selective for a wide range of heavy metal ions while remaining resistant to interfering cations even at high ionic strength and low pH. Other benefits of MoS2 nanosheets over graphene-based nanosheets include higher selectivity- MoS2 nanopores are inherently charged due to electron redistribution between the Mo and S atoms, which can boost ion selectivity through repulsive membrane ion interactions40. Another way to enhance the selectivity of the MoS2 membrane for water desalination is through pore functionalization.
Pore chemistry/functionalization
Design of pore chemistry allows applications beyond filtration68. Pore functional group effects on water flux such as sites with hydrophilic groups enhance water permeation compared to hydrophobic groups, which presents entropic barrier to transport83. The pore chemistry of the nanoporous MoS2 membrane plays a significant role in regulating the water flux and ion rejection9,37. More precisely, the molybdenum-only pores had the highest water velocity and density among the three pore configurations: molybdenum (Mo) only, sulphur only, and mixed. It was found that pore with only Mo in their edges in a MoS2 membrane has a higher water flux ∼70% compared to graphene of similar pore sizes32. The hydrophilic property of molybdenum, which pulls water to the pore interior, was attributed to this performance32. In addition, small hydrophobic functional groups, such as the methyl group, play a very important role in improving water flow in MoS2 membranes, according to experimental findings81.
Increased fouling and/or chemical resistance is, as well as permeability and selectivity, are a core concern in RO membrane1,101. Foulants like protein particles stick to the surface of the membrane through hydrophobic interactions. The effects of such can be minimized through the modification of the surface or by using hydrophilic materials102. For instance, Fathima et al.103 used 2D MoS2 nanoplatelets for fouling resistant membrane surfaces. Utilizing non-toxic l-Cysteine as a sulphur source, the 2D MoS2 nanoplatelets were produced using a green bottom-up technique. The MoS2 coated membrane surface demonstrated excellent resistance with a significant antifouling effect. Foulants could also be trapped in the open pores of rough membranes; this can be reduced by using very smooth surfaces. The lack of conjugate structure in MoS2 may help prevent scaling and organic fouling that is typical in graphene-based membranes due to the cation interactions55.
In addition, a way to use 2D MoS2 membrane, is the incorporation of these materials as additives with other materials such as polyamide to enhance performance of water desalination. A hybrid membrane system incorporates a membrane filter device with other systems, including coagulation, ion exchange adsorption, and another membrane system104.
Pore functionalization can alter ionic transport drastically, particularly if the pore is smaller than the hydrated ion size; the partly charged or charged functional groups will lower the energy barrier for opposite-charge ions along the edges of the pore and raise the barrier for similar-charge ions, which affects ion selectivity83,105. Li et al.29 modified the surface of thin-film nanocomposite (TFN) with lamellar MoS2, and the MoS2-TFN RO membrane had increased surface hydrophilicity and surface roughness. It exhibited an optimal 6.2 L m−2 h−1 bar−1 water permeability, 98.6% salt rejection, and improved fouling resistance.
Hydrophilicity is a significant feature that affects desalination performance63. In improving the hydrophilicity of the MoS2 membrane, Polyethyleneimine (PEI) was used, and the resulting MoS2/PEI created a neat and highly stacked nanostructure. The composite had a good desalination performance with permeability of 4.6 Lm−2 h−1 bar−1 and 95.5% MgCl2 rejection63.On the other hand, because of the hydrophilic sites and the strongly negatively charged properties given by the Mo and S atoms, the physiochemical properties of the MoS2 can be used to change the surface of the TFC RO membrane, such as the electrostatic charging, the hydrophilicity and the roughness, which are some of the major factors affecting the fouling resistance and membrane permeability106. Hirunpinyopas et al.64 functionalized MoS2 membranes with different dyes; the membranes showed improved water permeation rates. This important result was attributed to the improvement in surface chemistry on the surface of the MoS2 membrane after functionalization. Later work by the same group functionalized MoS2 with naphthalene sulfonate dye. The functionalization of MoS2 membranes not only yielded an excellent size/charge selective ions sieving but also enabled the cation-selective tunability of about 80% under alkaline conditions67. Ries et al.81 carried out a study on the use of MoS2 membranes for water desalination via covalent functionalization. The functionalized MoS2 membranes reveal that micropollutants and NaCl are respectively >90% and ~87% rejected in RO conditions.
It was common practice to determine the mass flow through porous materials per unit area using the Hagen-Poiseuille equation107. This equation’s fundamental assumptions are laminar flow and no-slip at the boundary layer108.
Q is the volumetric flow rate, d is the diameter of the pore, L is the thickness of the membrane, μ is the viscosity of water, and ∆P is the pressure drop109
For a variety of scenarios, including flow through hydrophobic capillaries, moderate departures from the no-slip boundary requirement have been obtained, allowing for the creation of membranes with modest pore size and better liquid transport. The equation’s implications for nanoporous materials (small radius) is that the pressure drop across the pores limits the transportation rate108. The surface energies prevent water from flowing into the transport channels and would need significant overpressures to wet the surface108. As demonstrated in some studies, permeability does not scale with the inverse of the membrane thickness, contrary to the conventional hydrodynamic behaviour64,110. The nanopore design has a significant impact on membrane permeability, revealing valuable information on how future desalination membranes should be made.
Fabrication and scalability challenges of MoS2 membrane for water desalination
Graphene and other 2D materials such as MoS2 offer new ways at nanoscale to control mass transport. These materials, with high mechanical and chemical robustness, could be used in addressing the challenges of membrane technology83. Even at the laboratory scale, several of the materials discussed here have failed to generate defect-free membranes, despite significant advancements in membrane production and performance111. Two main approaches have been used in 2D materials synthesis, the top-down approach (mechanical and the liquid-phase exfoliation) and the bottom-up approach (chemical vapour deposition and atomic layer deposition)75,112,113. Among the challenges of the top-down approach are the inevitable inherent defects during manufacturing. However, advances in the manufacture of superior separation performance membranes can be accomplished by using the bottom-up approach33,114; therefore, the focus is on the bottom-up approach for the synthesis of MoS2.
Chemical vapour deposition (CVD)
Chemical vapour deposition (CVD) is a deposition method commonly used for thin films development, amorphous or crystalline, of gaseous, liquid, or solid precursors of several materials115. It has emerged as a tunable and effective process for generating continuous, uniform, and a large area of 2D materials83,116 According to Chen et al., CVD methods employed in growing graphene thin film are summarised as (a) transfer with a support layer (b) transfer without a support and (c) direct growth on the target substrate. Depending on the method employed, the transfer process could affect the properties of the transferred material116.
