Abstract
The ocean has often been announced as a sustainable source of important materials for civilization. Application of the same extraction processes to desalination concentrate, rather than to unconcentrated seawater, will necessarily be more energetically favorable, so the expansion of seawater desalination in recent decades brings this dream closer to reality. However, there is relatively little concrete commercial development of ‘concentrate mining’. This review assesses the technical and economic prospects for utilization of commercially viable products from seawater. The most important technologies for economic use of products from desalination plant concentrate are technologies for more economic separation and technologies for more economic concentration. The most promising separation technologies are those, such as nanofiltration, which separate brine into streams enriched/depleted in entire classes of constituents with minimal input of energy and reagents. Concentration is becoming more economic due to rapid advances in Osmotically-Assisted RO technology. Despite very active research on many aspects of desalination concentrate utilization, it is likely that commercial development of the non-NaCl components of desalination brine will depend on the available market for NaCl, as the challenges and costs of extracting the other mineral components from bitterns in which they are highly enriched are so much less than those faced in direct treatment of brines.
Similar content being viewed by others
Introduction
The world economy is heavily reliant on the sustainable supply of rare metals and valuable minerals and the development and deployment of sustainable products to the advanced manufacturing industries of the 21st century will require increased amounts of these materials. Advances in resource recovery technology over the last ten years have made extraction of minerals and metals from seawater desalination brine more cost-competitive in comparison to terrestrial mining1,2,3,4. However, the distribution of chemical compounds present in seawater (Table 1, Fig. 1) is dominated by a few abundant species of relatively low economic value. There have been periodic bursts of research enthusiasm into the isolation of low-abundance species from seawater since the 19th century—gold5, then uranium6, and more recently lithium7—but only the high-abundance species have ever given a commercial return. The goal of this review is to assess qualitatively which products from seawater desalination are commercially realistic in the medium term and which processes and technologies are most critical for enabling commercial production.
Concentration of chemical species in seawater and their commercial value, as estimated in September 2021. Rare earth data for North Atlantic surface water, Crocket187. Copper, Alexander & Corcoran188; Germanium, mbari.org/chemsensor/ge/germanium.html; Gold, Falkner & Edmond189 and references therein; Titanium, Croot190.
The metallic elements found in the highest concentration are sodium, magnesium, calcium and potassium, which have been commercially extracted as the chlorides, sulfates, and carbonates1 while magnesium has been extracted as the hydroxide8.
Recent overviews of brine mining possibilities have shown graphs similar to Fig. 1, with a line separating ‘economically feasible’ from ‘economically challenging’ target elements9,10,11. Such figures have sometimes used prices for pure metals which are not significant commercial products; in Fig. 1, the concentrations and prices of the most commercially relevant salts have been used wherever possible. Concentrations in Fig. 1 are based primarily on the Standard Sea Water (SSW) composition available online at Stanford University (https://web.stanford.edu/group/Urchin/mineral.html) with price estimates for materials not traded on a publicly available exchange estimated from current price ranges on Indiamart and Alibaba.
The line of ‘economic feasibility’ cannot be a straight line over the entire range of the figure, as production of sodium chloride for a miniscule fraction of a cent would clearly be uneconomic; however, over a range of concentrations corresponding to typical ores a straight line is feasible. While processing costs for terrestrial mining typically scale with tonnage (W) according to W0.5–0.7 12, below a certain minimum threshold energy costs per kg of product have been found to scale with concentration to the power of -3 13. While these are not the only costs involved in mining elements such as Li, Sr, and Rb, these dependencies suggest that a separation line with a slope >1, as in Loganathan et al.10, is more realistic than the shallower separation lines given in Shahmansouri et al.9 or Kumar et al. 11. The lowest grade of ore at which gold mines can operate profitably is about 0.5 ppm14, corresponding to about 0.025 ppm on seawater solids.
As can be seen from the two lines presented by Shahmansouri et al., the economics of brine mining will depend significantly on the amount of material processed. A plant producing in excess of 1 million m3 brine per day will be able to implement processes which would be uneconomic for a plant producing 10,000 m3 brine per day. While these lines differ greatly, they clearly delineate two sets of species with only a few doubtful intermediate cases, and only species lying to the right of all lines will be considered further. Note that bromine and sodium chloride, which have demonstrated profitability as products from seawater with current technologies, are present at concentrations more than an order of magnitude greater than all lines.
The potential income obtainable from a given volume of seawater from different sources is not immediately clear from Fig. 1 and has not been quantified explicitly in recent reviews of seawater mining. The relative economic importance of various chemical species extractable from seawater can be roughly estimated by multiplying the potential price range of a product by the total amount of limiting species present (Table 2). Only those compounds with a potential value of more than 1 USD per 1000 m3 seawater are shown. Note that in almost every case, additional chemical and energy inputs will be required to get to a final saleable product.
Brine (concentrate) from desalination plants contains large quantities of minerals, enriched in concentration compared to sea water—thus the figures appearing in Table 2 could be multiplied by a factor between 1.5 and 2.5. In mining seawater directly, energy equivalent to the energy expended in seawater desalination plant operation would need to be explicitly added to the system to achieve such an increase in concentration. Extraction of mineral products from desalination plant concentrate has potential advantages compared to terrestrial mining of the same compounds. These include: the essentially inexhaustible scale of the ocean; the constant composition of the ocean; the vast capacity of the ocean to dilute treated waste streams; and the stable and fixed footprint of the mining operation.
Much of the discovered shallow high-grade mineral ore worldwide has been mined over many decades leaving poorer quality, more difficult to access, and less mineral-enriched ores for future extraction. Mining operations have become progressively more costly over the past few decades because of the increasing depth and scarcity of the mined ores, high costs for environmental impact mitigation, and lower quality ores remaining available for extraction15. Conventional mining can create a multitude of environmental problems including the generated wastes and their associated health risks. Even more stringent environmental regulations associated with terrestrial mining are likely to be applied in the future, which would further make terrestrial mining more challenging and costly.
As technologies for seawater brine mining develop, desalination concentrate as a source of minerals becomes more economically and environmentally viable. The economic gain obtained by extracting minerals is proportional to the increase in the concentration of minerals in the concentrate as well as the market price of these minerals. In this respect, mining of compounds of elements including Mg, Na, Ca, K, Sr, Li, Br, B, and Rb could potentially be economically attractive for harvesting from concentrate, if suitable methods of brine concentration and extraction are developed10. Economically, the cost of extraction needs to be weighed against the revenue achievable, which relies on market fluctuations of commodity prices. Environmentally, extraction from brine is less intrusive than conventional mining with the added benefit of reduction in brine volumes. Commercial viability has been assessed to be likely for a number of products, including bromine, chlorine, sodium hydroxide, magnesium, potassium salts, and uranium, many of which are currently or were historically produced economically from seawater11.
Process intensification is likely to result in further improvement in technologies, specifically membrane technologies, to sustainably recover minerals from concentrate9,16. Publications in the brine mining area have tended to take a high-level view with a goal of generating excitement in the area, or are narrowly targeted to address specific issues. The goal of this review is to address the most promising current and emerging technologies for brine mining and assess as realistically as possible the prospect for commercial application of these technologies to target minerals.
Mineral recovery economics
Historically, several minerals have been extracted commercially from seawater; some directly, and a larger number indirectly from bitterns after production of commercial NaCl. Due to the fascination of the ocean as a source of minerals, significant research effort has been put into isolation of a much broader range of chemical species than those that have been commercially exploited.
We have considered only those chemical substances appearing on the right-hand side of Fig. 1, where the combination of price and availability makes economic viability more likely, in the approximate order of viability according to our assessment. For each substance or class of substances common applications are briefly mentioned and the current sources described. Existing or emergent technologies available for production from seawater desalination concentrate are discussed and specific potential benefits of production from desalination concentrate rather than current sources, if any, are highlighted.
Sodium chloride
Virtually every person in the world has some contact with sodium chloride (NaCl, table salt) on a daily basis. Sodium chloride is found in many processed foods, where it is added as an osmotic preservative, fermentation-control additive, texture-control agent and color developer, and consumers routinely add NaCl to their food as a flavor enhancer. However, the diverse industrial uses of salt account most of the world’s consumption. NaCl is not only used directly in many industrial processes, but is a major source of sodium and chlorine compounds used as feed stocks for further chemical syntheses. The single largest use of NaCl is in the chlor-alkali process as feedstock for chlorine and caustic soda manufacture and these two inorganic chemicals are used to make many consumer-related end-use products17. Similarly, in the soda ash industry, NaCl is used in the Solvay process to produce sodium carbonate and calcium chloride18. Sodium carbonate, in turn, is used to produce glass, sodium bicarbonate, and dyes, as well as a myriad of other chemicals. In the Mannheim process19 and in the Hargreaves process20, NaCl is used for the production of sodium sulfate and hydrochloric acid. It is also used to make sodium chlorate, which is added along with sulfuric acid and water to manufacture chlorine dioxide for disinfection. Further applications of NaCl are in oil and gas exploration, textiles and dyeing, pulp and paper, metal processing, rubber manufacture and tanning and leather treatment. NaCl is also used extensively in water treatment, for softening of hard water, which contains excessive calcium and magnesium ions that contribute to the build-up of a scale or film of alkaline mineral deposits in household and industrial equipment and pipes21. Finally, large quantities of NaCl are used for de-icing and anti-icing of roads in sub-freezing weather22.
NaCl is currently produced by mining of rock salt, evaporation of seawater, or evaporation of brine from brine wells and salt lakes. In 2020, world production was estimated at 270 million tons, the top five producers (in million tons) being China (60.0), United States (39.0), India (28.0), Germany (14.0), and Australia (12.0)23. Although NaCl is a relatively low-value commodity, the locations of production are often not near consumers and hence transportation costs significantly add to the price. The shipping cost for oceanic, rail, or truck transportation can be an important determining factor when attempting to secure supply sources. In some cases, pumping NaCl brine through pipelines can be the most economical solution when distances are relatively short. Large bulk shipments of dry NaCl in ocean freighters or river barges are relatively low in cost but are restricted by points of origin and consumption. The type of NaCl (e.g., vacuum, rock, solar), its purity, production, processing, and packaging factors can influence the selling prices. The relevance of both NaCl transport costs and pricing variability to brine mining lies in the fact that NaCl is the bulk of the available product both in terms of amount and net value in seawater (Table 2). Where a market can be established for NaCl, the availability of bitterns in which the other components of seawater are further concentrated by more than an order of magnitude dramatically increases the commercial viability of extracting these other components.
Desalination increases the concentration of salt in desalination brine by as much as 100%. Most modern sea water desalination plants use reverse osmosis membranes with a recovery of 30–50%, depending on initial concentration of the sea water and other process factors. As a result, desalination brines can contain as much as 8% NaCl. This significant increase in concentration makes recovery of NaCl from desalination concentrate inherently more economical than conventional production of NaCl from seawater.
Bromine
Bromine was extracted from seawater on an industrial scale throughout the 20th century as a critical ingredient in additives for leaded gasoline24. The process involved oxidation of the bromine present in seawater to bromine, followed by blowing air through the seawater to extract the volatile bromine. The bromine vapor was absorbed using alkali solution or sulfur dioxide to generate a concentrated liquor, which was then distilled to generate bromine product25,26. Ion-exchange27 and membrane-based28 methods for bromine extraction from brines have been extensively researched, but have not been applied to commercial production29. With the phasing out of leaded gasoline, the bromine market contracted significantly and plants extracting bromine from seawater ceased operations. More recently the bromine market has been expanding rapidly with continuing demand for bromine-containing flame retardants and emerging applications in clear brine fluids for oilfield completion, additives to reduce mercury emissions from coal-burning power plants, and salts for storage batteries for renewable energy. Bromine today is extracted using the same technologies once used from seawater, but from brines that have a higher bromide concentration: the main sources are the Dead Sea, underground brines in Arkansas and Shandong, and the bittern obtained from sea salt manufacture (chiefly in India, China and Japan)30. About 430,000 tons of bromine are produced worldwide per year. The volume of bittern produced by salt manufacture will clearly be limited by the available markets for sodium chloride, and many of the sources currently exploited are rapidly being depleted (e.g., the Dead Sea and the Bohai Gulf underground brine formations)31. The growing market for bromine products, the limited availability of natural brines with high bromide concentrations, and the increasing volumes and concentrations available from the output of desalination plants make desalination brine a plausible source to meet the growing demand for bromine.
