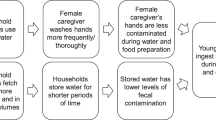
Abstract
Sanitation facility conditions and waste disposal practices are hypothesized to affect the fecal contamination of drinking water and kitchenware. The present study aimed to examine the physical conditions of sanitation facilities and waste disposal locations as well as determine the concentrations of Escherichia coli in drinking water, cups, dishes, flies, toilet floors, and kitchen floors. A total of 336 samples were collected from 17 households in peri-urban Lusaka. Generalized linear mixed models showed that six out of seven physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices had significant effects on the contamination of either kitchenware or drinking water. The results highlighted that improving the physical pit-latrine conditions, dumpsite location, washing water, and kitchenware-drying location may potentially reduce fecal contamination of drinking water, cups, and dishes.
Similar content being viewed by others
Introduction
Inadequate water, sanitation, and hygiene are important causes of diarrheal deaths that account for more than 1 billion mortalities reported globally and annually1. Owing to the inadequate sanitation conditions, ~431,000 out of 1.4 million reported diarrheal deaths occurred in low-and middle-income countries in 20162,3. Out of these 431,000 deaths, ~54.7% were reported from sub-Saharan African countries2. In the rapidly growing urban population of Sub-Saharan Africa, low-income settlements, where the majority have low sanitation coverage, may have high burden of diarrhea. Furthermore, poor sanitation conditions in such areas may result in widespread fecal contamination in the living environment as the contamination can be transmitted from one medium to another, thus causing fecal exposure, as described in F-diagram4,5.
Owing to global efforts, there has been progressive improvement in global sanitation coverage6. However, these sanitation facilities may be in poor condition. For example, not only shared latrines but also certain individual household latrines were observed to be non-functional due to poor management of sanitation facilities, as reported in urban areas of Ghana7, India8, and Nepal9. In addition, human waste has been reported to be mixed and handled with solid waste due to poor waste disposal practices in urban low-income settings, such as in urban areas of Kenya10,11 and Mozambique12. Such poor waste disposal practices may lead to contamination of the living environment.
Besides the reduction in diarrhea frequency, fecal contamination has been considered as a meaningful indicator to evaluate the impacts of sanitation improvement13,14. A study investigated the association of sanitation facility conditions with sanitary status in the living environment. Latrines in Tanzania15 with slabs had significantly less fecal contamination of hand contact surfaces inside latrines than those without slabs. Moreover, the number of flies, as a vector for fecal transmission4,5, can also be influenced by the conditions of sanitation facilities. In rural Kenya16 and Tanzania17, roofed latrines had significantly fewer flies than non-roofed latrines. Although sanitation facility conditions potentially affect fecal contamination in the living environment, little is known about their effect, especially on important exposure media such as drinking water and kitchenware, which are yet to be thoroughly investigated. Furthermore, studies on the effects of household waste disposal practices on fecal contamination of drinking water and kitchenware are lacking.
This study aimed to fill the knowledge gap by testing the hypothesis that the physical conditions of sanitation facilities and the location of waste disposal affect fecal contamination of drinking water and kitchenware. A cross-sectional study was conducted in peri-urban communities in Lusaka, Zambia, to test the hypothesis. Zambia has a coverage of 65.4, 31.9, and 17.9% for basic water, sanitation, and hygiene, respectively18. The specific objectives of the present study were to (1) assess the sanitation facility conditions, waste disposal practices, and fecal contamination, using Escherichia coli (E. coli) as an indicator of various media in the living environment, especially in drinking water and kitchenware; (2) investigate E. coli concentration levels by five types of sanitation facility conditions and waste disposal practices, and two types of kitchen hygiene practices; and (3) examine the multi-factor association of the mentioned conditions and practices on fecal contamination of drinking water and kitchenware using generalized linear mixed models (GLMMs).
Results
Conditions and contamination in sanitation facilities
A total of 17 sanitation facilities were examined based on their physical conditions: the type of sanitation, presence of a roof, type of wall, type of entrance, type of floor, and location of dumpsites from sanitation facilities and houses (Supplementary Table 1). According to the JMP classifications19 of the type of sanitation facilities, 15 (88.2%) of 17 facilities were improved sanitation facilities, which included 13 pit latrines with slabs, one septic tank, and one urine-diverting dry toilet (UDDT), whereas the remaining two were unimproved sanitation facilities, that is, pit latrines with no slabs and only soil ground.