Using the transfer and integration method, large area of few layers 2D MoS2 has been grown successfully with the chemical and microstructural integrity preserved after the integration. The few-layer 2D MoS2 shows good desalination performance attributed to dimensional and geometric effect and the electrostatic interaction resulting from the CVD induced atomic vacancies that govern the ionic permeation selectivity at the solution and membrane interface117. Figure 9 shows the desalination mechanism suggested based upon intrinsic nanopores and van der Waals gaps of the CVD-2D MoS2. To an extent, the quality expectations for atomically thin materials for membrane applications are stricter than those for electronic applications, as small pinhole defects can seriously compromise selectivity118.
a Water molecules translocation through nanopores (b) hydrated ions rejection and water molecules translocation in the interlayer spacing. c–g Shows the TEM images, confirming intrinsic nanopores presence within the CVD-2D MoS2 grain boundaries revealing layers. Reproduced with permission from Nano Lett. 19, 8 (2019). Copyright 2019 American Chemical Society118.
Park et al.73 outlined some key design criteria for new membranes: (a) properly sized pore, (b) narrow pore distribution, (c) active thin layer (d) highly tuned interactions between the membrane surface and the permeates of interest. This near-atomic thickness membrane which has both exceptional water permeability and good selectivity can be achieved using methods such as CVD. Applying some of these design criteria, Li et al.17 realized experimentally few layers of 2D MoS2 membrane of near-atomic thickness using the CVD method for high-efficiency water desalination. The permeable membrane thickness is ∼7 nm. The near-atomic thick membrane showed excellent permi-selectivity capability (rejection >99% and permeability >322 L·m−2·h−1·bar−1), surpassing 2D MoS2 membranes previously developed with larger thickness. They concluded that the superiority of this method is the collective result of the inherent near-atomic vacancies of the CVD-grown 2D MoS2 and the membrane thickness. According to Wang and Mi55, depending on the precursor and substrate variation, CVD synthesis of MoS2 could be classified into (i) the vapourization and decomposition of the precursor of S and Mo and the subsequent formation of the MoS2 grown on a substrate and (ii) direct sulphurization of the Mo-based film (iii) the thermolysis of the precursors that contain S and Mo atoms. It is worth saying that complex processes like CVD have the ability to add substantial costs to the fabrication process119.
Atomic layer deposition (ALD)
ALD is a thin film deposition method where the chemical precursors are introduced in sequence to the surface of a substrate material where they react chemically with the surface, forming sub-monolayers of film120. It is becoming attractive as a deposition process due to its unique high uniform deposition, excellent conformity, and precise control growth films on complex three-dimensional surfaces121,122,123,124 and its scalability. Engineered nanomaterials (ENMs) can be used to control biofouling- a major limiting factor for membrane125. Controlling the surface chemistry of exfoliated 2D materials provides room for further exploration of the nanofluidic processes inside nanolaminate membranes for water purification or osmotic energy81. Lu et al.75 developed a well-controlled fabrication process wherein was the lamellar structure could be tuned carefully. This is critical to achieving both defects-free structure and highly selective 2D desalination membrane. ALD provides nano-engineering capabilities and flexibility that traditional approaches cannot achieve121.
The chemistry of ALD and CVD are similar; however, the reaction in ALD breaks the CVD reaction to two half-reactions, keeping the precursor materials separate126. The difference lies in the precursor’s adsorption, alternative and sequential addition of precursors and reactants, and the self-limiting characteristics127. CVD has been shown to have intrinsic structural variations such as the atomic vacancies and the grain boundaries, which inevitably present in CVD-grown 2D MoS2 layers65,66,128,129,130; this can compromise membrane integrity; however, this can be corrected using ALD. A detailed comparison of ALD, CVD, and other thin film deposition methods are presented in120,131,132.
Membrane flux scales inversely with the thickness of membrane, and new types of ultrathin membranes offer the promise of much higher water permeability13,133. The permeability, selectivity, tunability, stability, and fouling which are among the challenges currently faced in membrane development, can be addressed using ALD with advantages such as ultra-thin membrane to aid in permeability the tenability-which can be used to enhance fouling and selectivity. ALD are in particular applicable in membranes such as MoS2, as they enable: (a) synthesis of membrane materials with complex designs (b) control over the surface or fluid interaction enhancing the membrane hydrophilicity or organophilicity (c) Preparing coatings for specially designed compositions and nanostructures (d) functionality tuning which can alter the pore surface and the reactivity properties121.
Although ALD has the promise of thin-film fabrication, there are currently very few studies on ALD application in MoS2 membrane development. ALD has been applied in the deposition of MoS2 in several applications134,135,136,137,138,139. Its use for water desalination needs further study to benefit from its huge potentials. Figure 10 illustrates the application of ALD in developing a membrane for water desalination. ALD is a technology with numerous possibilities that could be explored by using different precursors and parameters to achieve desired properties, including the advantage of functionalization and tuning of the pore size to enhance membrane performance140.
The top images show the transmission electron microscopy (TEM) of a modified pore geometry through ALD process, which are limited to the mouth of the pore, while the bottom image is a further modification of the internal pore geometry using the polypeptide groups through the ALD process resulting in pore active sites with dimensions and chemical functionality that are comparable to those seen in natural biological pores. (Susan Rempe et al. 2010 Sandia laboratory publication on Biomimetic membrane for water purification 2010)140.
Engineering challenges of scalability
The atomic thickness of 2D materials makes them the thinnest possible barriers, combined with their mechanical strength, chemical robustness, selectivity potential, and nanometre-scale pores promise membrane with potential for high selectivity, high permeability, and good chemical resistance83. CVD and ALD have the potential routes to address the defect-free membranes; however, in practical applications, the same critical characteristics of these membranes, such as pore size and interlayer spacing, need examination. Industrial applications have proven to be difficult since scalability is currently insufficient to meet industrial requirements - there are few experimental studies of 2D MoS2 layered membrane of near-atomic thickness in water desalination because of the difficulty in fabricating and integrating such with their integrity preserved117. Some literature1,119,141 did outline challenges as regards scalability. Although it has been particularly difficult to make large-scale continuous (> cm2) 2D MoS2 layers with thickness of ∼1−10 nm, with the advance of technology, such could be achieved17,118. Like the TFC polyamide desalination membrane, integrating 2D or ultrathin materials on top of a porous support layer may give structural support for these materials, hence assisting with scalability and defect issues. In addition, these bottom-up approaches can be used in controlling the nanopore or nanochannels of the 2D materials118,142,143.