In desalination brine, bromide obviously exists at a higher concentration than in seawater, which was economically used as a source for bromine until recently. This reduces the volume of material which needs to be handled with concomitant decreases in capital and operating expenditure. Most seawater desalination plants are also located in regions with year-round warm water, giving more efficient removal of bromine by air blowing than historical plants producing bromine from seawater (the Octel Amlwch plant, on the Irish Sea, could not operate at full capacity in the winter months (N. Summers, personal communication)).
Further improvement in the quality of brine as a feedstock for bromine extraction can be achieved by brine concentration, with brines obtained at the Desalination Technologies Research Institute (DTRI) in Saudi Arabia with bromide concentrations up to eight times that of standard seawater. The nanofiltration pre-treatment needed to obtain these concentrations also ensures that the scale-forming ions (primarily Ca2+ and SO42‒) that are a source of operational problems in other brine sources of bromine are removed: scale formation is a limitation to the current efficiency of bromine extraction, particularly from underground brines32. Whether brine concentration is cost-effective to maximize bromine production in the absence of a market for other brine components will clearly depend on the details of the market. Where a sufficient market for sodium chloride exists, bromine production can readily be implemented within an integrated process for adding value to desalination brine. This is most cost-effectively done by treating the bittern or crystallizer purge remaining after removal of the commercially available sodium chloride component of the brine.
Magnesium and magnesium salts
Magnesium compounds obtainable in seawater have a variety of useful applications in the agricultural, nutritional, chemical, construction and industrial industries. Magnesium itself is a low-density and therefore lightweight metal that produces strong alloys, which in recent years have replaced aluminum in many products in the construction, automotive, and consumer goods sectors. About 1 million metric tons of magnesium were produced worldwide in 202133. Epsomite (MgSO4·7H2O) has economic value principally as a fertilizer, while bischofite (MgCl2·6H2O) is used in dust and ice control and brucite (Mg(OH)2) is used as a fire retardant and in wastewater treatment. Approximately 3 million tons of epsomite, 3 million tons of bischofite, and 1 million tons of brucite were produced worldwide in 202134.
Historically, magnesium and magnesium salts have been extracted on an industrial scale from seawater and are still extracted commercially from brines. The historical process for producing magnesium from seawater involved precipitation of magnesium as magnesium hydroxide through the addition of lime or dolime35. The magnesium hydroxide could then be treated in one of two ways: reacted with hydrochloric acid to generate magnesium chloride solution (Dow Process), or heated at high temperature to generate magnesium oxide which could then be reacted with hydrochloric acid or chlorine to generate anhydrous magnesium chloride (Norsk Hydro Process)36. In either case, the magnesium chloride was then used as a feedstock for the electrolytic generation of magnesium metal. Despite the greater energy consumption in obtaining a feedstock for electrolysis in the Norsk-Hydro process, the relative simplicity of the electrolysis step in comparison to the Dow process meant that both processes were economic until the 1990s. Production of magnesium salts from desalination brines has been seen as attractive since desalination brines first became available in significant volumes, but has not yet been commercialized37,38. Over the past quarter-century the production of magnesium and magnesium salts from brines has shrunk in importance with the advent of inexpensive magnesium produced from mined magnesite and dolomite, chiefly from China. Where magnesium metal is still extracted from brines, it is obtained from saline waters with a much higher magnesium content than seawater where large volumes of bischofite or carnallite can be precipitated directly without addition of chemicals (e.g., the Great Salt Lake and the Dead Sea). Magnesium hydroxide is still produced commercially from seawater in China, Japan, Ireland, the United States, and elsewhere, accounting for about 60% of world magnesium hydroxide production.
Key to the profitability of any process based on extraction of magnesium from desalination brine is avoiding as much as possible the chemical costs incurred in producing magnesium chloride and the energy costs in drying magnesium chloride for electrolysis39. Within the context of an integrated facility where sodium chloride is also produced, the magnesium-rich bitterns derived from desalination brines could be commercially viable sources for magnesium manufacture40. A transformative technology which could greatly improve the viability of this process is nanofiltration: Separation of desalination brines into divalent-rich and monovalent-rich streams by nanofiltration can generate an inexhaustible source of saline water approximating the composition of magnesium-rich lakes, and hence open the way to restoring the ocean as the main source of magnesium salts and magnesium metal. Sequential crystallization of the nanofiltration reject stream can in principle produce gypsum, epsomite, and bischofite requiring relatively little purification before use, although this process is yet to be implemented commercially.
Potassium salts
Potassium salts are in demand worldwide as fertilizer: potassium sulfate (sulfate of potash, SOP) and potassium ammonium sulfate are more attractive for this application than potassium chloride (muriate of potash, MOP) and command higher prices41. The total production of potash fertilizers worldwide is over 30 million tons per year. While these are critical materials and shortages have been forecast due to exhaustion of readily accessible evaporite deposits, the relatively low price of these salts means that they have not attracted as much attention as potential products from desalination brines42. Processes for sequential evaporation of bitterns to produce KCl have been patented43. As with other ions of interest, a variety of electrochemical, membrane-based, and adsorption-based methods have been investigated to the removal of potassium from seawater. Battery deionization using a Fe[Fe(CN)6] electrode has been demonstrated to give 70% removal of K+ with a 140:1 K:Na selectivity from synthetic seawater, but this is likely to be a very capital and energy-intensive method of producing KCl44. A polyamide membrane incorporating zeolite was found to give 4:1 K:Na selectivity and was proposed for continuous extraction of potassium from seawater45, and diatomite has shown selective adsorption of potassium and been suggested as a pathway to produce fertilizer46, but these processes are also unlikely to be economically viable.
Potassium sulfate can be produced from sea salt bitterns by treatment of a kainite (KCl·MgSO4) mineral precipitate formed after removal of NaCl with sulfuric acid47 and versions of this process have been applied commercially to underground brines. More capital-intensive methods of producing SOP from seawater investigated on the laboratory scale include removal of sulfate from an anion exchange membrane with KCl solution48 and absorption of K+ on clinoptilite followed by elution with ammonium sulfate49.
A process where potassium ammonium sulfate is generated from seawater by reaction of magnesium sulfate (produced by precipitation from chilled bitterns), aqueous ammonia, and potassium tartrate has been proposed; its viability would depend on the efficiency with which tartaric acid could be recycled in the process50.
Calcium salts
The calcium salts that could potentially be obtained from brine have market prices of the same order of magnitude as to the magnesium salts obtainable, but the amounts available are significantly less. The potential products are all readily available commercially from mining plentiful reserves (CaCO3, limestone, and CaSO4·2H2O, gypsum)51,52 or as a by-product from the Solvay process for production of sodium carbonate (CaCl2)18, and are used in bulk in construction, agriculture, and chemical processes. More than 200 million tons of gypsum is used worldwide annually, overwhelmingly in low value construction applications. Calcium carbonate is used primarily in the production of cement and in roadbuilding aggregate and as a filler in plastics, with more than 100 million tons consumed annually. There appears to have been minimal academic or commercial interest in utilizing desalination brine as a source of these salts. As of 2017 a brackish water treatment facility in Southern California was producing calcium carbonate pellets economically from treatment of water with TDS of ~1000 ppm, but Ca clearly comprised a much larger proportion of the solids in the source water than is found in seawater53.
Lithium salts
Lithium extraction has attracted a great deal of research attention in recent years, due to the rapidly increasing market for Li-containing batteries for consumer electronics and electric cars. Production of lithium salts (in terms of amount of elemental lithium) has increased from 30,000 to ~100,000 metric tons since 201054. The fact that the ocean contains an essentially inexhaustible store of lithium has exerted a hypnotic effect on scientists and funding agencies, despite the widespread availability of non-oceanic sources. While lithium is commercially extracted largely from brines, these are underground brines formed under unusual geological conditions which have concentrations thousands of times greater than that found in seawater—hundreds or thousands of ppm rather than high ppb55. This difference in concentration means that existing brine treatment technologies, which rely on precipitating Li salts, cannot be directly applied to seawater or desalination brines56. Attempts to achieve selective absorption57, selective permeation58, and/or exploit the electrochemical behavior59 of lithium have been the main strategies investigated for extraction of Li from dilute solutions.
Manganese dioxide-based ion-sieve materials have been the most extensively investigated strategy for separation of lithium from complex aqueous solutions60. The small size of the Li+ ion means it can penetrate the spinel structure of MnO2 and thus exhibit a higher selective adsorption on MnO261. Various strategies have been employed to improve the selectivity and efficiency of this innate property of manganese dioxide, including combining it with graphene oxide62, cellulose63, or cellulose acetate64 membranes, intercalating titanium into the MnO2 lattice65, and using electrolytic approaches based on MnO2 electrodes66.
Other materials that have been shown promise for selective lithium absorption are polydopamine67, polymeric 1,3-diketones68, and ruthenium complexes embedded in a poly(methacrylic acid) resin69. Methods based on selective complexation of lithium followed by liquid-liquid extraction70,71, transport through a liquid membrane72, or transport through a solid membrane73 have also attracted significant research interest.
Recently a few studies have reported very large Li+:Na+ selectivities in seawater treatment. An electrochemically driven intercalation process using titania-coated iron (III) phosphate electrodes has achieved a Li+:Na+ selectivity of 18,000 and near quantitative removal of Li+ from a 300 mL sample of salt water over ten cycles of extraction74. On a larger scale, gram quantities of Li3PO4 have been obtained by precipitation of a solution in which Li was concentrated 43,000 times by iterative electrically-driven membrane sieving75. While both these studies are exciting from the proof-of-concept view, the energy involved in simply moving the large volumes of water required to obtain viable amounts of lithium by either of these process makes them uncompetitive. For this reason it has been suggested that sufficiently-selective technology relying on passive uptake of Li+ to a material submerged in the ocean was the only economically viable way to recover Li from seawater1. One possibility that could be suitable for such a process is direct electrochemical reduction of metallic lithium from seawater. Lithium sieves that can also serve as solid-state electrolytes based on materials of formula Li1−xAlyGe2−y(PO4)3 have been shown to be robust under environmental conditions and can generate pure lithium in quantities of the order of 20–50 μg cm−2 h−1 from seawater on the laboratory scale using a variety of metal oxide anodes7,58. Rates of production of order 200 μg cm−2 h−1 from seawater have been reported using a similar process and material of formula Li7La3Zr2O1276. If Li is collected not directly from seawater or brine, but from the more concentrated solution remaining after the principal components are removed, the hundredfold increase in Li concentration will make many of the approaches currently being investigated much more practicable77.
About 60% of current Li comes from hard-rock mines, chiefly in Australia, and about 30% from brines in the Andes of South America, with concentrations at least three orders of magnitude greater than seawater, and there are significant unexploited reserves that have not been fully assessed78.
Strontium salts
Studies of strontium recovery from seawater and brine have historically focused on methods for analysis of radioactive 90Sr79,80, which is of significant concern in monitoring nuclear plant safety. Extraction into organic solvent79,81 or a solid membrane82 using crown ethers or tertiary amides has been found to be effective in separating strontium from similar ions in seawater. However, the cost of these Sr-complexing compounds means that considerable work in optimizing their regeneration is needed before they could be applied to commercial recovery of strontium salts.