In terms of the presence of a roof, six out of 17 (35.3%) facilities had roofs, and 11 (64.7%) had no roofs. For floor type, 15 out of 17 (88.2%) facilities had concrete floors, whereas only two (11.8%) had soil ground. Regarding the type of walls, 13 out of 17 (76.5%) facilities had solid concrete walls, whereas four (23.5%) had wooden frames hung with curtains made of polysack bags. Regarding the type of entrance, 11 of 17 (64.7%) facilities had solid wooden doors, whereas 6 (35.3%) had polysack curtains. For the location of dumpsites, 4 out of 17 (23.5%) dumpsites were located around houses, whereas 13 (76.5%) were distant from houses. Further analysis was performed with a focus on only 15 pit latrines, excluding one septic tank and one UDDT toilet.
E. coli concentrations on the surfaces of the inside- and outside-latrine-space floors of pit latrines are shown in Table 1. For every 100 cm2 floor surface area, the E. coli-positive proportions were 77.3% for inside-latrine-space floors and 71.4% for outside-latrine-space floors. Of the 22 inside-latrine-space floors, one floor sample (4.5%) had E. coli concentration exceeding 6000 CFU/100 cm2. E. coli concentrations on the inside-latrine-space floors were significantly higher than those on the outside-latrine-space floors (p < 0.05). The floor contamination inside- and outside-latrine-space was compared based on the physical pit-latrine conditions and the location of dumpsites from the pit latrines (Table 2). E. coli concentrations on inside-latrine-space floors (med: 2.97 log10 CFU/100 cm2) were significantly higher when the pit latrines had concrete walls than when they had polysack curtains (1.00 log10 CFU/100 cm2) (p = 0.02).
Conditions and contamination in kitchens
Hygiene practices in 17 kitchens were investigated (Supplementary Table 1). According to the JMP classifications, 7 out of 17 (41.2%) kitchens used an improved water source, that is, stored tap water collected from six public taps and one private tap, whereas 10 (58.8%) kitchens used an unimproved water source, that is, open-dug well water from unprotected wells. Stored tap water was used for both drinking and washing, whereas open-dug well water was used only for washing. After washing, cups and dishes were dried either inside or outside the house. Of the 17 kitchens, 11 (64.7%) dried their kitchenware outdoors, whereas six (35.3%) dried indoors. After drying, kitchenware from all 17 kitchens was kept indoors.
E. coli concentrations were obtained from six types of samples found in the kitchen environment (Table 3). In general, 82 out of 148 samples were positive for E. coli. Among the two types of dishwashing water, the E. coli-positive proportions per 100 ml were 12 out of 22 (54.5%) for stored tap water and 13 out of 15 (86.7%) for open-dug well water. Stored tap water was significantly less contaminated than open-dug well water (p < 0.001).
Among the cups and plates, for every inner surface of a medium, the E. coli-positive numbers were 18 out of 34 (47.1%) cups and 11 out of 21 (52.4%) dishes. No significant differences were found between E. coli concentrations in the cups and dishes.
For floor surfaces, the E. coli-positive numbers per 100 cm2 were 16 out of 22 (72.7%) floors in kitchens and 13 out of 22 (59.1%) floors at the house entrance. Floors in kitchens (p < 0.01) and at the house entrance (p < 0.01) were significantly less contaminated than those inside the latrine space.
The E. coli concentrations in cups, dishes, and drinking water (stored tap water) were compared with the washing and drying practices of kitchenware, physical pit-latrine conditions, and the location of dumpsites from kitchens (Table 4).
For kitchen hygiene practices, E. coli concentrations in dishes (med: 2.27 log10 CFU/medium) were significantly higher when the kitchenware was dried indoors than when it was dried outdoors (0.40 log10 CFU/medium) (p = 0.02). For the physical pit-latrine conditions, E. coli concentrations in cups and drinking water (med: 1.14 log10 CFU/medium for cups; 1.08 log10 CFU/100 ml for drinking water) were significantly higher when pit latrines had solid walls than when pit latrines had polysack curtains (0.40 log10 CFU/medium; −0.30 log10 CFU/100 ml) (p = 0.02 for cups; p = 0.03 for drinking water). Furthermore, E. coli concentrations in cups and drinking water (1.38 log10 CFU/medium; 1.08 log10 CFU/100 ml) were significantly higher when pit latrines had solid doors than that when pit latrines had polysack curtains (0.40 log10 CFU/medium; −0.30 log10 CFU/100 ml) (p < 0.01; p = 0.05).