Another issue with the use of MoS2 for desalination is the creation of sub-nanometer pore. In creating sub-nanometer pores in MoS2, electron beam drilling using transmission electron microscope (TEM) was used144,145 as proof-of-concept. Although it gave good insight into nanopore functionality, the scalability is limited because it is relatively slow and expensive and requires precise control of the beam in the TEM86,146. Surwade et al.5 produced defect-free graphene using oxygen plasma to generate subnanometer pores in one study. This method resulted in ultra-high water fluxes, which is much greater than earlier research and among the highest ever documented; how such methods could work for MoS2 need further study. Thiruraman et al.147 recently demonstrated the fabrication of atomic vacancies in MoS2 using Ga+ ion irradiation. Other methods used for creating MoS2 nanopores in a controlled manner are electron beam144, ion bombardment148,149, and defect engineering55,66, though with challenges too. It is suggested that an efficient and scalable way for producing a large number of nanopores with generally consistent diameters is to use an electrochemical process that can remove individual atoms surrounding defects or single atom vacancies146. Chemical etching150, annealing under oxygen151, and chemical oxidations152 are some of the various nanopore fabrication techniques that can be used. Therefore, applying such MoS2 pores generation will require additional experimental capability in the process of design and fabrication9.
Small-scale samples are usually enough for characterization to get data; nevertheless, large area sizes are needed in industrial membrane production. Scaling up of defect-free materials such as MoS2 is a major problem and would worsen the use of the materials if the materials cannot be reliably turned into thin defect-free membranes73 structures needed for functional applications such that they can retain acceptable transport and selectivity properties when exposed to real-world, dynamic feed mixtures for a prolonged period. To achieve this, it is important to produce a high-performance asymmetrical cross-section consisting of a thin (100‒500 nm thick) self-assembled active layer assisted by a microporous layer underlying it68.
Currently, the layered-stacked MoS2 membrane is the feasible experimental option; this is because of the feasibility challenges of fabricating large area of MoS2 and the creation of homogeneous nanopores17,55,63,70. Although according to Lu et al.75, the mass transport within nanochannels of lamellar 2D films does not really occur as intended but rather, it is the microporous defects that are the results of imperfect stacking due to poor fabrication process that dominate the mass transport. To eliminate these defects and achieve the full benefits of 2D materials remains a serious challenge. Therefore, there is the need to scale up the fabrication process of the bottom-up methods to help mitigate these challenges and hence achieve the large-scale membrane.
Membranes are only one aspect of desalination system, and existing technologies are not designed to maximize the advantage of improved MoS2 selectivity and permeability, thus the need to integrate such in the desalination system. According to Epsztein et al.87, the mechanisms that affect the energy barrier for solute transport due to different elementary reactions, which can also be viewed as transport resistance, are: (a) steric hindrance, (b) electrostatic repulsion, (c) dielectric effects (d) weak van der Waals (e) Frictional and viscous effects. An ideal membrane should have these attributes: small thickness for maximum permeation, narrow pore size to maximize selectivity, and the mechanical strength to withstand pressure153. All these need to be considered in membrane fabrication.
A new mechanism that could break the permi-selectivity trade-off has been discovered. It was applied in rotating nanoporous graphene. When water molecules and ions have a high radial velocity at the border layers of membrane pores, they will escape through the pore opening. From the equation, the hydrated mass of the ions (which is higher than of water) would result in lower acceleration, resulting in lower penetration by the ions and thus higher rejection. Details of these mechanisms are presented in references39,154. The rotating graphene membrane had salt rejection of almost 100% even when the pore size was larger than the hydrated ions. The surface slip at the graphene/liquid interface exhibited a high permeability and outstanding selectivity154. This discovery can be used to overcome the limitation posed by pore size, opening new ways of designing membranes with high performance. MoS2 can be used too in such system to enhance the permi-selectivity.
Membranes need to be thermally and chemically tolerant to RO conditions during operations. Aqueous chlorine is usually used to control biological activities in fed water streams during desalination processes because of its costs and effectiveness. Current aromatic polyamide-based polymers desalination membranes suffer from chemical attacks due to poor resistance when exposed continually to oxidizing agents (chlorine). Chlorine also affects MoS2; however, when exposed to such, the reaction is much slower than the polyamide membrane40, which gives it a relative advantage. According to a study, hypochlorite etching in MoS2 begins at the edges with dangling bonds, and this progresses toward the center; however, morphology and thickness remain inert when such edges are covered155.
The focus should also be on improving the anti-fouling properties of MoS2 through functionalization with other materials, which can help reduce the capital cost20. Improving membrane fouling resistance will increase reliability, improve energy usage, and reduce the impact on the environment.
Other options are developing MoS2 such that it functions like a biomimetic membrane (The term “biomimetic” refers to the study of biological systems’ architecture and functions in order to establish a model for engineering solutions) with very high permeability and good selectivity. These biomimetic membranes are made up of discrete nanochannels that are aligned inside amphiphilic matrices on a strong support156,157,158,159,160. While biological components have been employed directly, substantial research has been carried out to create systems that mimic these protein channels and lipid bilayers. A robust biomimetic membrane might well be tuned for specific uses, ranging from having self-aligned channels that reject virtually all solutes for desalination to more specialized applications needing solute selective transport. In selecting the channels, stability, processability and performance are very important. The synthetic water channels could ideally behave similarly to aquaporin in terms of transport but with greater stability and processability161. The nanochannels/nanopores of MoS2 can be designed in terms of both pore and surface chemistry to have very high permeability and good selectivity.
Other factors that will help with scalability and market readiness, includes the mechanical robustness, fouling effect, chemical and thermal stability, need more studies. The efficient water transport and mechanical strength of MoS2 make it a top candidate for water desalination41. MoS2 nanomembranes have remarkable thermal stability and mechanical properties with elastic response comparable to graphene. The monolayer MoS2 has exceptional mechanical properties that are comparable to those of steel162. In addition to the outstanding properties mentioned, monolayer MoS2 also has some remarkable mechanical properties, which include well-balanced stiffness and flexibility, which makes it a suitable nanomaterial for the nanoporous filter for water desalination9,163. However, the inherent properties of MoS2 with regards to its ability to preserve its mechanical integrity under extreme hydraulic pressures in a reverse osmosis RO desalination process needs to be explored. Mechanical strengths of the membranes correlate with their pore sizes and geometries164. While the presence of water has been observed to reduce the fracture toughness of some materials like oxide ceramics, it has also been observed to improve the fracture toughness of materials such as dentine165. Hence, understanding if a MoS2 nanomembrane can maintain its mechanical strength and integrity at high pressures similar to that obtained in RO desalination process is key to its utilization as a material in the desalination process. The manufacturing scalability, materials and fabrication cost, breakeven points, quality control, and assurance, and others are still open before practical applications.
Another important fact in the course of MoS2 membrane production for water desalination is the study of environmental and health risks. Given the importance of this material for desalination, it lacks studies pointing in that direction. Although studies have suggested that MoS2 has low toxicity17, the variability of MoS2 nanosheets, such as thickness, phase, lateral size, and defects, may complicate the toxicity effects and need further studies on the effects and the underlying mechanisms55. There is also need for further study to see if the freshwater produced meets the world health organization (WHO) standard for desalination166.