Strontium recovery from synthetic seawater was studied using alginate microspheres83,84, which not surprisingly showed significant competition from other common cations in seawater and achieved a maximum uptake of 147 mg dm−3 of alginate. The Sr2+ was eluted from the alginate with HCl solution. It was found to be accompanied by approximately ten times as much Cr2+, suggesting strongly that unless Ca2+ is removed from seawater before treatment this would not be a viable strategy for harvesting Sr. In similar work, a magnetite/MnO2/fulvic acid nanocomposite was found to absorb up to 6.4 mg g−1 Sr from natural seawater85 which was desorbed with hydrochloric acid, but the degree of separation of Sr from Ca and Mg was not assessed. Hydrothermally structured titanate nanotubes86 have also shown a high sorbent capacity (92 mg g−1), but when applied to seawater were found to have a Sr2+:Ca2+ selectivity of only approximately two.
Overall, the similar chemical properties of Sr and Ca mean that selective membrane rejection or resin absorption strategies are unlikely to be successful, with selective precipitation of insoluble Ca salts such as gypsum (CaSO4·H2O) the most promising strategy to obtain a Sr-enriched solution. Key to the success of such a strategy will be the degree to which Sr is incorporated in the gypsum: if a significant proportion of the Sr is lost in this way such a strategy will be unviable.
Strontium sulfate (celestite) is used in drilling fluids for oil and gas extraction and is the principal strontium ore mined, with a production of about 200,000 tons per annum, principally in China, Iran, Mexico and Spain87. There is a significant market for strontium salts and there may be a brine mining opportunity from the geographical concentration of desalination plant brine in areas that are also important for oil and gas extraction.
Rubidium salts
Studies of Rb+ recovery from synthetic seawater have shown that it can be effectively adsorbed using potassium cobalt hexacyanoferrate88 or potassium copper hexacyanoferrate (KCuFC)89. While adsorption of Rb+ was affected only slightly by high concentrations of Ca2+, Na+, and Mg2+, sorption of Rb+ was significantly reduced in the presence of K+. To compensate for the effect of K+, the column adsorptive removal of Rb+ was investigated with a polyacrylonitrile-encapsulated KCuFC. Using 0.1 M KCl, the adsorbed Rb+ was desorbed and solution of 68% pure Rb+ was produced by passing through a resorcinol formaldehyde column and subsequently leaching with HCl which kinetically separated the Rb+ from the K+90. The commercially available hexacyanoferrate-based ion-exchange resin CsTreat, designed for removal of radioactive cesium from nuclear reactor wastewaters, has shown a high sorption capacity for Rb+ from SWRO brine2.
Extraction of Rb into the organic phase from brine using the selective ligands BAMBP [4-tert-butyl-2-(α-methylbenzyl) phenoxide]91 or dicyclohexano-18-crown-692 has been extensively investigated. BAMBP has been found to be about 12–20 times more selective for Rb+ than K+ (and about 100 times more selective for Cs+ than K+)93. Calixarenes have also been shown to be effective in selectively extracting Rb+ from brines94.
The current market for rubidium products is relatively small and the extremely high prices for metallic Rb quoted in some previous analyses of brine-mining viability95 apply to a miniscule market. Rubidium salts are used primarily for specialty glasses, with an annual consumption of only about 4 tons, which is supplied almost entirely as a by-product from hard-rock mining of the lithium-rich ores pollucite and lepidolite; there is currently no production of Rb salts outside of China96.
Boric acid and borate salts
There is a significant literature on the extraction of boron from seawater, as boron can have negative effects on plant and animal health and historically very low limits (0.5 ppm) were required for desalinated drinking water97. However, the absorbed or rejected borate from desalinated water treatment has only hitherto been returned to the waste stream, rather than being converted into a saleable product. The principal strategies for removing boron have been complexation of borate with a resin incorporating vicinal diol ligands, which are highly selective for borate and have little uptake of other species present in seawater98; rejection of borate using RO membranes99; electrodialysis100; and combination methods where borate is complexed with polymers101 or nanoparticles97, which can be removed from the seawater stream by UF or a coarser filtration system. Combination methods avoid the increased concentration of scale-forming ions that would otherwise arise from NF: as the pH must be above the pKa of borate in seawater (~8.6 @30 °C102) in order for any of these removal methods to function, and as the scaling potential of Mg2+ and Ca2+ increases with pH, scaling has historically been of concern in boron rejection systems. Figure 1 suggests that boric acid could be a commercially viable product, and complexation of borate with particles that can be readily removed and regenerated with high efficiency is probably the most appropriate strategy. Boric acid has metallurgical and pharmaceutical applications and is used as a fireproofing agent for wood. Commercially boric acid and borate salts are obtained primarily from deposits of the highly water-soluble mineral borax (Na2B4O7·10H2O), with production of about 4 million tons per year primarily from dry lakes in Turkey, the United States, and Chile103. In Russia and China there is significant production of boric acid from other minerals which require more significant processing, and it is industrial markets without ready access to borax where extraction of borate from seawater is likely to be most practicable104.
Existing brine mining technologies
Concentrate from desalination plants is still seen mainly as a waste product for disposal- a potential problem to be managed, rather than an opportunity105. The costs of using brine to generate useful products remains high, and terrestrial sources are still far cheaper for most products, e.g., gypsum for construction106. However, with advancing costs and more stringent regulation of land-based mining, and continuing improvements in water recovery leading to ever more concentrated brine, the beneficiation technologies described in this review are likely to become more competitive. With the appeal to brine producers of environmentally positive large-scale beneficial use of desalination plant concentrate, technologies are expected to evolve significantly.
As indicated in Tables 1 and 3, seawater contains most minerals in low concentration, and while desalination concentrates may be twice this concentration, they must be both concentrated to dryness and separated into their separate components in order to afford commercially viable products. The previous section has considered potential products from seawater desalination concentrate individually. In this section, established technologies of general application to separation of chemical products from seawater desalination brine will be considered on a process basis.
The main technologies applied or proposed to mine minerals from seawater are evaporation with sequential precipitation, selective sequential precipitation, membrane separation, electrodialysis, membrane distillation and crystallization (MDC), and adsorption/desorption/crystallization10. In all these technologies, the concentration of the metal targeted for extraction is first increased to the level of supersaturation to enable their crystallization. In all these technologies except the last, recovery of minerals requires that the solubility product of the salt needs to be less than the enriched ionic product of the constituent ions. Only the method of adsorption/desorption/crystallization is not dependent on concentration of the brine. It has been more frequently proposed for obtaining minerals containing less common elements such as Li, Sr, Rb, and U57,85,88,107. Adsorbents allow these minerals to be adsorbed with other minerals and later quantitatively desorbed and crystallized.
One recurring outcome of our assessments of proposed brine mining operations is that targeting a single product is less viable than integrated processes which allow the isolation of a number of commercial products from a process stream.
Evaporation with sequential precipitation
The purpose of the process of salt solidification and recovery is to selectively recover high purity beneficial salts from the desalination plant concentrate. Technologies most commonly used currently are based on fractional crystallization and precipitation. Salts are crystallized either through evaporation of concentrate, or, within limits, by temperature control or alteration of the solvent quality108.
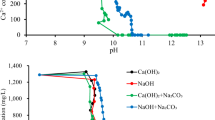
Minerals precipitate from seawater via evaporation in the order shown in Fig. 2. Calcium carbonate (aragonite or calcite) and calcium sulfate (gypsum) are most easily extracted, followed by sodium chloride (table salt). The remaining salts are precipitated in the last 2.5% of evaporation and in the conventional salt solidification process of seawater brines (Fig. 3) are deposited as mixed salts (e.g., MgCl2.KCl·H2O, carnallite) which require further processing and separation before use.
Sequence of precipitation of minerals from seawater, adapted with permission from Voutchkov and Kaiser180.
Schematic of salt solidification and recovery system, adapted with permission from Voutchkov and Kaiser180.
Solar evaporation in ponds is the oldest method for extraction of minerals such as sodium chloride from seawater and desalination plant concentrate. Evaporation ponds are designed as a system of shallow pools to concentrate and crystallize desalination plant brine. Evaporation pond systems are relatively easy to construct, require low maintenance and minimal mechanical equipment. Significant land area is however required, and the period for brine concentration and crystallization can be quite lengthy—typically at least two years of operation is required before product is obtained. To prevent groundwater pollution, the ponds must be lined with clay, poly(vinyl chloride), or polyethylene materials109. The main expenditure for solar evaporation ponds is the cost of land as such ponds are very land-intensive. Only minerals with high content (e.g., NaCl) can be economically recovered through this process alone, but this is the essential first step in producing bitterns highly enriched in potassium and magnesium minerals, as well as bromine.
Thermal evaporation and crystallization can be applied in an analogous manner to isolate first scale-forming salts, and then relatively pure sodium chloride, from seawater or desalination brine: however, the energy costs of such processes are prohibitively high and the process is commercially viable only for high-value salt intended for human consumption110.
Potash (MOP, potassium chloride), magnesium chloride, magnesium sulfate, magnesium hydroxide and bromine, may all be produced at a plausible cost for commercial production from bitterns remaining from solar salt production by thermal treatment111,112,113,114. While the bitterns remaining after crystallization of sodium chloride are more highly concentrated in magnesium and potassium salts than desalination brines, the mixed salts that precipitate on further concentration require further separation and chemical treatment in order to produce saleable products115.
The second stage bitterns remaining after crystallization of these minerals can be economically exploited for the recovery of rarer elements (specifically rubidium, for which bitterns remaining from potash extraction were historically an important source in the United States of America)116. Production of minerals from salt making bitterns has recently been reviewed by Bagastyo et al.117.
Selective sequential precipitation
Addition of counter-ions to produce insoluble salts can alter the sequence of fractional precipitation shown in Figs. 2 and 3, selectively removing a specific mineral from the concentrate: for example, Mg2+ as Mg(OH)2 or Ca2+ as CaCO3. Such magnesium and calcium salts have been selectively precipitated from desalination brine using several existing commercial technologies and assessed as commercial commodities118.
Chemical precipitation has been used to recover salts from seawater and RO reject brines using sodium carbonate and sodium phosphate119. Carbonate addition gave recovery of between 89 and 96% of the Ca2+ and between 86 and 91% of the Mg in seawater and two RO brines. Phosphate gave a similar recovery rate of Ca2+, but a lower recovery of Mg2+, and had much more variable results between seawater and brines. In seawater, sodium phosphate led to 98% recovery of calcium and 47% of magnesium while in concentrate, these rates were 75% and 24%, respectively119. Approximately 2 kg of calcium and magnesium salts were precipitated from concentrate per 1 kg of sodium carbonate used, while only 1.43 kg of calcium and magnesium salts could be obtained from seawater per 1 kg of sodium carbonate, indicating the greater cost-effectiveness of treating desalination concentrate120. In a similar study, a seawater RO brine containing 830 mg dm−3 Ca and 2620 mg dm−3 Mg was treated with 14 g dm−3 Na2CO3 at 25 °C, removing 94% of Ca and 70% of Mg; at 65 °C, due to the inverse solubility behavior of CaCO3 and Mg(OH)2, 8.5 g dm−3 of Na2CO3 removed 95% of Ca and 82% of Mg120. Attempts to push extraction to higher levels by increasing pH were ineffective, despite commercial modeling software suggesting that this would be effective. In addition, this research indicates that the recovery of calcium and magnesium is hindered by antiscalants and other metallic ions in the reject brine. To compensate for the inhibitory effect of antiscalants on precipitation of calcium and magnesium, the reject brine was further concentrated by electrodialysis (ED), reducing the amount of antiscalants in the brine. A range of 0.35–14 g dm−3 of sodium carbonate and 0.85 g dm−3 of sodium hydroxide were used to maximize the removal of calcium and magnesium. The residual from the ED-RO process contained 10 mg dm−3 of calcium and magnesium and the overall removal efficiency of these minerals from brine exceeded 95%121.