Conditions and contamination of flies
The number of flies caught per fly trap at five places in each of 22 households was determined. A total of 110 fly traps were set up, on which 256 flies were caught within an hour of setting up. The highest proportion of the fly-positive traps (Table 5), was observed at dumpsites (77.3%), and the lowest was observed inside kitchens (18.2%). Significant differences were observed in the proportions of fly-positive traps among the five locations (p < 0.01). Of the total 110 traps, 51 traps did not catch any flies, whereas 57 traps caught 10 or less flies. The remaining two traps found at the dumpsites carried an exceptionally high number of 50 flies each.
Of the 256 flies caught, 141 were tested for E. coli. As shown in Table 6, of the 141 flies tested, 65 (46.1%) were positive for E. coli. No significant differences were found in E. coli-positive proportions among the five locations. For the E. coli concentration of flies, certain flies had concentrations exceeding 1200 CFU/fly: 1 of 19 (5.3%) flies inside latrine spaces, 6 of 34 (17.6%) flies outside latrine spaces, 5 of 32 (15.6%) flies at house entrances, 1 of 15 (6.7%) flies inside kitchens, and 9 of 41 (22.0%) flies at dumpsites.
For each of the five fly trap locations, the number of flies per trap and the E. coli concentration in flies were compared by considering the physical pit-latrine conditions and dumpsite locations (Table 7). For fly traps inside kitchens, the fly number was significantly higher in households with dumpsites located nearby (med: 0 fly/trap) than in those with dumpsites located distantly (0 fly/trap) (p < 0.01), even though the median values of the same. E. coli concentrations in flies inside kitchens were not significantly different between any of the physical pit-latrine conditions or dumpsite locations. As for fly traps in the remaining four places, the number of flies inside latrine spaces was significantly higher for pit latrines with polysack curtains (1.5/trap) than those with solid doors (0 fly/trap) (p < 0.01). Opposing the results of fly numbers, E. coli concentrations of flies inside latrine spaces (3.08 log10 CFU/fly) were significantly higher in pit latrines having solid doors than those having polysack curtains (0.30 log10 CFU/fly) (p < 0.01). Besides, E. coli concentrations in flies inside latrine spaces were significantly higher in pit latrines having concrete walls (1.83 log10 CFU/fly) than those having polysack curtains (0.30 log10 CFU/fly) (p < 0.01). The E. coli concentrations in flies outside latrine spaces and at house entrances did not differ remarkably based on the physical pit-latrine conditions or dumpsite locations.
Sanitation vs drinking water and kitchen media
The association of seven types of physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices with the contamination of drinking water (stored tap water), cups, and dishes in kitchens were examined using GLMMs. The models for each of the three media were ranked based on the corrected Akaike information criterion (AICc). The models with the lowest AICc and those with an AICc difference of less than two from the lowest AICc models are listed in Table 8.
Nagelkerke’s R2 of the lowest AICc models was 0.743 for cups, 0.725 for drinking water, and 0.488 for dishes, indicating good fitting of the models, especially for drinking water and cups. Focusing on the best models for each media, out of seven physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices, the presence of pit-latrine roofs had the strongest negative effect on the E. coli concentrations of cups and drinking water; the condition significantly reduced the E. coli concentration of cups by −2.61 log unit, equivalent to e−6.02 of the estimate in Table 8, (95% CI: −3.18 to −2.05) and that of stored tap water by −1.51 log unit (95% CI: −2.07 to −0.96). The second strongest negative effect was observed for locating dumpsites far from houses, which significantly reduced the E. coli concentration of cups by −1.08 log unit (95% CI: −1.60 to −0.56). Besides those, washing with stored tap water also significantly reduced the E. coli concentrations in cups and dishes by −0.55 log unit (95% CI: −0.97 to −0.13) and −0.72 log unit (95% CI: −1.43 to −0.004), respectively.
In contrast, of the seven mentioned conditions and practices, the presence of solid doors had the strongest positive effect on the E. coli concentrations in cups and drinking water; it significantly increased the E. coli concentrations of cups and drinking water by 1.45 log unit (95% CI: 0.76 to 2.15) and 1.26 log unit (95% CI: 0.54 to 1.98), respectively. In addition, the presence of solid walls also significantly increased the E. coli concentrations in cups and drinking water by 1.13 log units (95% CI: 0.33 to 1.93) and 0.88 log units (95% CI: 0.16 to 1.60), respectively. In addition, indoors significantly increased the E. coli concentration of dishes by 1.78 log units (95% CI: 1.09 to 2.48). Based on the results of the GLMM analysis, six out of seven physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices had a significant effect on the contamination of drinking water, cups, and dishes, indicating a clear association among the physical pit-latrine conditions, waste disposal practices, or kitchen hygiene practices, and fecal contamination in kitchens.