The cost associated with MoS2 and environmental implications in its use as a desalination membrane needs critical examination. Methods such as the bottom-up have higher costs than other methods used. Although the cost of production would normally reduce when the process is scaled up and optimized. Furthermore, mechanistic insight into the possible swelling behaviour of the MoS2 membrane in aqueous and ionic environments has been lacking; this needs detailed study.
Outlook and SummarySeveral works have been done regarding permeability and selectivity; however other factors that will help with scalability and market readiness such as the mechanical robustness, fouling effect, chemical and thermal stability need more studies. The manufacturing scalability, materials and fabrication cost, breakeven points, quality control, and assurance, and others are still open before practical applications. In addition to having outstanding permeability, there is the pressing need for membranes with better selectivity, especially in water purification33,73. Opportunities for advancing membranes have been outlined as (a) materials with robust chemical, mechanical, and thermal properties, (b) excellent permeability and selectivity (c) emphasis on the fundamental relationships between the structure/property/processing73. Another important part of the production of new membrane materials such as MoS2 for water desalination is the study of environmental and health risks. Given the importance of this material for desalination, it lacks studies pointing in that direction. Although studies suggest that MoS2 has low toxicity17, the variability of MoS2 nanosheets, such as thickness, phase, lateral size, and defects, may complicate the toxicity effects and may need detailed further studies to explain the effects and the underlying mechanisms55. The transformation phase, the environmental impact, and possible human health risks that could be associated with MoS2 nanosheet need to be critically examined to minimise possible harmful effects. There is also need for further study to see if the freshwater produced meets the world health organization (WHO) standard for desalination166.
The cost associated with MoS2 and environmental implications in its use as a desalination membrane needs critical examination. Methods such as the bottom-up have higher costs than other methods used. Although the cost of production would normally reduce when the process is scaled up and optimized. Furthermore, mechanistic insight into the possible swelling behaviour of the MoS2 membrane in aqueous and ionic environments has been lacking; this needs detailed study.
In conclusion, 2D MoS2 has potential for water desalination because of its high permeability and good selectivity properties. MoS2 functionalized membranes possess better antifouling performance compared to commercial RO desalination membranes. It performed better than graphene-based membranes significantly in relation to energy, flux rate, and fouling; this was attributed to surface chemistry and absence of functional groups in MoS2. The exciting findings of outstanding characteristics and performance of 2D-based nanomaterials have sparked a surge in interest in utilizing 2D MoS2 nanomaterials for environmental applications. Along with understanding the principles of selectivity, continuous advancements in membrane manufacturing processes are critical for the creation of single-species selective membranes. At the same time, current attempts to precisely control the pore size and chemical functionality of new materials, such as 2D MoS2 sheets, should improve the solute–solute separation and the water–solute selectivity. In addition, MoS2 can be functionalized by integrating with other materials to improve membrane performance in desalination. The scaling-up of fabrication process of the bottom-up methods such as CVD and ALD is necessary. From the study, there are great benefits that could be derived from using methods such as ALD and CVD for the tuning and functionalization of different materials with MoS2. ALD and CVD can provide nano-engineering capabilities and flexibility that traditional approaches cannot provide; this will help mitigate the challenge and hence achieve the large-scale membrane for industrial membrane system.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analysed during this study are included in this published article.
References
Boretti, A. et al. Outlook for graphene-based desalination membranes. NPJ Clean Water 1, 1–11 (2018).
Homaeigohar, S. & Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 9, e427–e427 (2017).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 337–346 https://doi.org/10.1038/nature06599 (2008).
Raju, M., Govindaraju, P. B., Van Duin, A. C. T. & Ihme, M. Atomistic and continuum scale modeling of functionalized graphyne membranes for water desalination. Nanoscale 10, 3969–3980 (2018).
Surwade, S. P. et al. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 10, 459–464 (2015).
Simpson, G. B. & Jewitt, G. P. W. The development of the water-energy-food nexus as a framework for achieving resource security: a review. Front. Environ. Sci. 7, 8 (2019).
Hamiche, A. M., Stambouli, A. B. & Flazi, S. A review of the water-energy nexus. Renew. Sustain. Energy Rev. 65, 319–331 (2016).
IRENA. Renewable energy in the water, energy and food nexus. International Renewable Energy Agency https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2015/IRENA_Water_Energy_Food_Nexus_2015.pdf (2015).
Li, W., Yang, Y., Weber, J. K., Zhang, G. & Zhou, R. Tunable, strain-controlled nanoporous MoS2 filter for water desalination. ACS Nano https://doi.org/10.1021/acsnano.5b05250 (2016).
Oviroh, P. O. et al. Micro water-energy-food (MicroWEF) Nexus: a system design optimization framework for integrated natural resource conservation and development (INRCD) projects at community scale. Appl Energy 333, 120583 (2023).
Dervin, S., Dionysiou, D. D. & Pillai, S. C. 2D nanostructures for water purification: graphene and beyond. Nanoscale 8, 15115–15131 (2016).
Cheng, W., Ma, J., Zhang, X. & Elimelech, M. Sub-1 μm free-standing symmetric membrane for osmotic separations. Environ. Sci. Technol. Lett. https://doi.org/10.1021/acs.estlett.9b00364 (2019).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608 (2012).
Ullah, I. & Rasul, M. G. Recent developments in solar thermal desalination technologies: a review. Energ. (Basel) https://doi.org/10.3390/en12010119 (2019).
Khan, S. et al. Nuclear Energy Powered Seawater Desalination. in Renewable Energy Powered Desalination Handbook: Application and Thermodynamics. https://doi.org/10.1016/B978-0-12-815244-7.00006-4 (2018).
Wang, Z. et al. Pathways and challenges for efficient solar-thermal desalination. Sci. Adv. 5, eaax0763 (2019).
Li, H. et al. Experimental realization of few layer two-dimensional MoS 2 membranes of near atomic thickness for high efficiency water desalination. Nano Lett. 19, 5194–5204 (2019).
Qasim, M., Badrelzaman, M., Darwish, N. N., Darwish, N. A. & Hilal, N. Reverse osmosis desalination: a state-of-the-art review. Desalination 459, 59–104 (2019).
Fritzmann, C., Löwenberg, J., Wintgens, T. & Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007).
Elimelech, M. & Phillip, W. A. The future of seawater desalination: Energy, technology, and the environment. Science https://doi.org/10.1126/science.1200488 (2011).
Voutchkov, N. Energy use for membrane seawater desalination – current status and trends. Desalination 1, 2–12 (2018).
The Role of Desalination in an Increasingly Water-Scarce World. The Role of Desalination in an Increasingly Water-Scarce World. https://doi.org/10.1596/31416 (2019).
Li, Y., Yang, S., Zhang, K. & Van der Bruggen, B. Thin film nanocomposite reverse osmosis membrane modified by two dimensional laminar MoS2 with improved desalination performance and fouling-resistant characteristics. Desalination 454, 48–58 (2019).