A further precipitation technology patented by GEO-Processors has found application in Australia and the United States. Through the SALPROC process, salts are precipitated through a combination of chemical reactions with repeated evaporation and cooling steps. In this way, salts such as magnesium carbonate, calcium carbonate and gypsum are recovered from concentrate122,123.
Careful control of pH and stoichiometry is needed to precipitate CaCO3 without co-precipitation of Mg(OH)2. As separation of precipitates into relatively pure fractions of a single salt is required for most applications, there is little scope for technologies that precipitate Ca and Mg salts simultaneously. These technologies are all unlikely to be economically competitive for extraction of minerals from brine, due to the requirement of addition of stoichiometric (or greater than stoichiometric) quantities of other reagents in order to generate the product(s) of interest. These required reagents, such as calcium hydroxide, sodium carbonate, sodium phosphate and sodium hydroxide, tend to be only slightly less costly than the relatively low-value target minerals.
Membrane-based separations
In seawater and brine, elements of greater value and lower concentration may exist as cationic or anionic species. Some of the high-value metals that are frequently present as cationic species in seawater and brine include copper, nickel, cobalt, and lithium. In contrast, uranium, platinum, molybdenum, and vanadium are present in brine as anionic species. Table 3 presents the concentration of key rarer elements contained in seawater and the rejection of these elements by NF and SWRO membranes. As seen from this table, NF brine has a high rejection of most key high-value elements except for lithium and rubidium. Usually, NF membranes reject over 85% of the calcium and magnesium in the seawater, with a similar rejection of other multivalent ions, and only reject 15–20% of sodium, chloride, and other monovalent ions.
Brine can be further concentrated by membrane osmotically-assisted RO when the concentrations of the minerals approach saturation124 or by Forward Osmosis (FO) membranes125. The technical limit of such methods is the point of crystallization of salts from the brine; they become uncompetitive with thermal concentration methods at a slightly lower TDS. Membrane-based methods will be treated in more detail below.
Electrodialysis (ED)
Electrodialysis (ED) can be applied for brine concentration and is applied commercially for concentration of seawater in Japan, Korea, and Kuwait126. Electrodialysis is fundamentally more energy-consuming than membrane-based methods that rely on movement of water though a membrane rather than the movement of ions through a membrane and has almost twice the power consumption per ton of salt produced of the most advanced membrane-based methods127,128.
Electrodialysis has however an additional benefit, as in combination with selective monovalent cation and anion permeable membranes, it can be used to separate monovalent ions, such as Na+ and Cl−, from divalent ions, such as Ca2+, Mg2+, and SO42- 129. This produces both a concentrated solution enriched in NaCl and an NaCl-depleted solution with Mg2+ concentration which is four to six times higher than that in seawater. These solutions can then be treated by other methods to obtain solid product. Evaporation of the NaCl-rich stream will obtain crystalline NaCl with greater purity and a lower energy input than direct evaporation of brine, while Mg2+ can be precipitated as Mg(OH)2 from the Mg-enriched stream. This has been done electrolytically by decomposing water to H2 and OH−, giving Mg(OH)2 of ~99% purity130. Mg(OH)2 can also be obtained by increasing pH to 11 by the addition of Ca(OH)2 or NaOH. While Ca2+ can inhibit the precipitation of Mg(OH)2, this can be avoided by pre-treatment with an appropriate stoichiometric amount of Na2CO3 to precipitate CaCO3, or by deaeration at an appropriate pH to remove CO32− as CO2130.
Appropriate combination of cation-selective and anion-selective membranes can separate a brine stream into product streams where the divalent cations and anions are sent in different directions131: e.g., where one stream can be used to obtain the sulfate component of the brine as Na2SO4, and another can obtain the calcium component of the brine as CaCl2. While this additional flexibility in obtaining desired mineral products would be of great value, at the present time the energy requirements of such systems are prohibitively high for high TDS solutions (G. Qile, personal communication).
Ongoing development of monovalent cation-permeable and anion-permeable membranes which can separate monovalent and divalent ions has the prospect to improve ED as a process for mineral recovery. Membrane research is expected to yield further improvements in permeable membranes sensitive to specific individual ions, for example, Li+66, for coupling to ED, but the energy requirements for electrodialysis in concentrated brines remain such that only high value components will be of potential interest.
Membrane distillation crystallization (MDC)
Membrane distillation crystallization (MDC) is an innovative technique for implementation of membrane technology in crystallization processes132. MDC exploits the excellent ability of membrane distillation (MD) process, a thermally-driven operation, to concentrate the feed solution up to supersaturation133. In MDC, the saturated solute is crystallized out from the solution when the solution reaches the saturation state134. Hence the system attains suitable conditions for crystallization. The advantages of MDC include well-controlled nucleation and growth kinetics, fast crystallization rates and reduced induction time, and production of high-quality crystals135.
MDC has drawn attention as an attractive alternative method for water recovery as well as for crystal production, especially for high-value products136. MDC inherits all of the benefits embedded in MD, such as lower operating temperatures and energy requirements137. MDC is generally used at temperatures ranging from 30 to 85 °C, which is below conventional distillation138. This offers the possibility to use low-grade heat (e.g., solar and geothermal) or waste heat (e.g., surplus heat from industrial processes) for operation139,140, which can reduce the cost of the process significantly and also offers a “carbon-neutral” technique for processing different streams141.
The most typical membranes used in existing MDC processes are those fabricated from polymeric materials containing polypropylene, polyvinylidene fluoride, polytetrafluoroethylene, and polyethersulfone. Flat-sheet and hollow fiber membrane modules are commonly applied136. The flat-sheet membrane module has the advantages of simple structure, convenient cleaning, and low cost, but the specific surface area and packing densities are lower than in the hollow fiber membrane module. In principle, all MD configurations can be used as MDC142, including direct contact membrane distillation (DCMD), air gap membrane distillation, sweeping gas membrane distillation, and vacuum membrane distillation (VMD)142. The optimum configuration should be determined depending on feed solution and the operative conditions. DCMD is the main applied configuration of MDC due to its simplicity and low cost but VMD is preferred to achieve a high degree of concentration. In addition to these traditional MDC types, novel configurations have been also investigated, including submerged MDC143, percrystallization144, bubble MDC145, and MDC coupled with cooling crystallization146.
When MDC is applied to brines from seawater desalination plants, it leads to reduction in brine discharge, flexibility in site selection, production of dry salts of high quality and controlled properties, and increased production in fresh water147,148,149. There have been many reports on the use of MDC to recover minerals from either natural or synthetic seawater brines138. MDC has been widely applied to produce NaCl crystals145,150 and has been also used to harvest MgSO4151, CaCO3152, CaSO4153, and Na2SO4143. Moreover, MDC had potential to increase the recovery of fresh water by controlling the membrane scaling due to mineral crystallization137,151. The introduction of MDC units on both brines in a NF-SWRO system increases the water recovery so much that it can reach values higher than 90% and also allows the production of NaCl, CaCO3, and MgSO4, etc.154. Recent research focus on MDC has shifted to recover more valuable components such as Li155 and Rb156.
Although there are many advantages in MDC for mineral recovery from brines, it also has shortcomings associated with membrane fouling and pore wetting135,157. The lack of appropriate membrane materials and modules is also a critical issue133. Unlike other membrane systems such as NF and RO, scale-up of MDC is still difficult, which results from insufficient information and experience157. Due to these challenges, most work on MDC has been carried out on bench scales and only a handful of experiments have been done on pilot scales148,149. In addition, a serious consideration in application of MDC to brine mining is that it has no inherent mechanism for separating out particular crystalline species from a complex mixture such as seawater, so cannot produce pure minerals except for those with clearly separated crystallization points, such as NaCl and CaSO4.
Adsorption/desorption
Minerals are naturally found in low concentration in seawater, which is a major reason why land mining has generally been economically favored. Separating most individual minerals by precipitation or crystallization given their low concentrations in seawater is difficult with existing technologies. Thus, adsorbent materials that can selectively bind particular chemical species in solution have been an attractive goal for research10. Once adsorption is complete, the selected mineral must be desorbed and precipitated to form the crystalized salt. The desorbed solution may contain other minerals which in turn need to be removed through applying adsorbents specifically to these minerals. Very high selectivity is required for adsorption/desorption processes, due to the low concentration of the target ions relative to the main species present in seawater. In assessing the viability of the processes, it is necessary to consider the operational costs of regenerating the adsorbent material, which will usually require the use of stoichiometric quantities of acid, or very much greater than stoichiometric quantities of fresh water. While increased concentration of the feed brine will enhance the extent and rate of adsorption of dissolved ions to the adsorbent, it will not improve selectivity or significantly reduce the relative amount of reagent needed for desorption. Adsorption studies of potassium45, lithium61, strontium86, and rubidium89 have already been discussed above with reference to those elements. Branched polyethyleneimine (PEI) macromolecules have been suggested as one class of adsorbents with selectivity for metal ions in seawater, specifically Cu2+ and UO22+3. PEI has also been embedded in high-capacity chelating resins and membrane absorbers have been studied for selective recovery of boron from seawater and brine158. It has been suggested that to avoid the high energy costs of pumping seawater or brine, adsorbent material could be placed directly in a static body of seawater or brine and removed when saturated1. In one embodiment of this approach to selectively extract Rb+, a submersible device combining membrane distillation to produce a concentrated brine gave ~87% recovery of Rb+ from a spiked solution of SWRO brine containing 5 ppm Rb159.
Brine concentration and nanofiltration: the keys to brine mining
Two key transformative technologies are discussed in this chapter: Nanofiltration (NF), to separate brine into mixed dissolved ion stream into mono-valent enriched and multi-(di-)valent enriched streams; and Membrane Brine Concentration (MBC), to concentrate the brine into higher salinity in a more energy-efficient way than a conventional thermal evaporator. While individually these technologies can improve the performance of brine mining operations, the combination of NF and MBC technologies can make a significant impact on the feasibility of brine mining, with an electricity consumption of 75–79 kWh per ton of NaCl isolated calculated in comparison with 165 kWh per ton salt for commercial ED systems127,160.
Nanofiltration (NF)
The single most important technology for improving brine mining is selective nanofiltration membranes which have a significantly greater rejection of divalent ions than monovalent ions. These allow separation of one stream, NF reject, with a significantly higher concentration of divalent ions such as Ca2+, Mg2+, and SO42−, and a second stream, NF permeate, with greatly reduced concentrations of these ions. In terms of any process aimed at recovering a particular mineral, this reduces the volume of liquid that must be treated in order to obtain the mineral and reduces the number of interfering species. Processes for extracting bromine, for example, need only be applied to the permeate stream, while processes for extracting magnesium hydroxide need only be applied to the reject stream, in each case using reduced quantities of reagents for pH adjustment and requiring smaller volumes of liquid to be processed.
In application of methods such as MDC or solar evaporation following NF, concentration of NF reject would not form significant quantities of the mixed salts kainite (KMg(SO4)Cl·3H2O) or carnallite (KCl.MgCl2·6H2O)—which would require further processing to obtain saleable mineral products—but would lead directly to the precipitation of saleable bischofite (MgCl2·6H2O) well-separated from the other precipitated salt products. Similarly, on the permeate side, the removal of Mg makes it possible for direct precipitation of sylvite (KCl) rather than mixed salts with magnesium. As the divalent ions are the principal scale forming species, their removal into the reject stream also makes it possible to concentrate the permeate stream to higher levels by brine concentration, reducing further the volume which must be handled in brine mining operations.
Exemplary mineral recovery steps on the NF reject stream could be as follows:
-
1.
Recovery of CaSO4·2H2O (gypsum). Because CaSO4 is the most likely scale-forming ion in the reject of NF applied to seawater, the typical saturation at NF reject will easily exceed 100% and could be up to 400% depending on the composition in the raw seawater, NF recovery and ion rejection ratios. Although antiscalant is usually dosed to prevent CaSO4 scale deposition, several technologies, e.g., Membrane Crystallization, might be considered to harvest CaSO4.
-
2.
