Abstract
Increasing human activity, including commercial and noncommercial use of pharmaceuticals, personal care products, and agricultural products, has introduced new contaminants that can be challenging to remove with currently available technologies. Pharmaceuticals, in particular, can be especially challenging to remove from the water supply and can pose great harm to people and local ecosystems. Their highly stable nature makes their degradation with conventional water treatment techniques difficult, and studies have shown that even advanced treatment of water is unable to remove some compounds. As such, decontamination of water from pharmaceuticals requires the development of advanced technologies capable of being used in indirect and direct potable water reuse. In this review, we discuss pharmaceutical removal in indirect potable water treatment and how recent advancements in adsorption and photocatalysis technologies can be used for the decontamination of pharmaceutical-based emerging contaminants. For instance, new materials that incorporate graphene-based nanomaterials have been developed and shown to have increased adsorptive capabilities toward pharmaceuticals when compared with unmodified graphene. In addition, adsorbents have been incorporated in membrane technologies, and photocatalysts have been combined with magnetic material and coated on optical fibers improving their usability in water treatment. Advancements in photocatalytic material research have enabled the development of highly effective materials capable of degradation of a variety of pharmaceutical compounds and the development of visible-light photocatalysts. To understand how adsorbents and photocatalysts can be utilized in water treatment, we address the benefits and limitations associated with these technologies and their potential applicability in indirect potable water reuse plants.
Similar content being viewed by others
Introduction
Potable water can be considered the most important human need. However, human activities have introduced dangerous contaminants in water systems requiring a multibarrier treatment approach to purify water for potable use. From the Ganges River Basin in India to the surface water in Milan, contaminants such as pharmaceuticals and personal care products have been detected.1,2,3,4,5,6,7,8,9 These contaminants are difficult to remove and can cause harm not only to humans but to wildlife and local ecosystems as well. Pharmaceuticals, personal care products, persistent organic pollutants, methanesulfonic acids, artificial sweeteners, transformation products, and engineered nanomaterials have all been identified as current contaminants of emerging concern (CECs).10,11,12,13 In this review, we focus on emerging pharmaceutical contaminants (EPCs) because of their potential adverse effects to humans and the ecosystem (Table 1). For instance, EPCs such as antibiotics can give rise to antibiotic resistant bacteria, which can cause irreparable harm to humans and the ecosystem.
Although detection of alarming concentrations of EPCs in wastewater streams has been a major concern for years, the true fate of some EPCs continues to be understudied. With the currently available information, it can be clearly seen that EPCs bioaccumulate in animal and plant tissues and often persist in the environment.14,15 For example, antibiotic presence in water and related ecosystems is already leading to an increase in antibiotic resistant bacteria.9,16 More alarming is the amount of these contaminants ending up in effluent streams as a result of their continuous usage in the treatment of various diseases. As such, the existence of EPCs in water sources is a globally important issue requiring increased attention on how non-target organisms are affected and how EPCs can be removed from potable water.
Due to the multiple concerns surrounding the decline of freshwater resources and increasing water demand, water reclamation and reuse projects are widely popularizing all around the world.17,18,19 With CEC detection in freshwater sources and revelations about CEC harm on human health and safety, potable water treatment facilities require careful design of additional steps to ensure water is safe for consumption.20,21,22 Conventionally, harmful contaminants are removed from wastewater with a multiple barrier approach.20,22,23 Primary and secondary treatment techniques are well established and capable in removing dissolved organic matter as well as larger particles (suspended particles and biodegradable solids are removed via physical and biological means, respectively).19,24 In the case of CECs, many stable and non-biodegradable compounds can survive these steps requiring further treatment.23,25
The next treatment step is determined by different water reuse downstream approaches, which can be categorized as unplanned, direct, and indirect. The unplanned potable reuse water cycle is the simplest, where treated water is released to a natural water system after the primary and secondary treatment steps.19,23,24 Both direct and indirect potable reuse plants contain a tertiary (advanced) treatment step before being released from the plant. This step can include one or more of the following processes: membrane filtration, carbon adsorption, ion exchange, chlorination, and advanced oxidation processes (AOPs), such as ozone and UV radiation.23,24,26 Selection of the appropriate combination of tertiary operations in a water treatment plant is important as contaminants that are not removed by primary and secondary processes, such as CECs, are often removed with advanced processes.26,27 However, even these energy-intensive methods may not fully decontaminate water from CECs and may result in the generation of harmful byproducts.28,29
While direct potable reuse water plants feed treated water from the tertiary step to the distribution system located before a drinking water treatment plant, indirect potable reuse plants purposely release it to a natural water source such as a surface water reservoir, river, sea, or groundwater aquifer19,23,24,30 (Fig. 1). Direct potable water reuse is a common practice in areas with few source waters and high demands. Indirect potable reuse plant operation is plausible only when there is an adequate natural system downstream. The effluent from the treatment plant is expected to be held in the environmental buffer for a specified retention time where the water can be treated by natural processes such as direct photolysis, adsorption, filtration through natural media, and natural microbiota.30,31,32 Certain CECs can travel through the water subsurface for up to 60 days, therefore, a longer time in the buffer may reduce CEC concentrations in the source water making it cleaner for the subsequent drinking water treatment step.33,34 However, communities with limited natural recharge opportunities may be unable to accommodate long lag times between the discharge and reuse steps.31 The possibility of artificial recharge systems resembling natural buffers has been raised as a method overcoming such limitations.31,35
Contaminants such as pharmaceuticals, microorganisms, and harmful ions can be present in water. To prepare contaminated water for potable use, it first goes through a wastewater treatment plant and is subsequently released in the environment (in lakes, reservoirs, and groundwater) where decontamination of water can occur through natural processes. To ensure all microorganisms and harmful contaminants do not reach the end user, the water undergoes a drinking water treatment before being released to the community.