Rana, D., Kim, Y., Matsuura, T. & Arafat, H. A. Development of antifouling thin-film-composite membranes for seawater desalination. J. Memb. Sci. 367, 110–118 (2011).
Misdan, N., Ismail, A. F. & Hilal, N. Recent advances in the development of (bio)fouling resistant thin film composite membranes for desalination. Desalination 380, 105–111 (2016).
Choi, H., Son, M. & Choi, H. Integrating seawater desalination and wastewater reclamation forward osmosis process using thin-film composite mixed matrix membrane with functionalized carbon nanotube blended polyethersulfone support layer. Chemosphere 185, 1181–1188 (2017).
Zhang, Y. et al. Surface modification on thin-film composite reverse osmosis membrane by cation complexation for antifouling. J. Polym. Res. 26, 1–12 (2019).
Darling, S. B. Perspective: interfacial materials at the interface of energy and water. J. Appl Phys. 3, 030901 (2018).
Li, Y., Yang, S., Zhang, K. & van der Bruggen, B. Thin film nanocomposite reverse osmosis membrane modified by two dimensional laminar MoS2 with improved desalination performance and fouling-resistant characteristics. Desalination https://doi.org/10.1016/j.desal.2018.12.016 (2019).
Köhler, M. H., Bordin, J. R. & Barbosa, M. C. Ion flocculation in water: from bulk to nanoporous membrane desalination. J. Mol. Liq. 277, 516–521 (2019).
Cohen-Tanugi, D., Lin, L. C. & Grossman, J. C. Multilayer nanoporous graphene membranes for water desalination. Nano Lett. 16, 1027–1033 (2016).
Heiranian, M., Farimani, A. B. & Aluru, N. R. Water desalination with a single-layer MoS 2 nanopore. Nat. Commun. 6, 1–6 (2015).
Werber, J. R., Osuji, C. O. & Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 1–15 (2016).
Teow, Y. H. & Mohammad, A. W. New generation nanomaterials for water desalination: a review. Desalination 451, 2–17 (2019).
Anis, S. F., Hashaikeh, R. & Hilal, N. Functional materials in desalination: a review. Desalination 468, 114077 (2019).
Yang, Y., Li, W., Zhou, H., Zhang, X. & Zhao, M. Tunable C 2 N membrane for high efficient water desalination. Sci. Rep. https://doi.org/10.1038/srep29218 (2016).
Cao, Z., Liu, V. & Farimani, A. B. Water desalination with two-dimensional metal-organic framework membranes. Nano Lett. https://doi.org/10.1021/acs.nanolett.9b03225 (2019).
Cohen-Tanugi, D. & Grossman, J. C. Nanoporous graphene as a reverse osmosis membrane: recent insights from theory and simulation. Desalination https://doi.org/10.1016/j.desal.2014.12.046 (2015).
Oviroh, P. O. et al. Nanoporous MoS2 membrane for water desalination: a molecular dynamics study. Langmuir 37, 7127–7137 (2021).
Sapkota, B. et al. High permeability sub-nanometre sieve composite MoS2 membranes. Nat. Commun. 11, 1–9 (2020).
Cao, Z., Liu, V. & Barati Farimani, A. Why is single-layer MoS2 a more energy efficient membrane for water desalination? ACS Energy Lett. 5, 2217–2222 (2020).
Köhler, M. H., Bordin, J. R. & Barbosa, M. C. 2D nanoporous membrane for cation removal from water: effects of ionic valence, membrane hydrophobicity, and pore size. J. Chem. Phys. 148, 222804 (2018).
Kou, J. et al. Nanoporous two-dimensional MoS2 membranes for fast saline solution purification. Phys. Chem. Chem. Phys. https://doi.org/10.1039/c6cp01967f (2016).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011).
Chen, Y. et al. Tuning electronic structure of single layer MoS2 through defect and interface engineering. ACS Nano https://doi.org/10.1021/acsnano.7b08418 (2018).
Lembke, D., Bertolazzi, S. & Kis, A. Single-layer MoS2 electronics. Acc. Chem. Res. https://doi.org/10.1021/ar500274q (2015).
Lukowski, M. A. et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. https://doi.org/10.1021/ja404523s (2013).
Mao, J., Wang, Y., Zheng, Z. & Deng, D. The rise of two-dimensional MoS2 for catalysis. Front. Phys. https://doi.org/10.1007/s11467-018-0812-0 (2018).
Yin, F. et al. Functionalized mos2 nanosheets as multi-gene delivery vehicles for in vivo pancreatic cancer therapy. Nanotheranostics https://doi.org/10.7150/ntno.27308 (2018).
Wang, X., Chang, J. & Wu, C. 7 - MoS2-based biomaterials for cancer therapy. In Woodhead Publishing Series in Biomaterials (eds. Yang, L., Bhaduri, S. B. & Webster, T. J. B. T.-B. in T. M.) 141–161 (Academic Press, 2019). https://doi.org/10.1016/B978-0-12-813477-1.00007-4.
Shi, Z. T. et al. Hierarchical nanotubes assembled from MoS2-carbon monolayer sandwiched superstructure nanosheets for high-performance sodium ion batteries. Nano Energy https://doi.org/10.1016/j.nanoen.2016.02.009 (2016).
Xie, X., Ao, Z., Su, D., Zhang, J. & Wang, G. MoS2/Graphene composite anodes with enhanced performance for sodium-ion batteries: the role of the two-dimensional heterointerface. Adv. Funct. Mater. 25, 1393–1403 (2015).
Theerthagiri, J. et al. Recent advances in MoS2 nanostructured materials for energy and environmental applications – a review. J. Solid State Chem. https://doi.org/10.1016/j.jssc.2017.04.041 (2017).
Huang, H. H., Fan, X., Singh, D. J. & Zheng, W. T. First principles study on 2H-1T′ transition in MoS2 with copper. Phys. Chem. Chem. Phys. https://doi.org/10.1039/c8cp05445b (2018).
Wang, Z. & Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.7b01466 (2017).
Lin, Y. C., Dumcenco, D. O., Huang, Y. S. & Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS 2. Nat. Nanotechnol. https://doi.org/10.1038/nnano.2014.64 (2014).
Deng, S., Sumant, A. V. & Berry, V. Strain engineering in two-dimensional nanomaterials beyond graphene. Nano Today https://doi.org/10.1016/j.nantod.2018.07.001 (2018).
Bertolazzi, S., Brivio, J. & Kis, A. Stretching and breaking of ultrathin MoS 2. ACS Nano https://doi.org/10.1021/nn203879f (2011).
Conley, H. J. et al. Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. https://doi.org/10.1021/nl4014748 (2013).
Jiang, J. W. & Park, H. S. Mechanical properties of MoS2/graphene heterostructures. Appl Phys. Lett. https://doi.org/10.1063/1.4891342 (2014).
Eknapakul, T. et al. Electronic structure of a quasi-freestanding MoS2 monolayer. Nano Lett. https://doi.org/10.1021/nl4042824 (2014).