MBC as an intermediate step, which produces a less saline stream (to be recycled to NF system or to be considered as additional water production if the considered MBC is a dewatering (e.g., RO) type) and a more saline stream (limited only by the scale deposition risk).
-
3.
Evaporation ponds and sequential bittern concentration steps to recover NaCl, MgCl2·6H2O, CaCl2·2H2O, and so on.
A commercial-scale project is being undertaken by the Saline Water Conversion Corporation (SWCC) to construct a NF plant in Shoaiba, Saudi Arabia (https://idadesal.org/ida-academy-webinar-on-innovation-in-desalination-brine-mining-with-swcc/). The main purpose is to harvest magnesium for two major beneficial uses. The first is to for supplementation of drinking water. There have been numerous studies which have found a link between Mg content in drinking water and human health, with low Mg content in drinking water being linked to negative outcomes in bone and cardiovascular health161,162 and high content giving more positive outcomes in diabetes and cancer treatment163,164. Desalination product water is usually deficient in Mg2+ (<1 ppm) and the level of 15 ppm Mg2+ associated with improved health outcomes is being targeted by installing a multi-stage NF system with inter-stage dilution165, similar to the system proposed by Birnhack et al.166. As the Mg2+ in seawater is vastly higher than 15 ppm (~1500 ppm), in comparison to the capacity of the seawater intake in a desalination plant, only a small fraction of seawater or brine needs to be treated to extract the required Mg for this post-treatment. When 400,000 m3 per day desalinated water is considered, for example, where its seawater intake capacity would be 1,000,000 m3 per day with 40% of overall recovery, for example, the intake seawater contains ~1500 ton per day Mg. 15 ppm of 400,000 m3 per day indicates 6 tons per day of Mg is required, which can be harvested by treating only 0.4% of the intake seawater. In a practical design, due to non-perfect rejection of Mg ion in NF membranes, around 0.8% of intake seawater will be treated by multi-stage NF system to harvest and supply Mg to the desalination product water.
The second beneficial use is to supply the Mg-enriched low-salinity brine as a liquid fertilizer. Many acidic soils contain very low levels of soluble magnesium, which is essential for photosynthesis, and crop yields generally increase by of order 10% when magnesium fertilizer is added167. Certain tropical fruits, such as mango, are more heavily dependent on magnesium levels, with fruit quality declining if magnesium is deficient (www.ks-minerals-and-agriculture.com/uken/fertiliser/advisory_service/crops/mango.html; www.mango.org/wp-content/uploads/2018/04/Magnesium_Fertilization_Final_Report_Eng.pdf). Irrigated farms in the vicinity of desalination plants, especially for tropical fruit and at large scales, can realize significant cost savings by replacing commercial magnesium sulfate fertilizer with a liquid fertilizer system utilizing the Mg-enriched low-salinity brine from a multi-stage NF system.
Membrane brine concentration (MBC)
A second key technology to make brine mining more attractive is membrane brine concentration. Davenport et al.168 have reported that a membrane-based technology would require less than half of the energy consumption by conventional thermal evaporation technology in the application of hypersaline brine desalination. In an example of concentrating 70,000 mg dm−3 feed brine to 250,000 mg dm−3, they estimated specific energy consumption (SEC) of 24 kWh m‒3 with two-stage MVC, while it would drop to 7.3 kWh m‒3 with two-stage high pressure RO (HPRO). They indicated the maximum operation pressure for typical SWRO as 80 bar and analyzed two scenarios—one with HPRO up to 150 bar (double the current operating limit) and the other with HPRO up to 300 bar (due to around 290 bar of osmotic pressure at 250,000 mg dm−3).
Practically, the maximum operating pressure of SWRO is a function of temperature as well, which varies between 70 and 82.7 bar. A higher temperature allows for a lower maximum operating pressure in order to minimize the risk from membrane compaction. This penalty could be partially moderated with improved membrane materials, e.g., with new core tube material. There are commercial HPRO membranes of up to 120–124 bar available in the market, such as Hydranautics’ PRO-XP1 (http://pureaqua.com/content/pdf/hydranautics-pro-xp1-membrane.pdf) (Fig. 4) and Dupont (Filmtec)’s XUS180808 (www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/45-D01736-en.pdf). Ultra-high-pressure RO of up to 200 bar has been reported in special applications, e.g., landfill leachate treatment as early as 2000169, and currently PWS (Pacific Water Solutions) is working on the same pressure range (https://pws-water.com/project-ultra-hgh-pressure-ro-uhpro-membrane-module-development-for-international-desalination-company/), which membranes are currently being tested by SWCC-DTRI (https://idadesal.org/ida-academy-webinar-on-innovation-in-desalination-brine-mining-with-swcc/).
Comparison of temperature and pressure operation limits for a conventional SWRO membrane and LG Chem SWRO membranes (www.lgwatersolutions.com/en/technical-document/technical-bulletins-tsb, technical service bulletin 106) and Hydranautics PRO-XP (HPRO) membrane (Hydranautics, PRO-XP1 membrane specification, (http://pureaqua.com/content/pdf/hydranautics-pro-xp1-membrane.pdf)).
Even though there are continuous efforts in the development and application of (U)HPRO, the practical application of (U)HPRO in a large scale will be challenging due to the need for the expensive materials in pump, pipe, valve, instruments and so on. A very high operating pressure presents an additional difficulty in materials where hypersaline brine is already a big challenge. In order to overcome this issue of very high pressures, osmotically assisted RO (OARO) has gained attention from many researchers and industrial players. There have been a number of proposals to overcome the limit of osmotic pressure when RO is applied170,171,172,173. The principle of OARO is to reduce an osmotic pressure gradient across the membrane by allowing a certain salinity brine flow on the conventional permeate side of the membrane. The feed to reject side of the membrane is pressurized while the permeate side has much lower pressure, and if the pressure difference between the two sides are higher than the osmotic pressure difference, mostly water molecules rather than other solutes (such as Na and Cl) will pass through the semi-permeable membrane (e.g., RO, FO, or pressure retarded osmosis), thus the feed side becomes more concentrated and the permeate side becomes more diluted. It is possible that even NF membranes could be used, depending on the solute of interest to be concentrated on the feed side.
The typical configurations of OARO are illustrated in Fig. 5. A single OARO is shown in (a) where the flow could be either co-current (a1) or counter-current (a2). When a series of OARO is considered, both (b) and (c) could be considered, where (c) has at least 1 recycling stream inside OARO124,174. As an example, if (b) configuration is considered, the leftward arrow on the right top (brown) could be considered as seawater, which flow rate increases and concentration is getting less through OAROs while receiving water flux from lower side of the diagram, thus the leftward arrow on the left end (blue), which could be SWRO feed is already diluted with increased flow rate, thus higher recovery of fresh water in SWRO can be expected. The rightward arrow on the left end (orange), which could be SWRO reject, is getting concentrated with losing its flow rates through OAROs, and the final rightward arrow (red) will be higher concentration with less flow rate compared to the typical SWRO reject. In this way, the concept of MBC is achieved within a limited maximum operating pressure.
Osmotically-assisted Reverse Osmosis (OARO) configurations. a Example of co-current (a1) and counter-current (a2) flows; b Example of multiple OARO in series, c example when OARO includes at least one recycling stream. (The block sky-blue arrows indicate the flux of permeate (usually water) thus indicating the pressure gradient on the membrane. Solid arrow color indicates salinity (higher towards red, lower towards sky-blue) and the thickness indicates flow rate).
Peters and Hankins175 analyzed several OARO processes and compared their theoretical energy consumption to multi-stage RO (MSRO, which is the combination of SWRO and HPRO in series). As the membrane modeling of OARO is similar to that of pressure assisted FO (PAFO), they adopted the model of B. Kim. et al.176, for water flux calculation and the model of J. Kim, et al.177, for solute flux calculation. Two scenarios were considered: 1) concentrating 35,000 mg dm−3 to 125,000 mg.dm−3 and 2) concentrating 70,000 mg.dm−3 to 125,000 mg dm−3. The theoretical SEC comparison showed that MSRO consumes less energy than OARO, where SECs by MSRO were 3.32 and 5.16 kWh m–3 while SECs by OARO were 4.09 and 6.37 kWh m–3 for scenario 1 and 2, respectively. The reduced energy efficiency was could be explained by the increase in entropy arising from dilution and mixing of the saline streams in OARO174 However, it should be noted that Peters and Hankins175 considered 48.3 bar as the maximum operating pressure of OARO as per the earlier study by other researchers on Pressure Retarded Reverse Osmosis (PRO) with commercially available TFC FO membrane. Membranes operating at 70 bar are already commercially available for OARO without additional high-cost components (e.g., a porous steel plate as feed spacer). Toyobo has a commercial membrane product for brine concentration purpose (Toyobo, FB10155FI) and FTS H2O also has a commercial product for OARO (FTSHBCR-01/04, https//:ftsh2o.com/products/hbcr-high-brine-concentration-and-recovery/). DTRI-SWCC has tested Toyobo’s HFF membrane product for more than nine months, and was able to concentrate 110,000 ppm HPRO reject (operated at 120 bar with Hydranautics HPRO membrane) to 170,000 ppm with two-stage OARO (70 bar with Toyobo HFF BC membrane) continuously and up to 220,000 ppm with three-stage OARO. The commercially available FTSHBCR membranes are already delivered to DTRI-SWCC and are undergoing testing long-term operation, with a pilot facility designed to concentrate 78,000 ppm SWRO reject to 220,000 ppm with three-stage OARO (https://idadesal.org/ida-academy-webinar-on-innovation-in-desalination-brine-mining-with-swcc/).
Comparing the two MBC candidates, HPRO could be more energy efficient, while OARO may reduce the capital and maintenance cost thanks to its operation at relatively lower pressure (70 bar). Also, the challenges on HPRO becomes much larger when higher levels of concentration are required, because much higher pressure is required, while for OARO, higher concentration forces to increase its number of stages but there is no technical challenge from high pressure. Therefore, further studies at DTRI-SWCC aim to determine an optimal concentration by HPRO, by OARO, and by the combination of the two methods.
Combination of nanofiltration and membrane brine concentration (NF-RO-MBC)
The idea of combining the above two key technologies was recently proposed by DTRI-SWCC168,178. The key concept of applying NF upstream of RO and MBC as the core of an integrated facility for seawater concentrate mining is illustrated in Fig. 6127,165. When “towards Zero Liquid Discharge” is discussed, it is essential to secure economic feasibility to realize an idea to a real life on a commercial scale. Therefore, the principal idea in this NF-RO-MBC system is to produce two commercially valuable concentrate steams in addition to a higher recovery of freshwater production. The high selectivity nature of the NF system is adopted to make the seawater which has mixed dissolved ions in nature into two streams—a high purity monovalent ions steam in its permeate and a highly concentrated multivalent ions stream in its retentate. With the following RO and/or MBC systems, both streams could be concentrated further to the level of concentration where downstream industries could utilize it as a source brine for their processes or where the following mineral harvesting steps could become economically feasible. The pilot plant using commercial-size membranes was successfully demonstrated to produce concentrated multivalent ions steam of about 90,000 mg dm−3 with high concentrations of divalent ions, i.e., 3.40 times Ca2+, 5.16 times Mg2+, and 6.56 times SO42− compared to these ions in seawater and NF feed, and to produce the high-purity highly-concentrated monovalent ions stream where the sum of Na and Cl portion in the TDS of about 170,000 mg dm−3 is increased to about 96.85% from 85.98% in seawater127.