It is important to note that uncertainties related to removal and potential hazards of unremoved contaminants can account for a considerably larger proportion of the associated risk of maintaining the plant.36 In terms of cost, indirect potable water treatment can cost more than the direct potable treatment mainly due to the environmental buffer used along with the indirect potable reuse plant. Although, the cost of water treatment after the environmental buffer is less for the indirect potable reuse plants as they receive much cleaner source water making it easier to treat. Furthermore, inclusion of reverse osmosis or other advanced treatment techniques increases treatment plant cost, however, currently, these techniques are the most successful in removing most pharmaceutical contaminants.31,37,38 Therefore, application of low-cost EPC removal techniques can have a clear effect on reducing water purification costs, and development of such techniques can potentially guarantee the complete removal of EPCs.
In this review, we focus on the advancements in nanotechnologies using adsorption or photocatalysis to decontaminate water from pharmaceutical contaminants. Adsorption and photocatalysis are the two most widely studied water purification methods due to their effectiveness and potential scalability. Due to the popularity of such material in research, literature presenting carbon-based adsorbents and/or photocatalysts for the removal of pharmaceuticals have been published in recent years.39,40,41,42 In this review, we compile additional recent studies on graphene-based adsorbents and a wide range of photocatalysts without limiting the material presented to TiO2-based photocatalysts. In addition, we present recent advancements on modifications that have been made on adsorbents and photocatalysts to increase their applicability in water treatment. For instance, we present studies incorporating adsorbents on membranes and studies on magnetic photocatalysts and photocatalysts immobilized on optical fibers. Furthermore, we focus on discussing the limitations of the material as well as the limitations of available research in determining whether these materials can be utilized in water treatment facilities to reduce EPCs released in the environment, which has not been previously discussed in other review articles.
Technological advancements
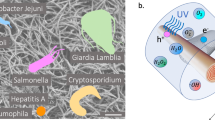
Utilization of nanomaterials such as graphene and metal-based nanoparticles in water treatment has shown promise due to their superior adsorptive and photocatalytic properties enabling removal and breakdown of harmful EPCs. Figure 2 shows a pictorial representation of the adsorptive and photocatalytic removal of contaminants.
In this section, we present some of the recent investigations on pharmaceutical removal from water using nanomaterials such as carbon-based nanomaterials and photocatalysts. We focus on understanding these technologies and their applicability in indirect potable water treatment processes. Additionally, we address the benefits and limitations of the nanomaterials and speculate about potential new research strategies.
Advancements in adsorption using nanomaterials
Adsorption processes utilizing carbon-based nanomaterials are considered effective in removing organic and inorganic matter from water. Adsorption is defined as a surface phenomenon where organic and inorganic matter attaches to an adsorbent’s surface by adhesion arising from physical-chemical forces mainly caused by van der Waals and electrostatic interactions.