Fang, A., Kroenlein, K. & Smolyanitsky, A. Mechanosensitive ion permeation across subnanoporous MoS 2 monolayers. J. Phys. Chem. C. https://doi.org/10.1021/acs.jpcc.8b11224 (2019).
Zhang, H. et al. Construction of MoS2 composite membranes on ceramic hollow fibers for efficient water desalination. J. Memb. Sci. 592, 117369 (2019).
Hirunpinyopas, W. et al. Desalination and nanofiltration through functionalized laminar MoS2 membranes. ACS Nano https://doi.org/10.1021/acsnano.7b05124 (2017).
Wang, Z. et al. Understanding the aqueous stability and filtration capability of MoS2 membranes. Nano Lett. 7, 7289–7298 (2017).
Zhou, W. et al. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. https://doi.org/10.1021/nl4007479 (2013).
Hirunpinyopas, W., Prestat, E., Iamprasertkun, P., Bissett, M. A. & Dryfe, R. A. W. Potential dependent ionic sieving through functionalized laminar MoS2 membranes. 2d Mater. https://doi.org/10.1088/2053-1583/ab5ad9 (2020).
Zhang, Y. et al. Fit-for-purpose block polymer membranes molecularly engineered for water treatment. npj Clean Water https://doi.org/10.1038/s41545-018-0002-1 (2018).
Thomas, M., Corry, B. & Hilder, T. A. What have we learnt about the mechanisms of rapid water transport, ion rejection and selectivity in nanopores from molecular simulation? Small https://doi.org/10.1002/smll.201302968 (2014).
Abal, J. P. K. Water desalination by MoS2 nanoporous membrane: a molecular dynamics analysis. http://hdl.handle.net/10183/211475 (2020).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput Phys. 117, 1–19 (1995).
Chu, C. et al. Precise ångström controlling the interlayer channel of MoS2 membranes by cation intercalation. J. Memb. Sci. https://doi.org/10.1016/j.memsci.2020.118520 (2020).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science https://doi.org/10.1126/science.aab0530 (2017).
Deng, M., Kwac, K., Li, M., Jung, Y. & Park, H. G. Stability, molecular sieving, and ion diffusion selectivity of a lamellar membrane from two-dimensional molybdenum disulfide. Nano Lett. https://doi.org/10.1021/acs.nanolett.6b05238 (2017).
Lu, X. et al. Relating selectivity and separation performance of lamellar two-dimensional molybdenum disulfide (MoS2) membranes to nanosheet stacking behavior. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.0c02364 (2020).
Zheng, S., Tu, Q., Urban, J. J., Li, S. & Mi, B. Swelling of graphene oxide membranes in aqueous solution: characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano https://doi.org/10.1021/acsnano.7b02999 (2017).
Guo, B. Y. et al. MoS2 membranes for organic solvent nanofiltration: stability and structural control. J. Phys. Chem. Lett. https://doi.org/10.1021/acs.jpclett.9b01780 (2019).
Ai, K., Ruan, C., Shen, M. & Lu, L. MoS2 nanosheets with widened interlayer spacing for high-efficiency removal of mercury in aquatic systems. Adv. Funct. Mater. https://doi.org/10.1002/adfm.201601338 (2016).
Safaei, J., Xiong, P. & Wang, G. Progress and prospects of two-dimensional materials for membrane-based water desalination. Mater. Today Adv. 8. https://doi.org/10.1016/j.mtadv.2020.100108 (2020).
Xu, G. R. et al. Two-dimensional (2D) nanoporous membranes with sub-nanopores in reverse osmosis desalination: latest developments and future directions. Desalination https://doi.org/10.1016/j.desal.2017.09.024 (2019).
Ries, L. et al. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. https://doi.org/10.1038/s41563-019-0464-7 (2019).
Ritt, C. L., Werber, J. R., Deshmukh, A. & Elimelech, M. Monte carlo simulations of framework defects in layered two-dimensional nanomaterial desalination membranes: implications for permeability and selectivity. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.8b06880 (2019).
Wang, L. et al. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. https://doi.org/10.1038/nnano.2017.72 (2017).
O’Hern, S. C. et al. Selective molecular transport through intrinsic defects in a single layer of CVD graphene. ACS Nano https://doi.org/10.1021/nn303869m (2012).
Zhao, Y. et al. Two-dimensional material membranes: an emerging platform for controllable mass transport applications. Small https://doi.org/10.1002/smll.201401549 (2014).
Ke, J. A., Garaj, S. & Gradečak, S. Nanopores in 2D MoS2: defect-mediated formation and density modulation. ACS Appl Mater. Interfaces https://doi.org/10.1021/acsami.9b03531 (2019).
Epsztein, R., DuChanois, R. M., Ritt, C. L., Noy, A. & Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. https://doi.org/10.1038/s41565-020-0713-6 (2020).
Verliefde, A. R. D. et al. Influence of solute-membrane affinity on rejection of uncharged organic solutes by nanofiltration membranes. Environ. Sci. Technol. 43, 2400–2406 (2009).
Raza, A., Hassan, J. Z., Mahmood, A., Nabgan, W. & Ikram, M. Recent advances in membrane-enabled water desalination by 2D frameworks: Graphene and beyond. Desalination 531. https://doi.org/10.1016/j.desal.2022.115684 (2022).
Abdelkader, B. A., Antar, M. A. & Khan, Z. Nanofiltration as a pretreatment step in seawater desalination: a review. Arabian J. Sci. Engineer. 43. https://doi.org/10.1007/s13369-018-3096-3 (2018).
Bandini, S. & Vezzani, D. Nanofiltration modeling: the role of dielectric exclusion in membrane characterization. Chem. Eng. Sci. 58 (2003).
Yaroshchuk, A. E. Dielectric exclusion of ions from membranes. Adv. Colloid Interface Sci. 85. https://doi.org/10.1016/S0001-8686(99)00021-4 (2000).
Oatley-Radcliffe, D. L., Williams, S. R., Barrow, M. S. & Williams, P. M. Critical appraisal of current nanofiltration modelling strategies for seawater desalination and further insights on dielectric exclusion. Desalination 343 (2014).
Szymczyk, A. & Fievet, P. Investigating transport properties of nanofiltration membranes by means of a steric, electric and dielectric exclusion model. J. Memb. Sci. 252 (2005).
Yaroshchuk, A. E. Non-steric mechanism of nanofiltration: superposition of donnan and dielectric exclusion. Sep. Purif. Technol. 22–23 (2001).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science (1979) https://doi.org/10.1126/science.1245711 (2014).
Mi, B. Scaling up nanoporous graphene membranes. Science https://doi.org/10.1126/science.aax3103 (2019).
Sun, L., Huang, H. & Peng, X. Laminar MoS2 membranes for molecule separation. Chem. Commun. https://doi.org/10.1039/c3cc46136j (2013).