Summary and outlook
Since as long ago as the 19th century the imagination of researchers has been seized by the potential of obtaining useful minerals and metals from the sea. Exploiting desalination concentrate, rather than direct use of seawater, is necessarily going to be more energetically favorable: the energy that would have otherwise removed the amount of water produced by the desalination plant has already been expended. Thus the expansion of seawater desalination in recent decades brings this longstanding dream a step closer to commercial reality. Research developments around the world are taking further small steps in this direction, despite a perception that resource recovery from brine may be entering a “trough of disillusionment”125. Processing large volumes of seawater desalination brine to extract a single component—with the exception of NaCl—will be less competitive than integrated processes designed to obtain several commercial species from concentrate. For a long time to come, it is likely that commercial utilization of the non-NaCl components of desalination brine will depend on the available market for NaCl, as the challenges and costs of extracting the other mineral components from bitterns in which they are highly enriched are so much less than those faced in direct treatment of brines.
The most important technologies for economic use of products from desalination plant concentrate are technologies for more economic separation and technologies for more economic concentration. In terms of separation, a long sequence of complex steps treating the entire volume of concentrate95 is unlikely ever to be viable, so the most promising separation technologies are those, such as NF, that separate the brine into streams enriched/depleted in entire classes of constituents with the least possible input of energy and reagents. In terms of concentration, rapid advances in OARO technology that allow the application of low-energy membrane-based methods of concentration to ever more concentrated brines are a transformative development for sustainable mining of seawater.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bardi, U. Extracting minerals from seawater: an energy analysis. Sustainability 2, 980–992 (2010).
Gilbert, O., Valderrama, C., Peterkóva, M. & Cortina, J. L. Evaluation of selective sorbents for the extraction of valuable metal ions (Cs, Rb, Li, U) from reverse osmosis rejected brine. Solvent Extr. Ion-. Exc 28, 543–562 (2010).
Diallo, M. S., Kotte, M. R. & Cho, M. Mining critical metals and elements from seawater: opportunities and challenges. Environ. Sci. Technol. 49, 9390–9399 (2015).
Nakazawa, N., Tamada, M., Ooi, K. & Akagawa, S. In Proceedings of the 9th ISOPE Ocean Mining Symposium. 184–189 (2011).
Dudley, W. C. Gold from the sea. J. Geol. Educ. 34, 4–6 (1986).
Kim, J. et al. Recovery of uranium from seawater: a review of current status and future research needs. Separ Sci. Technol. 48, 367–387 (2013).
Yang, S., Zhang, F., Ding, H., He, P. & Zhou, H. Lithium metal extraction from seawater. Joule 2, 1648–1651 (2018).
Quist-Jensen, C. A., Macedonio, F. & Drioli, E. Membrane crystallization for salt recovery from brine - an experimental and theoretical analysis. Desalination Water Treat. 57, 7593–7603 (2016).
Shahmansouri, A., Min, J., Jin, L. & Bellona, C. Feasibility of extracting valuable minerals from desalination concentrate: a comprehensive literature review. J. Clean. Prod. 100, 4–16 (2015).
Loganathan, P., Naidu, G. & Vigneswaran, S. Mining valuable minerals from seawater: a critical review. Environ. Sci. Water Res. 3, 37–53 (2017).
Kumar, A. et al. Metals recovery from seawater desalination brines: technologies, opportunities, and challenges. ACS Sustain. Chem. Eng. 9, 7704–7712 (2021).
O’Hara, T. A. & Suboleski, S. C. In SME Mining Engineering Handbook Vol. 1 (ed. Howard L. Hartmann) (Society for Minerals, Mining, and Exploration, 1992).
Koppelaar, R. H. E. M. & Koppelaar, H. The ore grade and depth influence on copper energy inputs. Biophys. Econ. Sustain 1, 1–16 (2016).
Ulrich, S. et al. In Proceedings of the 49th AusIMM New Zealand Branch Annual Conference 438–453 (Australian Institute of Mining and Metallurgy, Wellington, 2016).
Lala, A., Moyo, M., Rehbach, S. & Sellschop, R. In Metals and Mining Practice (2015). http://mckinsey.com/¬/media/mckinsey/industries.
Quist-Jensen, C. A., Ali, A., Drioli, E. & Macedonio, F. Perspectives on mining from sea and other alternative strategies for minerals and water recovery—the development of novel membrane operations. J. Taiwan Inst. Chem. Eng. 94, 129–136 (2018).
Crook, J. & Mousavi, A. The chlor-alkali process: a review of history and pollution. Environ. Forensics 17, 211–217 (2016).
Kostick, D. In US Geological Survey Minerals Yearbook, 70.71–70.75 (Reston, 2004).
Plessen, H. In Ullman’s Encyclopedia of Industrial Chemistry, (Wiley-VCH, 2000).
Neumann, B. & Kunz, J. The reaction in the Hargreaves process. Angew. Chem.—Ger. Ed. 42, 1085–1087 (1929).
Bakshi, B., Doucette, E. M. & Kyser, J. S. Centralized softening as a solution to chloride pollution: an empirical analysis based on Minnesota cities. PLOS One 16, 0246688 (2021).
Ramakrishna, D. M. & Virarghavan, T. Environmental impact of chemical deicers—a review. Water Air Soil Pollut. 166, 39–63 (2005).
Bolen, W. P. Salt. United States Geological Survey—Mineral Commodity Summaries (2020). pubs.usgs.gov/periodicals/mcs2020/mcs2021-salt.pdf.
Fossett, H. Extraction of bromine from sea water. Chem. Ind. 35, 1161–1171 (1971).
Stewart, L. C. Commercial extraction of bromine from seawater. Ind. Eng. Chem. 26, 361–369 (1934).
Heath, S. B. Process of producing bromine. United States of America patent US2143223A (1939).
Zhang, N. & Qiu, J. Research on the thermodynamics of bromine adsorption on 201 × 7 strong-base anion exchange resin. Ion-. Exch. Absorpt. 27, 26–32 (2011).
Zhang, Q. & Cussler, E. L. Microporous hollow fibers for gas absorption: I. Mass transfer in the liquid. J. Membr. Sci. 23, 321–332 (1985).
Ge, F., Li, Y., Ye, X. & Liu, H. In Proceedings of the International Symposium on Energy Science and Chemical Engineering, 23–27 (Guangzhou, 2015).
Wisniak, J. The history of bromine from discovery to commodity. Ind. J. Chem. Technol. 9, 263–271 (2002).
Abu Ghazleh, S., Hartmann, J., Jansen, N. & Kempe, S. Water input requirements of the rapidly shrinking Dead Sea. Naturwissenschaften 96, 637–643 (2009).
Zhang, Y. et al. In: Proceedings of the International Conference on Oil & Gas Engineering and Geological Sciences. 012114 (2019).
Bray, E. L. Magnesium. United States Geological Survey - Mineral Commodity Summaries (2021). https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-magnesium-metal.pdf.
Merrill, A. Magnesium Compounds. United States Geological Survey - Mineral Commodity Summaries (2021). https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-magnesium-compounds.pdf.
Robinson, H. A., Friedrich, R. E. & Spencer, R. S. Magnesium hydroxide from sea water. United States of America patent US2405055A (1946).
Wulandari, W., Brooks, G., Rhamdhani, M. & Monaghan, B. In Chemeca: Australasian Conference on Chemical Engineering (Engineers Australia, Adelaide, 2010).
Barba, D., Brandani, V., Di Giacomo, G. & Foscolo, P. U. Magnesium oxide production from concentrated brine. Desalination 33, 241–250 (1980).
Al-Mutaz, I. & Wagialia, K. M. Production of magnesium from desalination brines. Resour. Conserv. Recycl. 3, 231–239 (1990).
Kipouros, G. J. & Sadoway, D. R. In Advances in Molten Salt Chemistry (eds G. Mamantov, C. B. Mamantov, & J. Braunstein) 127–209 (Elsevier, 1987).
Abdel-Aal, H., Zohdy, K. & Abdelkareem, M. Seawater bittern a precursor for magnesium chloride separation: discussion and assessment of case studies. Int J. Waste Resour. 7, 1000267 (2017).
Al-Rawashdeh, R. & Maxwell, P. Analysing the world potash industry. Resour. Policy 41, 143–151 (2014).
Ciceri, D., Manning, D. A. C. & Allanore, A. Historical and technical development of potassium resources. Sci. Total Environ. 502, 590–601 (2015).
Ishibashi, T., Matsui, A. & Moriyasu, K. Methods of producing bittern with low gypsum concentration and potassium chloride. Japan patent JP2021017382 (2021).
Shi, W. et al. Berlin green-based battery deionization—highly selective potassium recovery in seawater. Electrochim. Acta 310, 104–112 (2019).
Hou, J., Yuan, J. & Sheng, R. Surface-modified zeolite-filled poly(piperazine-amide)/poldisulfone composite membrane for potassium extraction. Key Eng. Mater. 531–532, 186–189 (2013).
Wang, Z. Method for producing compound fertilizer by seawater potassium extraction using diatomite. People’s Republic of China patent CN106518504 (2017).
Scherzberg, H. & Schultheis, B. Procedure for the production of potassium sulphate from bittern. Germany patent DE10200918956B4 (2014).
Korngold, E. Sulfate removal from the sea by anion exchange process combined with K2SO4 precipitation. Desalination Water Treat. 201, 150–154 (2020).
Yuan, J. et al. Process for production of potassium sulfate from seawater. People’s Republic of China patent CN1792797A (2006).
Maiti, P., Ghara, K. K. & Ghosh, P. K. A process of production of potassium ammonium sulfate compound fertilizer in cost-effective manner directly from concentrated sea bittern. PCT Patent WO2016059651A1 (2016).
Bliss, J. D., Hayes, T. S. & Orris, G. J. Limestone - a crucial and versatile industrial mineral commodity. USGS Fact Sheet (2008). http://pubs.usgs.gov/fs/2008/3089.fs29008-3089.pdf.
Survey, U. G. Gypsum. United States Geological Survey - Mineral Commodity Summaries (2021). pubs.usgs.gov/periodicals/mcs2021/mcs2021-gypsum.pdf.
Paxton, C. ABC’s of Water Presentation, 23 May 2017. http://mojavewater.org/files/Salty_Conversation_20170523.pdf.
Jaskula, B. W. Lithium. United States Geological Survey - Mineral Commodity Summaries (2021). https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-lithium.pdf.
Garrett, D. E. Handbook of Lithium and Natural Calcium Chloride—Their Deposits, Processing, Uses and Properties. 1st edn, (Elsevier Academic Press, 2004).
Alsabbagh, A., Aljarrag, S. & Almahsaneh, M. Lithium enrichment optimization from Dead Sea end brine by chemical precipitation technique. Mater. Eng. 170, 1097038 (2021).
Samadiy, T. & Deng, T. Lithium recovery from water resources by ion exchange and sorption method. J. Chem. Soc. Pak. 43, 406–416 (2021).
Zhang, Y., Wang, L., Sun, W., Hu, H. & Tang, H. Membrane Technologies for Li+/Mg2+ separation from salt-lake brines and seawater: a comprehensive review. J. Ind. Eng. Chem. 81, 7–23 (2020).
Zhao, X., Yang, H., Wang, Y. & Sha, Z. Review on the electrochemical extraction of lithium from seawater/brine. J. Electroanal. Chem. 850, 113389 (2019).
Weng, D. et al. Introduction of manganese-based lithium-ion sieve: a review. Prog. Nat. Sci: Mater. Int. 30, 139–152 (2020).
Chitrakar, R., Kanoh, H., Miyai, Y. & Ooi, K. Recovery of lithium from seawater using manganese oxide adsorbent (H1.6Mn1.6O4). Ind. Eng. Chem. Res. 40, 2054–2058 (2001).
Cheng, M. et al. Synthesis of membrane-type graphene oxide immobilized manganese dioxide adsorbent and its adsorption behavior for lithium ion. Chemosphere 279, 130487 (2021).
Tang, L. et al. Highly efficient, stable, and recyclable hydrogen manganese oxide/cellulose film for extraction of lithium from seawater. ACS Appl Mater. Inter. 12, 9775–9781 (2020).
Qiu, Z. et al. Li4Mn5O12 doped cellulose acetate membrane with low Mn loss and high stability for enhancing Li extraction from seawater. Desalination 506, 115003 (2021).