Discussion
This study analyzed the fecal contamination of drinking water (stored tap water) and kitchenware (cups and dishes), along with unimproved sources of water, surfaces, and flies in a peri-urban community in Lusaka city, Zambia. The association of physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices with fecal contamination of drinking water and kitchenware were examined.
Based on the GLMM results, six out of seven physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices were significantly associated with the contamination of at least one of these: drinking water, cups, or dishes (Table 8). The presence of pit-latrine roofs significantly reduced the E. coli concentrations in cups and drinking water. The regulatory effect of latrine roofing could be explained by fecal transmission by flies. Previous studies in rural Kenya16 and Tanzania17 found that lower fly density was associated with the presence of roofs on latrines. Furthermore, a study in rural Kenya16 found that the lower the number of flies in latrines, the lower the number of flies in the kitchens of the same households. Roofing may act as an effective barrier by blocking the transmission of feces via flies, leading to an increase in sanitation. The number and E. coli concentration in flies inside kitchens did not show statistical differences between roofed and non-roofed pit latrines. This may be due to the limited sample number obtained in this study, as only five roofed latrines were analyzed, even though more roofed latrines could have been obtained. Further studies are required to determine the role of roofing in controlling flies that transmit E. coli.
Based on the GLMM results (Table 8), dumpsites distant from houses significantly reduced the E. coli concentration in cups. This was similar to the effect of roofing but was statistically clearer and could also be explained by fecal transmission by flies. Distant dumpsites from houses had a significantly lower number of flies inside kitchens compared to dumpsites around houses (p < 0.01) (Table 7), showing a regulatory effect on the number of flies inside kitchens. Feces were found disposed along with solid wastes at the dumpsites in this study as well as in other poor sanitation areas such as rural India20,21 and urban Nigeria22. Flies may breed at dumpsites and increase their E. coli concentration through contact with existing feces. By distancing dumpsites from houses, the frequency of fly movement from the dumpsite to the kitchens can be reduced, thereby reducing the chance of contamination of the kitchenware by the contaminated flies. Our results suggest that distancing dumpsites from houses could prevent fecal transmission of flies and reduce kitchenware contamination.
Furthermore, washing kitchenware with stored tap water significantly reduced the contamination of cups and dishes according to GLMM analysis (Table 8). This association could be explained by the E. coli concentration in washing water. As a water source, stored tap water was significantly less contaminated than open-dug well water (Table 3). A previous study23 found that E. coli in contaminated washing water may be transferred to kitchenware surfaces. In this study, washing with less contaminated stored drinking water could have reduced the transfer of E. coli to the surfaces of the cups and dishes. The findings suggest that the cleanliness of dishwashing water influenced kitchenware contamination.
Based on the GLMM results (Table 8), the presence of solid doors and walls increased E. coli concentrations in cups and drinking water. These association could be explained by the proximity of the pit-latrine spaces. From Table 1, E. coli concentrations of inside-latrine-space floors were significantly higher due to the presence of walls (p < 0.05); although not significant, the E. coli concentration of inside-latrine-space floors were higher due to the presence of solid doors. A study in urban Tanzania24 reported that sun exposure on the surfaces of latrines and lower moisture inside may reduce surface contamination inside the latrines compared to those shaded. In our study, the presence of solid doors and walls may cause an increase in humidity and block sunlight, leading to a higher concentration of bacteria, although the exposure to sun and humidity on surfaces inside latrine spaces was not measured. Furthermore, floor samples in the study site were contaminated: inside-latrine-space (med = 2.31 log10 CFU/100 cm2), outside-latrine (1.00), house-entrance (0.85), and kitchen floors (1.10). The E. coli concentrations on the house-entrance floors were positively correlated with those on the inside-latrine-space floors (correlation coefficient: 0.64) (Supplementary Table 2). As house-entrance floors were geographically close to kitchen floors in each household, these two floor surfaces could be inter-correlated; the E. coli concentration on the inside-latrine-space floors may also be positively associated with that on kitchen floors. A study in a rural Alaskan community25 found that footwear was able to transfer feces from one place to another. Although the present study did not analyze foot transfer, higher contamination of inside-latrine-space floors may potentially cause higher floor contamination at house entrances and kitchens by foot transfer. Contamination may potentially be transferred to cups and stored tap water via accidental contact with floor surfaces, such as floor-cleaning activities26,27 and indoor playing on the floor28,29. This study suggests that the construction of well-ventilated latrines is important for reducing floor contamination in latrines and kitchens, leading to less contamination in kitchenware and stored tap water.