An effective adsorbent must present a number of different properties such as being inert, biocompatible, resistant to mechanical forces, and needs to exhibit a high adsorption capacity to guarantee waste removal. These features are important as they can determine the utility of the material. Adsorption processes depend on a number of factors including: temperature, pH, concentration of pollutants, contact time, particle size, and the physical and chemical nature of the adsorbate and adsorbent. For example, pH can influence adsorption capacity by altering the surface groups present on the adsorbent and the pollutant charge,43 and an increase in temperature can improve adsorption capacity in endothermic reactions. In ibuprofen adsorption on activated carbon (AC), adsorption is more favorable at pH 3 than at pH 7.43 Additionally, as temperature is increased at pH 3, adsorption of ibuprofen has shown to increase.43 Depending on the adsorbent utilized, increasing contact time with the pollutant can increase the adsorbed amount since the time required for the adsorbent to become saturated varies depending on the surface and solution chemistry. Thus, a material can be a good adsorbent in a certain system and not in other systems.44
The number of aromatic rings and the chemical structure of EPCs make the adsorption process suitable for their removal from water. EPCs with more aromatic rings show faster adsorption rates.45 Generally, in graphene-based nanomaterials, this process is dominated by non-electrostatic interactions such as π–π interactions between the aromatic rings,46 hydrophobic interactions,47,48 H-bonding interactions due to the presence of COOH, OH and NH2 functional groups,49 and electrostatic interactions.50,51
Nanotechnology, while unexplored in industrial scale adsorption processes, creates a great opportunity to guarantee effectiveness of water treatment processes for EPC removal. AC is the current industrially used adsorbent. However, there are different adsorbents suitable for EPC removal that can replace AC including materials such as graphene, carbon nanotubes (CNTs), clay minerals, siliceous adsorbents, and polymeric materials. Graphene and graphene-based nanomaterials are being considered above all as good candidates for water treatment applications due to their unique structures and properties.52,53,54,55 They demonstrate appreciably fast adsorption kinetics due to their large surface area to volume ratio and other physiochemical properties, such as the π–π electron donor acceptor and electrostatic interaction with contaminants.56,57 The conjugated π region of graphene is capable of removing organic and inorganic contaminants by attracting aromatic pollutants.58 Graphene has been employed for several applications and is receiving increasingly more attention in water treatment. Different attempts have been made to modify graphene’s surface to increase its adsorption capacity and reusability (see Table 2).
Reduced graphene oxide57,58,59 and graphene52,60 have shown lower adsorption capacities for the majority of the reported EPCs compared to graphene oxide.61,62 This can be attributed to the increased hydrophobicity and decreased number of oxygen functional groups on the surface, which would hinder adsorption of EPCs present in water. The modification of graphene oxide with Fe3O4,53 MnO2,54 Fe/Cu55 and the preparation of graphene hydrogels56 exhibit low surface area, however, show larger adsorption capacities compared to the unmodified graphene. These modifications can alter the hydrophobicity of the composites and introduce different functional groups on its surface that promote more EPC removal. It is worth to note that while these material properties seem to enhance the adsorption capacity, the surface area can also play a role in the adsorption process. Increasing the surface area can effectively increase the number of sites EPCs can adsorb to, thus, increasing the adsorption capacity of the material. Comparing the adsorption capacity of magnetic chitosan grafted GO composite63 and activated graphene,64 we can see that activated carbon has a higher adsorption capacity towards ciprofloxacin (194.6 mg/g) than the magnetic chitosan grafted GO composite material (36.17 mg/g). This could be due to the differences in surface area. Activated graphene has a larger surface area (512.65 m2/g) than the magnetic chitosan grafted GO composite (388.3 m2/g), which can allow for increased adsorption of ciprofloxacin. While surface area can play an important role, comparison of material based on the resulting adsorption capacity can be more informative since it can be a better indicator as to the performance of the material. For instance, several materials with large surface area have lower adsorption capacities than materials with smaller surface areas. For example, graphene hydrogel65 (surface area of approximately 231.38 m2/g) has an adsorption capacity of 235.6 mg/g of ciprofloxacin, while the magnetic chitosan grafted graphene oxide composite66 (surface area of 388.3 m2/g) has an adsorption capacity of 36.17 m2/g.
As we can see in Table 2, the adsorption process by nanomaterials is a fast and effective method for EPC removal from aquatic environments. Most examples showed more than 50% removal of different EPCs, and several demonstrated removal efficiencies of more than 99%. However, adsorption processes have the disadvantage that the EPC attaches to the adsorbent limiting material reusability and creating a potential new environmental contaminant after disposal. While the interaction between the EPC and the adsorbent is not permanent, an extra step in the removal process must be included to separate the two. Some investigations propose the use of organic solvents or changes in the pH of the media to remove the organic molecule from the adsorbent.46,67
Currently, high cost and reusability are the two main problems associated with (graphene oxide) GO and GO-based nanomaterial since the preparation and subsequent purification of such material are exhausting and time-consuming processes. While production of reusable nanomaterial can reduce the overall cost, the strong electrostatic interactions of the material might influence the adsorption/desorption equilibrium and also influence its reusability making this nanomaterial inefficient for reuse.68 For example, long washing process periods69 and variation in the pH46,70 need to be performed to remove the CEC. Thus, making large-scale production, high cost, and reusability some of the unresolved problems associated with GO and GO-based nanomaterials, which can hinder their use in environmental pollution management.