Cohen-Tanugi, D., McGovern, R. K., Dave, S. H., Lienhard, J. H. & Grossman, J. C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. https://doi.org/10.1039/c3ee43221a (2014).
Faucher, S. et al. Critical knowledge gaps in mass transport through single-digit nanopores: a review and perspective. J. Phys. Chem. C. 123, 21309–21326 (2019).
Alam, I., Guiney, L. M., Hersam, M. C. & Chowdhury, I. Pressure-driven water transport behavior and antifouling performance of two-dimensional nanomaterial laminated membranes. J. Memb. Sci. https://doi.org/10.1016/j.memsci.2019.117812 (2020).
Huang, X. High-performance water filtration membranes using surface modification and new materials. Preprint at (2016).
Arshad, F., Aubry, C., Ravaux, F. & Zou, L. 2D MoS2 nanoplatelets for fouling resistant membrane surface. J. Colloid Interface Sci. 590, 415–423 (2021).
Hilal, N. & Wright, C. J. Exploring the current state of play for cost-effective water treatment by membranes. NPJ Clean. Water https://doi.org/10.1038/s41545-018-0008-8 (2018).
Konatham, D., Yu, J., Ho, T. A. & Striolo, A. Simulation insights for graphene-based water desalination membranes. Langmuir https://doi.org/10.1021/la4018695 (2013).
Kang, G. D. & Cao, Y. M. Development of antifouling reverse osmosis membranes for water treatment: a review. Water Res. https://doi.org/10.1016/j.watres.2011.11.041 (2012).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater 23 (2013).
Majumder, M., Chopra, N. & Hinds, B. J. Mass transport through carbon nanotube membranes in three different regimes: Ionic diffusion and gas and liquid flow. ACS Nano 5, 3867–3877 (2011).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science (1979) 312 (2006).
Abal, J. P. K., Dillenburg, R. F., Kohler, M. H. & Barbosa, M. C. Molecular dynamics simulations of water anchored in multilayered nanoporous MoS2 membranes: implications for desalination. ACS Appl Nano Mater. 4, 10467–10476 (2021).
DuChanois, R. M., Porter, C. J., Violet, C., Verduzco, R. & Elimelech, M. Membrane materials for selective ion separations at the water–energy nexus. Adv. Mater. n/a, 2101312 (2021).
Momeni, K. et al. Multiscale computational understanding and growth of 2D materials: a review. npj Computat. Mater. https://doi.org/10.1038/s41524-020-0280-2 (2020).
Sun, J. et al. Synthesis methods of two-dimensional MoS2: a brief review. Crystals https://doi.org/10.3390/cryst7070198 (2017).
Mauter, M. S. et al. The role of nanotechnology in tackling global water challenges. Nat. Sustain. https://doi.org/10.1038/s41893-018-0046-8 (2018).
Ferrari, A. C. et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale https://doi.org/10.1039/c4nr01600a (2015).
Chen, M., Haddon, R. C., Yan, R. & Bekyarova, E. Advances in transferring chemical vapour deposition graphene: a review. Mater. Horizons https://doi.org/10.1039/c7mh00485k (2017).
Li, H. Interfacial behavior in polymer derived ceramics and salt water purification Via 2D MOS2. (University of Central Florida, 2019).
Li, H. et al. Experimental realization of few layer two-dimensional MoS2 membranes of near atomic thickness for high efficiency water desalination. Nano Lett. https://doi.org/10.1021/acs.nanolett.9b01577 (2019).
Stevens, D. M., Shu, J. Y., Reichert, M. & Roy, A. Next-generation nanoporous materials: progress and prospects for reverse osmosis and nanofiltration. Indus. Engineer. Chem. Res. https://doi.org/10.1021/acs.iecr.7b02411 (2017).
Oviroh, P. O., Akbarzadeh, R., Pan, D., Coetzee, R. A. M. & Jen, T. C. New development of atomic layer deposition: processes, methods and applications. Sci. Technol. Adv. Mater. https://doi.org/10.1080/14686996.2019.1599694 (2019).
Weber, M., Julbe, A., Ayral, A., Miele, P. & Bechelany, M. Atomic layer deposition for membranes: basics, challenges, and opportunities. Chem. Mater. https://doi.org/10.1021/acs.chemmater.8b02687 (2018).
Miikkulainen, V., Leskelä, M., Ritala, M. & Puurunen, R. L. Crystallinity of inorganic films grown by atomic layer deposition: overview and general trends. J. Appl Phys. 113, 21301 (2013).
George, S. M. Atomic layer deposition: an overview. Chem. Rev. https://doi.org/10.1021/cr900056b (2010).
Johnson, R. W., Hultqvist, A. & Bent, S. F. A brief review of atomic layer deposition: from fundamentals to applications. Mater. Today https://doi.org/10.1016/j.mattod.2014.04.026 (2014).
Alvarez, P. J. J., Chan, C. K., Elimelech, M., Halas, N. J. & Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. https://doi.org/10.1038/s41565-018-0203-2 (2018).
Rao, C. N. R. & Biswas, K. Chemical vapour deposition and atomic layer deposition. Essentials of Inorganic Materials Synthesis 103–106 https://doi.org/10.1002/9781118892671.ch12 (2015).
Kim, H., Lee, H. B. R. & Maeng, W. J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Films https://doi.org/10.1016/j.tsf.2008.09.007 (2009).
Najmaei, S. et al. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. https://doi.org/10.1038/nmat3673 (2013).
Van Der Zande, A. M. et al. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. https://doi.org/10.1038/nmat3633 (2013).
Hong, J. et al. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. https://doi.org/10.1038/ncomms7293 (2015).
French, P., Krijnen, G. & Roozeboom, F. Precision in harsh environments. Microsyst. Nanoengineer. https://doi.org/10.1038/micronano.2016.48 (2016).
Zhuiykov, S., Kawaguchi, T., Hai, Z., Karbalaei Akbari, M. & Heynderickx, P. M. Interfacial engineering of two-dimensional nano-structured materials by atomic layer deposition. Appl Surf. Sci. https://doi.org/10.1016/j.apsusc.2016.09.040 (2017).
Suk, M. E. & Aluru, N. R. Water transport through ultrathin graphene. J. Phys. Chem. Lett. https://doi.org/10.1021/jz100240r (2010).
Zhan, Y., Liu, Z., Najmaei, S., Ajayan, P. M. & Lou, J. Large-area vapor-phase growth and characterization of MoS 2 atomic layers on a SiO 2 substrate. Small https://doi.org/10.1002/smll.201102654 (2012).
Tan, L. K. et al. Atomic layer deposition of a MoS2 film. Nanoscale https://doi.org/10.1039/c4nr02451f (2014).