In addition, indoor drying of kitchenware increased the E. coli concentration in the dishes (Table 8). This association could be caused by less exposure to the open-air environment compared with outdoor drying. Previous studies have found that a lower concentration of microorganisms is associated with the presence of open-air drying30,31 as well as sunlight32,33. In this study, outdoor drying of kitchenware may be associated with exposure to sunlight and natural ventilation to inactivate E. coli. Open-air drying of kitchenware should be practiced reducing fecal contamination and to keep the kitchenware safe for use.
According to the GLMM results (Table 8), the contamination of cups and drinking water (stored tap water) had a similar direction of effects under three physical pit-latrine conditions: the presence of latrine roofs, solid walls, and solid doors. The similarity in the effects of the two kitchen media suggests that the media may have a common source of contamination. This was further validated as the contamination of cups and stored tap water was positively correlated (correlation coefficient: 0.71). At the study site, cups were used to collect drinking water from water containers and for drinking purposes. Owing to the practice of cups, cross-contamination between cups and drinking water may have occurred. Such cross-contamination was also observed because of the handling and storage practices of water in households in urban and rural Bolivia34, rural Kenya35, and rural Uganda36.
In conclusion, this study quantified the association of physical pit-latrine conditions and waste disposal practices with fecal contamination of drinking water and kitchenware using GLMMs. Based on the results of the models, six out of seven physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices were significantly associated with contamination of either kitchenware or drinking water. The results highlighted that improving the physical pit-latrine conditions, dumpsite location, washing water, and kitchenware-drying location may potentially reduce fecal contamination of drinking water, cups, and dishes. This study has several limitations. As the sample size of roofed pit latrines was small, the association of roofing with fly numbers inside the latrines—which can likely cause the contamination of cups and drinking water—could not be confirmed, even though more roofed pit latrines could have been observed. The role of flies should be carefully investigated to track fly movement in contributing to fecal contamination in the living environment. Further, this is a case study, and the findings of this study cannot be directly generalized in other contexts. Nevertheless, this study successfully demonstrated the quantitative association of physical pit-latrine conditions and waste disposal practices with the contamination of drinking water and kitchenware.
Methods
Ethics
The protocol of this study was approved by the Excellence in Research Ethics and Science (ERES) Converge (2019-Feb-009) in Zambia and the Research Institute for Humanity and Nature (RIHN) (2017-2) in Japan. All participants were informed about the aim and procedures of this study, and written consent was obtained before conducting the survey in each household.
Study area
Lusaka is the capital city of Zambia with approximately 2 million residents; over 70% of this population lives in 33 settlements located in the outskirt areas of Lusaka37,38. As cases of peri-urban communities, two settlements, namely Chawama and Kanyama compounds, were selected in the dry season of June-July 2019. These settlements were selected as the study sites as severe cholera outbreaks have been reported in these settlements of Lusaka, Zambia; moreover, they have low sanitation coverages39,40. In addition, most residents had irregular income patterns (74.6%), regardless of the amount of income obtained41.
Public taps in communities are common sources of water shared among households. Tap water was collected for daily use, such as drinking, cooking, dishwashing, and laundry. After the collection of tap water using plastic pails, the filled pails were kept in the kitchens before use. On the other hand, open-dug wells were constructed either with or without the physical protection of linings and covers and shared in the community. Well water was used as an alternative for daily activities, such as dishwashing and laundry, excluding drinking and cooking.
Pit latrines are shared by several neighboring households in the community. Other types of sanitation facilities include septic tanks and dry UDDT. However, the scattering of human feces was still observed around certain houses, indicating the practice of open defecation.
Kitchenware, such as cups and dishes, were used in each household, and they were commonly made of plastic. The cups were used to drink and collect stored tap water from the filled pails. The kitchenware was washed using stored tap water or open-dug well water and dried either indoors or outdoors. After drying, they were kept on open shelves in the kitchens before use.
Household wastes in the communities were mainly food waste, plastic waste, and sanitary wastes, such as diapers and menstrual wastes. These wastes are disposed of in polysack bags or metal containers located outside the houses, which are known as dumpsites.
Flies were found in the living environment of households and moving freely within locations such as inside kitchens, outside the houses, inside and outside sanitation facilities, and around the dumpsites.