Ryu, T., Shin, J., Ghoreishian, S. M., Chung, K.-S. & Huh, Y. S. Recovery of lithium in seawater using a titanium intercalated lithium manganese oxide composite. Hydrometallurgy 184, 22–28 (2019).
Mu, Y., Zhang, C., Zhang, W. & Wang, Y. Electrochemical lithium recovery from brine with high Mg2+/Li+ ratio using mesoporous λ-MnO2/LiMn2O4 modified 3D graphite felt electrodes. Desalination 511, 115112 (2021).
Yu, C. et al. Bio-inspired fabrication of ester-functionalized imprinted composite membrane for rapid and high-efficient recovery of lithium ion from seawater. J. Colloid Inter. Sci. 572, 340–353 (2020).
Kato, T. & Kawata, K. Polymer composition for capturing lithium from seawater to use as lithium ion battery. PCT Patent WO2018-JP36499.
Katsutai, S. & Nakamura, T. Solid-phase extraction of lithium in seawater with porous polymer resin impregnated with lithium-ion selective metallohost. Nippon Kaisui Gakk 71, 298–299 (2017).
Harvianto, G. R., Kim, S.-H. & Ju, C.-S. Solvent extraction and stripping of lithium ion from aqueous solution and its application to seawater. Rare Met. 35, 948–953 (2016).
Kurniawan, Y. S. et al. A rapid and efficient lithium-ion recovery from seawater with tripropyl monoacetic acid calix[4]arene derivative employing droplet-based microreactor system. Sep Purif. Technol. 211, 925–934 (2019).
Zante, G., Boltoeva, M., Masmoudi, A., Barillon, R. & Trebouet, D. Highly selective transport of lithium across a supported liquid membrane. J. Fluor. Chem. 236, 109593 (2020).
Paredes, C. & Rodriguez de San Miguel, E. Selective lithium extraction and concentration form diluted alkaline aqueous media by a polymer inclusion membrane and application to seawater. Desalination 487, 114500 (2020).
Liu, C. et al. Lithium extraction from seawater through pulsed electrochemical intercalation. Joule 4, 1459–1469 (2020).
Li, Z. et al. Continuous electrical pumping membrane process for seawater lithium mining. Science 14, 3152–3155 (2021).
Zhao, X., Zhang, H., Yuan, Y., Ren, Y. & Wang, N. Ultra-fast and stable extraction of Li metal from seawater. Chem. Commun. 56, 1577–1580 (2020).
Cippolina, A., Bevacqua, M., Micale, G., Papapetrou, M. & Tamburini, A. Procedure for the extraction of minerals from sea water, plant for extraction and minerals obtained through the extraction procedure. European Patent, EP2020-189069 (2021).
Kramer, D. Fears of a lithium supply crunch may be overblown. Phys. Today 74, 20 (2021).
Naganawa, H., Suzuki, H., Yanase, N., Nagano, T. & Noro, J. Reversed-micellar extraction of strontium (II) from model solutions of seawater. Anal. Sci. 27, 321–324 (2011).
Korneikov, R. I. & Ivanenko, V. I. Extraction of cesium and strontium cations from solutions by titanium (IV) phosphate-based ion exchangers. Inorg. Mater. 56, 502–506 (2020).
Jeong, S.-K. & Ju, C.-S. Extraction of strontium ions from sea water by contained liquid membrane permeator. Korean J. Chem. Eng. 19, 93–98 (2002).
Wu, S.-C. et al. Separation of strontium from associated elements with selective specific resin and extraction chromatography. Chin. Chem. Lett. 24, 633–635 (2013).
Hong, H. et al. Investigation of the strontium (Sr(II)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater. J. Environ. Manag. 165, 263–270 (2016).
Hong, H. S. /,J., Pak, I. S., Ryu, T., Jeong, H. S. & Ryu, J. Demonstration of seawater strontium (Sr(II)) extraction and enrichment by a biosorption technique through continuous column operation. Ind. Eng. Chem. Res. 57, 12909–12915 (2018).
Ghaeni, N., Taleshi, M. S. & Elmi, F. Removal and recovery of strontium (Sr(II)) from seawater by Fe3O4/MnO2/fulvic acid nanocomposite. Mar. Chem. 213, 33–39 (2019).
Ryu, J. et al. Strontium ion (Sr2+) separation from seawater by hydrothermally structured titanate nanotubes: Removal vs. recovery. Chem. Eng. J. 304, 503–510 (2016).
Ober, J. A. Strontium. United States Geological Survey - Mineral Commodity Summaries (2021). https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-strontium.pdf.
Naidu, G., Nur, T., Loganathan, J., Kandasamy, J. & Vigneswaram, S. Selective sorption of rubidium by potassium cobalt hexacyanoferrate. Sep Purif. Technol. 163, 238–246 (2016).
Naidu, G. et al. Rubidium extraction using an organic polymer encapsulated potassium copper hexacyanoferrate sorbent. Chem. Eng. J. 306, 31–42 (2016).
Naidu, G. et al. Rubidium extraction from seawater by an integrated membrane distillation-selective sorption system. Water Res. 123, 321–331 (2017).
Huang, D., Zheng, H., Li, B. & Ma, G. Extraction kinetics of rubidium in brine solutions by 4-tert-butyl-(α-methylbenzyl)phenol/sulfonated kerosene using a single drop method. Chem. Phys. Lett. 713, 105–110 (2018).
Mohite, B. S. & Khopkar, S. M. Solvent extraction separation of rubidium with dicyclohexano-18-crown-6. Talanta 32, 565–567 (1985).
Liu, S.-M., Liu, H.-H., Huang, Y.-J. & Yang, W.-J. Solvent extraction of rubidium and cesium from lake brine with t-BAMBP-kerosene solution. Trans. Nonferr. Met. Soc. 25, 329–334 (2015).
Nisan, S., Laffore, F., Poletiko, C. & Simon, N. Extraction of rubidium from the concentrated brine rejected by integrated nuclear desalination systems. Desalination Water Treat. 8, 236–245 (2009).
Jeppesen, T., Shu, L., Keir, G. & Jegatheesan, V. Metal recovery from reverse osmosis concentrate. J. Clean. Prod. 17, 703–707 (2009).
Tuck, C. C. Rubidium. United States Geological Survey - Mineral Commodity Summaries (2021). https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-rubidium.pdf.
Wolska, J., Bryjak, M. & Kabay, N. Polymeric microspheres with N-methyl-d-glucamine ligands for boron removal from water solution by adsorption-membrane filtration process. Environ. Geochem. Health 32, 349–352 (2010).
Bicak, N., Bulutçu, N., Senkal, B. F. & Gazi, M. Modification of crosslinked glycidyl methacrylate-based polymers for boron-specific column extraction. React. Funct. Polym. 47, 175–184 (2001).
Agashichev, S. & Osman, E. Low pressure RO for boron elimination: impact of pH on the degree of rejection of boron and monovalent ions. Desalination Water Treat. 57, 4701–4707 (2016).
Landsman, M. R., Lawler, D. F. & Katz, L. E. Application of electrodialysis pretreatment to enhance boron removal and reduce fouling during desalination by nanofiltration/reverse osmosis. Desalination 491, 114563 (2020).
Smith, B. M., Todd, P. & Bowman, C. N. Hyperbranched chelating polymers for the polymer-assisted ultrafiltration of boric acid. Sep. Sci. Technol. 34, 1925–1945 (1999).
Culberson, C. H. Pressure Dependence of the Apparent Dissociation Constants of Carbonic and Boric Acids in Seawater. Ph.D. thesis, Oregon State University, (1968).
Brioche, A. S. Boron. United States Geological Survey - Mineral Commodity Summaries (2021). https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-boron.pdf.
Helvaci, C. In Encyclopedia of Geology, (ed. R. C. Selley) (Academic Press, 2004).
Voutchkov, N., Kaiser, G., Stover, R., Leinhart, J. & Awerbuch, L. In The International Desalination Association World Congress on Desalination and Water Reuse (International Desalination Association, Dubai, 2019).
Batte, M. T. & Forster, L. D. Old is new again: the economics of agricultural gypsum use. J. Am. Soc. Farm Manage. Rural Appraisers, 56–74 (2015).
Leggett, C. J. & Rao, L. Complexation of calcium and magnesium with glutarimidedioxime: implications for the extraction of uranium from seawater. Polyhedron 96, 54–59 (2015).
Epstein, J. A., Altaras, D., Feist, E. M. & Rosenzweig, J. The recovery of potassium chloride from Dead Sea brines by precipitation and solvent extraction. Hydrometallurgy 1, 39–50 (1975). saltworkconsultants.com/marine-brine-modern/.
Singh, J. & Christen, E. W. Minimising the cost of evaporation basins: Siting, design, and construction factors. (CSIRO Land and Water, Griffith, NSW, 1999).
Oram, R. The sea-salt industry in Medieval Scotland. Stud. Medieval Renaiss. Hist. 3, 210–228 (2012).
Li, X. Preparation of industrial grade magnesium sulfate from bittern. Sea-Lake Salt Chem. Ind. 31, 26–27 (2002).
Trivedi, K. et al. Evaluation of fertilizer potential of different K compounds prepared utilizing sea bittern as feed stock. Front. Plant Sci. 8, 01541 (2017).
Mustafa, A. M. K. & Abdullah, W. R. Preparation of high purity magnesium oxide from sea bittern residual from NaCl production in Al-Basrah saltern. South Iraq. Iraq Bull. Geol. Min. 9, 129–146 (2013).
Dave, R. H. & Ghosh, P. K. Enrichment of bromine in sea-bittern with recovery of other marine chemicals. Ind. Eng. Chem. Res. 44, 2903–2907 (2005).
Marx, H., Kaps, S., Schultheis, B. & Pfander, M. Potassium sulfate—a precious by-product for solar salt works. Bull. Soc. Seawater Sci.-Jpn. 73, 89–93 (2019).
Society, A. C. Cesium and rubidium hit market. Chem. Eng. N. 37, 50–56 (1959).
Bagastyo, A. Y. et al. Resource recovery and utilization of bittern wastewater from salt production: a review of recovery technologies and their potential applications. Environ. Technol. Rev. 10, 294–321 (2021).
Mickley, M. C. Treatment of concentrate. (United States Department of the Interior, Bureau of Reclamation, Technical Service Center, Water Treatment and Research Group, Denver, Colorado, 2009).
Sorour, M. H., Hani, H. A., Shaalan, H. F. & Al-Bazedi, G. A. Schemes for salt recovery from seawater and RO brines using chemical precipitation. Desalination Water Treat. 55, 2398–2497 (2014).
Giwa, A., Dufour, V., Al Marzooqi, F., Al Kaabi, M. & Hasan, S. W. Brine management methods: recent innovations and current status. Desalination 407, 1–23 (2016).
Casas, S. et al. Valorisation of Ca and Mg byproducts from mining and seawater desalination brines for water treatment applications. J. Chem. Technol. Biotechnol. 89, 872–883 (2014).
Svensson, M. Desalination and the environment: Options and considerations for brine disposal in inland and coastal locations. (Swedish University of Agricultural Sciences, Department of Biometry and Engineering, Uppsala, Sweden, 2005).
Carollo Engineers, Water Desalination Management and Piloting. (South Florida Water Management District, Sunrise, Florida).
Bartholemew, T. V., Mey, L., Arena, J. T., Siefert, N. S. & Mauter, M. S. Osmotically-assisted reverse osmosis for high salinity brine treatment. Desalination 421, 3–11 (2017).
Zhan, M., Kim, Y. & Hong, S. Comprehensive review of osmotic dilution/concentration using FO membranes for practical applications. Desalination 515, 115190 (2021).
Kim, B. et al. Purification of high salinity brine by multi-stage ion concentration polarization desalination. Sci. Rep. 6, 21850 (2016).