Shin, S., Jin, Z., Kwon, D. H., Bose, R. & Min, Y. S. High turnover frequency of hydrogen evolution reaction on amorphous MoS2 thin film directly grown by atomic layer deposition. Langmuir https://doi.org/10.1021/la504162u (2015).
Valdivia, A., Tweet, D. J. & Conley, J. F. Atomic layer deposition of two dimensional MoS 2 on 150 mm substrates. J. Vac. Sci. Technol. A: Vac., Surf., Films https://doi.org/10.1116/1.4941245 (2016).
Demirtaş, M., Odacı, C., Shehu, Y., Perkgöz, N. K. & Ay, F. Layer and size distribution control of CVD-grown 2D MoS2 using ALD-deposited MoO3 structures as the precursor. Mater. Sci. Semicond. Process https://doi.org/10.1016/j.mssp.2019.104880 (2020).
Mattinen, M. et al. Atomic layer deposition of crystalline MoS2 thin films: new molybdenum precursor for low-temperature film growth. Adv. Mater. Interfaces https://doi.org/10.1002/admi.201700123 (2017).
Rempe, S., Brinker, C. J. & Jiang, Y. B. Biomimetic membrane for water purification. https://www.osti.gov/servlets/purl/1671540 (2011).
Koros, W. J. & Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 16, 289–297 (2017).
Zhou, X. et al. Controlled TiO 2 growth on reverse osmosis and nanofiltration membranes by atomic layer deposition: mechanisms and potential applications. Environ. Sci. Technol. 52, 14311–14320 (2018).
Hao, W., Marichy, C. & Journet, C. Atomic layer deposition of stable 2D materials. 2D Materials https://doi.org/10.1088/2053-1583/aad94f (2019).
Liu, K., Feng, J., Kis, A. & Radenovic, A. Atomically thin molybdenum disulfide nanopores with high sensitivity for dna translocation. ACS Nano https://doi.org/10.1021/nn406102h (2014).
Garaj, S. et al. Graphene as a subnanometre trans-electrode membrane. Nature https://doi.org/10.1038/nature09379 (2010).
Feng, J. et al. Electrochemical reaction in single layer MoS2: Nanopores opened atom by atom. Nano Lett. 15, 3431–3438 (2015).
Thiruraman, J. P. et al. Angstrom-size defect creation and ionic transport through pores in single-layer MoS2. Nano Lett. 18, 1651–1659 (2018).
Inoue, A., Komori, T. & Shudo, K. I. Atomic-scale structures and electronic states of defects on Ar +-ion irradiated MoS2. J. Electron Spectros Relat. Phenom. 189, 11–18 (2013).
Yin, K. et al. Generating sub-nanometer pores in single-layer MoS2 by heavy-ion bombardment for gas separation: a theoretical perspective. ACS Appl Mater. Interfaces 10, 28909–28917 (2018).
Masih Das, P., Thiruraman, J. P., Chou, Y. C., Danda, G. & Drndić, M. Centimeter-scale nanoporous 2D membranes and ion transport: porous MoS 2 monolayers in a few-layer matrix. Nano Lett. 19, 392–399 (2019).
Yamada, Y. et al. Subnanometer vacancy defects introduced on graphene by oxygen gas. J. Am. Chem. Soc. 136, 2232–2235 (2014).
Li, Y. et al. Thermally reduced nanoporous graphene oxide membrane for desalination. Environ. Sci. Technol. 53, 8314–8323 (2019).
Lim, J. S. & Kim, G. First-principles modeling of water permeation through periodically porous graphene derivatives. J. Colloid Interface Sci. 538, 367–376 (2019).
Zhang, Z., Li, S., Mi, B., Wang, J. & Ding, J. Surface slip on rotating graphene membrane enables the temporal selectivity that breaks the permeability-selectivity trade-off. Sci. Adv. 6, eaba9471 (2020).
Zhang, P. et al. Chemically activated MoS2 for efficient hydrogen production. Nano Energy 57, 535–541 (2019).
Tang, C. Y., Zhao, Y., Wang, R., Hélix-Nielsen, C. & Fane, A. G. Desalination by biomimetic aquaporin membranes: review of status and prospects. Desalination https://doi.org/10.1016/j.desal.2012.07.007 (2013).
Shen, Y. X., Saboe, P. O., Sines, I. T., Erbakan, M. & Kumar, M. Biomimetic membranes: a review. J. Memb. Sci. 454, 359–381 (2014).
Giwa, A. et al. Biomimetic membranes: a critical review of recent progress. Desalination https://doi.org/10.1016/j.desal.2017.06.025 (2017).
Porter, C. J., Werber, J. R., Zhong, M., Wilson, C. J. & Elimelech, M. Pathways and challenges for biomimetic desalination membranes with sub-nanometer channels. ACS Nano 14. https://doi.org/10.1021/acsnano.0c05753 (2020).
Werber, J. R. & Elimelech, M. Permselectivity limits of biomimetic desalination membranes. Sci. Adv. 4, eaar8266 (2018).
Werber, J. R., Porter, C. J. & Elimelech, M. A path to ultraselectivity: support layer properties to maximize performance of biomimetic desalination membranes. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.8b03426 (2018).
Zeng, F., Zhang, W.-B. & Tang, B.-Y. The electronic structure and elastic property of monolayer and bilayer transition metal dichalcogenides MX$_2$ (M=Mo,W;X=O,S,Se,Te): a comparative first-principles study. Chin. Phys. B 24, 097103 (2015).
Subad, R. A. S. I., Akash, T. S., Bose, P. & Islam, M. M. Engineered defects to modulate fracture strength of single layer MoS2: an atomistic study. Physica B: Condensed Matter https://doi.org/10.1016/j.physb.2020.412219 (2020).
Sun, S., Shan, F., Lyu, Q., Li, C. & Hu, S. Theoretical prediction of mechanical strength and desalination performance of one-atom-thick hydrocarbon polymer in pressure-driven separation. Polym. (Basel) https://doi.org/10.3390/polym11081358 (2019).
Cohen-Tanugi, D. Nanoporous graphene as a desalination membrane. Massachusetts Institute of Technology (Massachusetts Institute of Technology, 2015).
World Health Organization. Safe Drinking-water from Desalination. World Health Organization (2011).
Acknowledgements
The authors would like to acknowledge the support from the National Research Foundation (NRF) of South Africa and the Global Excellence Scholarship (GES) and the Centre for High Computing Performance (CHPC) South Africa.
Author information
Authors and Affiliations
Contributions
T.C.J. supervised the project. P.O.O. did the information and data gathering and drafted the manuscript, J.R. did the paper restructuring and part writing, A.V.D. did the review and corrections of the paper. The paper was discussed and modified at different stages by the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oviroh, P.O., Jen, TC., Ren, J. et al. Towards the realisation of high permi-selective MoS2 membrane for water desalination. npj Clean Water 6, 14 (2023). https://doi.org/10.1038/s41545-023-00228-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00228-y