Onsite investigation of physical conditions of sanitation facilities, waste disposal practices, and kitchen hygiene practices
Target households were selected objectively based on the availability of kitchenware and stored tap water for sampling. Snowball sampling was used to collect target samples from those households. An onsite investigation was conducted in 17 households to observe six types of physical sanitation conditions and waste disposal practices: physical sanitation facility conditions (five types) and dumpsite locations (one type). The five types of physical conditions were roofs, floors, doors, walls, and the distance of sanitation from households. The roof conditions were classified as roofed and non-roofed sanitations; the floor conditions as ground and concrete floors; the door conditions as polysack curtains and solid wooden doors; the wall conditions as polysack curtains and solid concrete walls. In addition, the dumpsite location was classified based on the house walls as next to the house walls (around the house) and apart from the house walls (distant from house). Household interviews were also conducted to learn two types of hygiene practices in kitchens: handling kitchenware and storing tap water in each household. The method of drying the kitchenware were categorized as drying inside or outside the house, and the type of dishwashing water was categorized into stored tap water and well water.
Fecal contamination of environmental media
Focusing on 17 households, nine types of samples were collected: stored tap water at point-of-use, open-dug well water at point-of-source, cups, dishes, floors inside sanitation spaces, floors outside sanitation spaces, floors at the house entrance, floors in kitchens and flies. For water samples, 24 samples of stored tap water were collected from pails kept in kitchens using the existing cups used by local people to collect the water, and 15 samples of open-dug well water were collected directly from 12 wells using the existing pails used by local people to collect the well water. Water samples were collected in sterilized sampling bags. For kitchenware, 37 cups and 23 dishes were sampled from the inner surfaces using swab test kits (Pro-media ST25 PBS, ELMEX, Japan); these kitchenware were obtained from kitchen shelves. For floor samples, each of the 24 floor surfaces inside sanitation spaces, outside sanitation spaces, at house entrances, and in kitchens were sampled from each of 10 cm by 10 cm surface area using swab test kits. Sticky fly tapes were used to catch flies in each household at five places: inside sanitation spaces, outside sanitation spaces, at house entrances, in kitchens, and at dumpsites. In total, 22 fly tapes were collected from 17 households, with seven households repeatedly visiting after 1 h of setting up. The fly tapes were then stored in sterilized sampling bags.
All the samples were transported on ice to the laboratory in the Integrated Water Resources Management Center, the University of Zambia, within 4 h of sampling. In the laboratory, a maximum of five flies from each fly tape were picked using sterilized forceps and placed in individual 50 mL Falcon tubes. The flies were rinsed with 40 mL of phosphate buffer solution. All sample solutions for the water, surface, and fly samples were mixed well before being analyzed for E. coli. The sample solutions were filtered (0.45-μm pore size, EMD Millipore Microfil V Filtration Device, Fisher Scientific, USA) to cultivate E. coli on a specific enzyme substrate medium (XM-G agar, Nissui, Japan) and incubated at 37 °C for 22 h. Blue colonies on each agar plate were enumerated to obtain the E. coli concentrations. For quality control, field blanks and laboratory blanks were prepared using sterilized distilled water per sampling day. All blanks were tested for E. coli and none of the blanks were E. coli-positive. For every ten samples, the E. coli samples were triplicated and then the average of triplicated data was used as a result.
Data analysis
Of the 17 households visited, 15 used pit latrines, 1 used a septic tank, and 1 used a UDDT. The association of the above-mentioned physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices with the contamination of stored tap water, cups, and dishes in kitchens were analyzed using generalized linear mixed models (GLMMs). The generalized linear mixed model is an extension of a generalized linear model that allows more flexible handling of non-normal data and includes random effects in which sample values can be considered as random draws from a larger population; GLMM allows repeated sampling from a group42. In certain types of samples collected, more than one sample was collected from a single household; therefore, the GLMM was used to include the random effects of households.
In this study, the GLMM of the gamma distribution with a log-link function, as shown in Eq. (1), which can be transformed into Eq. (2).
where
Ci is the E. coli concentration of sample type i;
βo,j is the intercept of the model of sample type i;
βj,i is the coefficient of the explanatory variable of condition or practice j for sample type i.
xj is the explanatory variable of condition or practice j;
ri is the random effect of households for sample type i.
For each GLMM model for a sample type, submodels with all possible combinations of explanatory variables were generated using the dredge function in the R package MuMIn43. Then, these submodels were ranked by the lowest corrected Akaike information criterion (AICc) and further filtered by taking submodels with less than or equal to the value of 2 of the delta of AICc, which is the difference between the AICc of one submodel and the lowest AICc in all submodels44,45. Under these criteria, the top three submodels with the lowest AICc were selected and evaluated for the association of physical pit-latrine conditions, waste disposal practices, and kitchen hygiene practices with fecal contamination of stored tap water, cups, and dishes.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper.