Al-Amoudi, A. S., Voutchkov, N., Ihm, S., Farooque, A. M. & Al-Waznani, E. S. B. Dual brine concentration for the beneficial use of two concentrated streams from desalination plant - concept proposal and pilot plant demonstration. Desalination, in press (2022).
Garriga, S. C. Valorization of brines in the chlor-alkali industry: Integration of precipitation and membrane processes. Ph.D thesis, Universitat Politecnica de Catalunya (2011).
Liu, J. et al. Concentrating brine from seawater desalination process by nanofiltration-electrodialysis integrated membrane technology. Desalination 390, 53–61 (2016).
Sano, Y., Hao, Y. & Kuwahara, F. Development of an electrolysis based system to continuously recover magnesium from seawater. Heliyon 4, e00923 (2018).
Atkinson, S. Saltworks introduces Flex EDR following successful pilot project, membrane technology. Membr. Technol. 4, 8 (2018).
Macedonio, F. et al. Thermodynamic modeling of brine and its use in membrane crystallizer. Desalination 323, 83–92 (2013).
Chabanon, E., Mangin, D. & Charcosset, C. Membranes and crystallization processes: State of the art and prospects. J. Membr. Sci. 509, 57–67 (2016).
Edwie, F. & Chung, T. S. Development of hollow fiber membranes for water and salt recovery from highly concentrated brine via direct contact membrane distillation and crystallization. J. Membr. Sci. 421-422, 111–123 (2012).
Choi, Y., Naidu, G., Nghiem, L. D., Lee, S. & Vigneswaran, S. Membrane distillation crystallization for brine mining and zero liquid discharge: opportunities, challenges, and recent progress. Environ. Sci.-Water Res 5, 1202–1221 (2019).
Jiang, X., Shao, Y., Sheng, L., Li, P. & He, G. Membrane crystallization for process intensification and control: a review. Engineering 7, 50–62 (2021).
Edwie, F. & Chung, T. S. Development of simultaneous membrane distillation-crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 98, 160–172 (2013).
Ali, A. et al. A review of membrane crystallization, forward osmosis and membrane capacitive deionization for liquid mining. Resour. Conserv Recycl. 168, 105273 (2021).
Bouguecha, S. & Dhahbi, M. Fluidised bed crystalliser and air gap membrane distillation as a solution to geothermal water desalination. Desalination 152, 237–244 (2003).
Yadav, A., Labhasetwar, P. K. & Shahi, V. K. Membrane distillation using low-grade energy for desalination: a review. J. Environ. Chem. Eng. 9, 105818 (2021).
Edwie, F. & Chung, T.-S. Development of simultaneous membrane distillation–crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 98, 160–172 (2013).
Ruiz Salmón, I. & Luis, P. Membrane crystallization via membrane distillation. Chem. Eng. Process 123, 258–271 (2018).
Choi, Y., Naidu, G., Jeong, S., Lee, S. & Vigneswaran, S. Fractional-submerged membrane distillation crystallizer (F-SMDC) for treatment of high salinity solution. Desalination 440, 59–67 (2018).
Motuzas, J. et al. Novel inorganic membrane for the percrystallization of mineral, food and pharmaceutical compounds. J. Membr. Sci. 550, 407–415 (2018).
Liu, Z., Zhang, Y., Lu, X., Wang, X. & Zhao, X. Study of the bubble membrane crystallization process for zero-brine discharge. J. Membr. Sci. 563, 584–591 (2018).
Ali, A., Tufa, R. A., Macedonio, F., Curcio, E. & Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sust. Energy. Rev. 81, 1–21 (2018).
Curcio, E., Criscuoli, A. & Drioli, E. Membrane crystallizers. Ind. Eng. Chem. Res. 40, 2679–2684 (2001).
Sparenberg, M.-C., Chergaoui, S., Sang Sefidi, V. & Luis, P. Crystallization control via membrane distillation-crystallization: a review. Desalination 519, 115315 (2021).
Yadav, A., Labhasetwar, P. K. & Shahi, V. K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: opportunities, challenges and futuristic perspectives. Sci. Total Environ. 806, 150692 (2022).
Mericq, J. P., Laborie, S. & Cabassud, C. Vacuum membrane distillation of seawater reverse osmosis brines. Water Res. 44, 5260–5273 (2010).
Ji, X. et al. Membrane distillation-crystallization of seawater reverse osmosis brines. Sep Purif. Technol. 71, 76–82 (2010).
Bargeman, G. et al. Nanofiltration as energy-efficient solution for sulfate waste in vacuum salt production. Desalination 245, 460–468 (2009).
Naidu, G., Jeong, S. & Vigneswaran, S. Influence of feed/permeate velocity on scaling development in a direct contact membrane distillation. Sep Purif. Technol. 125, 291–−300 (2014).
Drioli, E., Criscuoli, A. & Macedonio, F. Membrane-Based Desalination: An Integrated Approach (MEDINA). (IWA Publishing, 2011).
Quist-Jensen, C. A., Ali, A., Mondal, S., Macedonio, F. & Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Membr. Sci. 505, 167–173 (2016).
Naidu, G., Zhong, X. & Vigneswaran, S. Comparison of membrane distillation and freeze crystallizer as alternatives for reverse osmosis concentrate treatment. Desalination 427, 10–18 (2018).
Das, P., Dutta, S. & Singh, K. K. Insights into membrane crystallization: a sustainable tool for value added product recovery from effluent streams. Sep Purif. Technol. 257, 117666 (2021).
Mishra, H. et al. Branched polymeric media: boron-chelating resins from hyperbranched polyethyleneimine. Environ. Sci. Technol. 46, 8998–9004 (2012).
Choi, Y., Ryu, S., Naidu, G. & Lee, S. Integrated submerged membrane distillation-adsorption system for rubidium recovery. Sep Purif. Technol. 218, 146–155 (2019).
Tanaka, Y. A computer simulation of ion exchange membrane electrodialysis for concentration of seawater. Membr. Water Treat. 1, 13–37 (2010).
Rosanoff, A. The high heart health value of drinking water magnesium. Med Hypotheses 81, 1063–1065 (2013).
Cotruvo, J. & Bartram, J. Calcium and Magnesium in Drinking Water: Public Health Significance. 1st edn, (World Health Organization Press, 2009).
Al-Baker, W. In Proceedings of the SWCC Workshop on Innovation in Desalination, (Jeddah, Saudi Arabia, 2022).
Liao, Y., Chen, P., Chiu, H. & Yang, C. Magnesium in drinking water modifies the association between nitrate ingestion and risk of death from esophageal cancer. J. Toxicol. Environ. Heal A 76, 192–200 (2013).
Alamoudi, A. S. M. et al. Multi-valent ion concentration using multi-stage nanofiltration. PCT patent WO2021034332A1 (2019).
Birnhack, L., Tang, S. C. N. & Lahav, O. Implementation, design and cost assessment of a membrane-based process for selectively enriching desalinated water with divalent seawater ions. Chemengineering 2, 2030041 (2018).
Wang, Z. et al. Magnesium fertilization improves crop yield in most production systems: a meta-analysis. Front. Plant Sci., 01727 (2020).
Davenport, D. M., Deshmukh, A., Werber, J. R. & Elimelech, M. High-pressure reverse osmosis for energy-efficient hypersaline brine desalination: Current status, design considerations, and research needs. Environ. Sci. Technol. Lett. 5, 467–475 (2018).
Fritzmann, C., Lowenberg, J., Wintgens, T. & Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007).
Wohlert, C. Apparatus and methods for solution processing using reverse osmosis. United States of America patent US 2013/0270186 A1 (2013).
Abusharkh, B. Method for purifying liquids. United States of America patent US 9206060 B1 (2015).
Wei, Q., Webley, J., Baker, E. & Carmignani, G. Osmotic pressure-assisted reverse osmosis process and method of using the same. PCT patent WO 2017/136048 A1 (2017).
Alamoudi, A. S. M., Ayumantakath, M. F., Voutchkov, N. & Ihm, S. Desalination brine concentration system and method. United States of America patent US 10947143 B2 (2021).
Chen, X. & Yip, N. Y. Unlocking high-salinity desalination with cascading osmotically mediated reverse osmosis: Energy and operating pressure analysis. Environ. Sci. Technol. 52, 2242–2250 (2018).
Peters, C. D. & Hankins, N. P. Osmotically assisted reverse osmosis (OARO): Five approaches to dewatering saline brines using pressure-driven membrane processes. Desalination 458, 1–13 (2019).
Kim, B., Gwak, G. & Hong, S. Analysis of enhancing water flux and reducing reverse solute flux in pressure assisted forward osmosis process. Desalination 421, 61–71 (2017).
Kim, J., Kim, J., Kim, J. & Hong, S. Osmotically enhanced dewatering-reverse osmosis (OED-RO) hybrid system: implication for shale gas produce water treatment. J. Membr. Sci. 554, 282–290 (2018).
Kurihara, M. Current status and future trend of dominant commercial reverse osmosis membranes. Membranes 11, 0906 (2021).
Dickson, A. G. & Govet, C. In Handbook of methods for the analysis of the various parameters of the carbon dioxide system in seawater; version 2, ORNL/CDIAC-74 (United States Department of Energy, 1994).
Voutchkov, N. & Kaiser, G. N. Management of concentrate from desalination plants. (Elsevier, 2020).
Wadekar, S. S. & Vidic, R. D. Insights into the rejection of barium and strontium by nanofiltration membrane from experimental and modeling analysis. J. Membr. Sci. 564, 742–752 (2018).
Kim, H.-J., Kim, S.-J., Hyeon, S., Kang, H. H. & Lee, K.-Y. Application of desalination membranes to nuclide (Cs, Sr, and Co) separation. ACS Omega 5, 20261–20269 (2020).
Li, Y., Zhao, Y., Wang, H. & Wang, M. The application of nanofiltration membrane for recovering lithium from salt lake brine. Desalination 468, 114081 (2019).
Llenas, L., Ribera, G., Martinez-Llado, X., Rovira, M. & de Pablo, J. Selection of nanofiltration membranes as pretreatment for scaling prevention in SWRO using real seawater. Desalination Water Treat. 51, 930–935 (2013).
Raff, O. & Wilke, R.-D. Removal of dissolved uranium by nanofiltration. Desalination 122, 147–150 (1999).
Basaran, G. et al. Comparative study of the removal of nickel (II) and chromium (VI) heavy metals from metal plating wastewater by two nanofiltration membranes. Desalination Water Treat. 57, 21870–21880 (2015).
Crocket, K. C. et al. Rare Earth element distribution in the NE Atlantic: Evidence for benthic sources, longevity of the seawater signal, and biogeochemical cycling. Front. Mar. Sci. 00147 (2018).
Alexander, J. E. & Corcoran, E. F. The distribution of copper in tropical seawater. Limnology 12, 236–242 (1967).
Falkner, K. K. & Edmond, J. M. Gold in seawater. Earth Planet Sci. Lett. 98, 208–221 (1990).
Croot, P. L. Rapid determination of picomolar titanium in seawater with catalytic cathodic stripping voltammetry. Anal. Chem. 83, 6395–6400 (2011).
Author information
Authors and Affiliations
Contributions
All authors contributed to ongoing discussion of the topics covered in this review and to the structure of the document, and provided substantive editorial contributions. C.F., S.I., and N.V. contributed the bulk of the first draft text, with sections provided by S.H.L. and M.F.A.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of the Saline Water Conversion Corporation, which is actively pursuing commercial opportunities in extracting minerals from desalination brine.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharkh, B.A., Al-Amoudi, A.A., Farooque, M. et al. Seawater desalination concentrate—a new frontier for sustainable mining of valuable minerals. npj Clean Water 5, 9 (2022). https://doi.org/10.1038/s41545-022-00153-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-022-00153-6
This article is cited by
-
Study of the Relationship Between New Ionic Interaction Parameters and Salt Solubility in Electrolyte Solutions Based on Molecular Dynamics Simulation
Journal of Ocean University of China (2024)