References
WHO, G. Global health estimates 2019: deaths by cause, age, sex, by country and by region, 2000–2019 (2020).
WHO, G. Global health estimates 2016: deaths by cause, age, sex, by country and by region, 2000–2016 (2018).
Prüss-Ustün, A. et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health 222, 765–777 (2019).
Wagner, E. G. & Lanoix, J. N. Excreta disposal for rural areas and small communities. Monograph Series. World Health Organization (1958).
Curtis, V., Cairncross, S. & Yonli, R. Review: domestic hygiene and diarrhoea - pinpointing the problem. Trop. Med. Int. Health 5, 22–32 (2000).
UNICEF/WHO. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020 (The UNICEF, 2021).
Hurd, J. et al. Behavioral influences on risk of exposure to fecal contamination in low-resource neighborhoods in Accra, Ghana. J. Water Sanit. Hyg. Dev. 7, 300–311 (2017).
Heijnen, M., Routray, P., Torondel, B. & Clasen, T. Shared sanitation versus individual household latrines in urban slums: a cross-sectional study in Orissa, India. Am. J. Trop. Med. Hyg. 93, 263–268 (2015).
McGinnis, S., Marini, D., Amatya, P. & Murphy, H. M. Bacterial contamination on latrine surfaces in community and household latrines in Kathmandu, Nepal. Int. J. Env. Res. Public Health 16, 257 (2019).
Bauza, V., Madadi, V., Ocharo, R. M., Nguyen, T. H. & Guest, J. S. Microbial source tracking using 16S rRNA amplicon sequencing identifies evidence of widespread contamination from young children’s feces in an Urban Slum of Nairobi, Kenya. Environ. Sci. Technol. 53, 8271–8281 (2019).
Ellis, A. et al. Practices and perspectives on latrine use, child feces disposal, and clean play environments in western Kenya. Am. J. Trop. Med. Hyg. 102, 1094–1103 (2020).
Holcomb, D. A. et al. Human fecal contamination of water, soil, and surfaces in households sharing poor-quality sanitation facilities in Maputo, Mozambique. Int. J. Hyg. Environ. Health 226, 113496 (2020).
Odagiri, M. et al. Human fecal and pathogen exposure pathways in rural Indian villages and the effect of increased latrine coverage. Water Res 100, 232–244 (2016).
Pickering, A. J. et al. The WASH benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob. Health 7, e1139–e1146 (2019).
Exley, J. L. R., Liseka, B., Cumming, O. & Ensink, J. H. J. The sanitation ladder, what constitutes an improved form of sanitation? Env. Sci. Technol. 49, 1086–1094 (2015).
Wolfe, M. K. et al. Adapting and evaluating a rapid, low-cost method to enumerate flies in the household setting. Am. J. Trop. Med. Hyg. 96, 449–456 (2017).
Irish, S., Aiemjoy, K., Torondel, B., Abdelahi, F. & Ensink, J. H. J. Characteristics of latrines in central Tanzania and their relation to fly catches. PLoS ONE 8, 1–7 (2013).
WHO & UNICEF. Progress on household drinking water, sanitation and hygiene 2000-2020. JMP for Water Supply Sanitation and Hygiene (2021).
WHO & UNICEF. Core questions on water, sanitation and hygiene for household surveys-2018 update. JMP for Water Supply Sanitation and Hygiene, 1–24 (2018).
Majorin, F. et al. Child feces disposal practices in rural Orissa: a cross sectional study. PLoS ONE 9, 1–7 (2014).
Bauza, V., Reese, H., Routray, P. & Clasen, T. Child defecation and feces disposal practices and determinants among households after a combined household-level piped water and sanitation intervention in rural Odisha, India. Am. J. Trop. Med. Hyg. 100, 1013–1021 (2019).
Ogundele, O. M., Rapheal, O. M. & Abiodun, A. M. Effects of municipal waste disposal methods on Community Health in Ibadan - Nigeria. Polytechnica 1, 61–72 (2018).
Mattick, K. et al. The microbiological quality of washing-up water and the environment in domestic and commercial kitchens. J. Appl. Microbiol. 94, 842–848 (2003).
Pickering, A. J. et al. Fecal contamination and diarrheal pathogens on surfaces and in soils among Tanzanian households with and without improved sanitation. Env. Sci. Technol. 46, 5736–5743 (2012).
Chambers, M. K., Ford, M. R., White, D. M., Barnes, D. L. & Schiewer, S. Transport of fecal bacteria by boots and vehicle tires in a rural Alaskan community. J. Environ. Manag. 90, 961–966 (2009).
Pickering, A. J., Julian, T. R., Mamuya, S., Boehm, A. B. & Davis, J. Bacterial hand contamination among Tanzanian mothers varies temporally and following household activities. Trop. Med. Int. Health. 16, 233–239 (2011).
Julian, T. R. & Pickering, A. J. A pilot study on integrating videography and environmental microbial sampling to model fecal bacterial exposures in Peri-Urban Tanzania. PLos ONE 10, e0136158 (2015).
Mattioli, M. C. M., Davis, J. & Boehm, A. B. Hand-to-mouth contacts result in greater ingestion of feces than dietary water consumption in Tanzania: a quantitative fecal exposure assessment model. Env. Sci. Technol. 49, 1912–1920 (2015).
Wang, Y., Moe, C. L. & Teunis, P. F. M. Children are exposed to fecal contamination via multiple interconnected pathways: a network model for exposure assessment. Risk Anal. 38, 2478–2496 (2018).
Himathongkham, S. & Riemann, H. Destruction of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes in chicken manure by drying and/or gassing with ammonia. FEMS Microbiol. Lett. 171, 179–182 (1999).
Sharpe, T. et al. Influence of ventilation use and occupant behaviour on surface microorganisms in contemporary social housing. Sci. Rep. 10, 1–13 (2020).
Maïga, Y., Denyigba, K., Wethe, J. & Ouattara, A. S. Sunlight inactivation of Escherichia coli in waste stabilization microcosms in a sahelian region (Ouagadougou, Burkina Faso). J. Photochem. Photobiol. B Biol. 94, 113–119 (2009).
Fisher, M. B. & Nelson, K. L. Inactivation of Escherichia coli by polychromatic simulated sunlight: evidence for and implications of a fenton mechanism involving iron, hydrogen peroxide, and superoxide. Appl. Env. Microbiol. 80, 935–942 (2014).
Rufener, S. et al. Quality of drinking-water at source and point-of-consumption-drinking cup as a high potential recontamination risk: a field study in Bolivia. J. Health Popul. Nutr. 28, 34–41 (2010).
Too, J. K., Sang, W. K., Ng’Ang’A, Z. & Ngayo, M. O. Fecal contamination of drinking water in Kericho District, Western Kenya: role of source and household water handling and hygiene practices. J. Water Health 14, 662–671 (2016).
Agensi, A., Tibyangye, J., Tamale, A., Agwu, E. & Amongi, C. Contamination potentials of household water handling and storage practices in Kirundo Subcounty, Kisoro District, Uganda. J. Env. Public Health 2019 (2019).
Central statistics office. Zambia 2010 census population and housing. 11, 1–117 (2012).
World Bank Group. Project Performance Assessment Report, Republic of Zambia, Water Sector Performance Improvement Project (2016).
Kabwe, P. et al. Descriptive characterization of the cholera outbreak in Lusaka District, 2016 Heal Press Zambia Bull. (2017).
Tembo, T. et al. Evaluating the costs of cholera illness and cost-effectiveness of a single dose oral vaccination campaign in Lusaka, Zambia. PLoS ONE 14, 1–16 (2019).
Nyambe, S., Agestika, L. & Yamauchi, T. The improved and the unimproved: factors influencing sanitation and diarrhoea in a peri-urban settlement of Lusaka, Zambia. PLoS ONE 15, 1–19 (2020).
Breslow, N. E. & Clayton, D. G. Approximate inference in generalized linear mixed models. J. Am. Stat. Assoc. 88, 9 (1993).
Barton, K. Mu-MIn: Multi-model inference. R Package Version 0.12.2/r18. http://R-Forge.R-project.org/projects/mumin/ (2009).
Richards, S. A. Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology 86, 2805–2814 (2005).
Symonds, M. R. E. & Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 65, 13–21 (2011).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP19H02274 and the Research Institute for Humanity and Nature (Project No. 14200107). The authors acknowledge Dr. Meki Chirwa for supporting this laboratory work in Lusaka.
Author information
Authors and Affiliations
Contributions
M.-L.C. conducted the fieldwork, sampled, analyzed the data, and wrote the manuscript. I.N., S.F., and T.Y. supported and supervised fieldwork. H.H. designed and supervised the overall study. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chua, ML., Nyambe, I., Fujii, S. et al. Association of latrine and waste disposal conditions with water and kitchenware contamination in peri-urban Lusaka. npj Clean Water 5, 54 (2022). https://doi.org/10.1038/s41545-022-00194-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-022-00194-x