However, keeping in mind the rapid growth and development in science and technology, material reusability problems are expected to be solved in the near future, which is an important factor for the potential application of GO and GO-based nanomaterials on a commercial scale. Although only a few studies investigate graphene-based adsorbent reusability for EPC, advancements in graphene nanomaterial reusability have allowed increased utilization of a single batch of material reducing the need for additional material purchases. For instance, GO has demonstrated high removal of metformin even after undergoing five sorption/desorption cycles in which sodium hydroxide and Milli-Q water were used to desorb metformin from the GO. The GO had a 31.60 mg/g absorption capacity after five cycles.46 Furthermore, a laccase-GO/alginate composite was used to remove cetirizine from solution where the material was washed with distilled water and acetate buffer after each removal experiment to recycle the adsorbent, and demonstrated a 23% reduction from the original 98% in cetirizine removal after four cycles.69 Adsorbents can also be modified with catalysts or photocatalysts to increase their removal capacity. Modification of graphene with catalysts, for instance, can make the sorption process easier and faster since CEC degradation will occur.71
Ultimately, as with any material, the lifetime of graphene-based material is finite, as such, its disposal will be required. Used graphene-based material can undergo similar disposal procedures to the currently utilized adsorbents in water treatment plants, which tend to forgo regeneration procedures in the United States. While the biocompatibility of graphene and graphene-based nanomaterials in terms of their antibacterial properties,72,73,74,75,76,77,78,79 antifungal properties,80,81 and cytotoxicity on human cells82,83,84,85,86 has been demonstrated for biomedical and environmental applications, only a few human cell lines have been studied. Hence, additional research is necessary before determining the health and environmental impacts of graphene.
Advanced application of adsorbents
As previously mentioned, there are several advanced processes for water treatment applications. Membrane processes are one of such advanced processes, which have gained significant popularity in EPC removal due to their effectiveness and scalability. Membranes can be used as part of microfiltration, ultrafiltration, nanofiltration, or reverse osmosis systems removing pollutants from water by acting as a physical barrier against contaminants. While nanofiltration and reverse osmosis membranes are highly effective, they require more energy than microfiltration and ultrafiltration membranes. As such, different membrane technologies are continuously being investigated, such as ceramic membranes,87,88 polymer membranes,89,90 metal-organic frameworks,67 or other advanced membranes.91 While a vast variety of membranes exist, polyamide-based membranes are one of the most commonly used membranes for EPC removal.92,93,94,95,96,97 Their properties, such as their porosity, fouling, stability, and hydrophilicity, can be altered by changing the polymerization conditions via the use of different monomers.98 Furthermore, these properties can be altered via the use of membrane coatings such as AC and graphene-based material. For example, graphene and GO have been incorporated into different polymer membranes to alter their properties and make them more effective for water treatment. In particular, poly(N-vinylcarbazole) (PVK), polyamide, and polysulfone membranes modified with GO have shown antimicrobial inactivation and improvement of water flux across the membrane.72,99,100,101,102,103
The modification of ceramic membranes with graphene was able to remove different pharmaceutical compounds from 32 to 99%.87 However, when the coating was with GO, the membrane was only able to remove approximately 50% of the EPC.88 Furthermore, polymer membranes such as polyamide membranes89 were able to remove 50 to 95% of the pharmaceutical compound studied.97 The modification of polysulfone with graphene oxide showed efficiencies in removal higher than 90%. The inclusion of GO and AC in polymer ultrafiltration membranes has also shown to improve the retention of pharmaceutical compounds. The oxygen rich structure of GO has been shown to increase hydrophilicity, decrease pore size, as well as increase electrostatic repulsion, thereby, improving its removal mechanism of contaminants for water treatment.88 In particular, GO coated membranes were shown to retain approximately 20% more of the pharmaceuticals (ibuprofen and sulfamethoxazole) than the uncoated ultrafiltration membrane.88 Furthermore, 0.22 μm membranes coated with GO and AC have shown 98.9% removal of tetracycline hydrochloride via vacuum filtration.104 Since very little information about pharmaceutical removal is out there, assumptions about the behavior towards pharmaceutical removal are complicated, however, the inclusion of graphene and graphene oxide have been a probe to exhibit good antimicrobial and durability properties.72,99,105
Although incorporation of AC and graphene-based nanomaterial onto membranes has shown increased EPC removal, research exploring such membranes is vastly underexplored. Immobilization of adsorbents on membrane technologies could enable effective EPC removal. Furthermore, the lifetime and potential reusability of the material could be improved. In particular, evaluation of sorbent material reusability is necessary due to the continuously increasing demand for sustainable wastewater treatment systems. Therefore, coated membrane technologies should be further studied, and desorption and recyclability tests should also be taken into consideration for potential reusability.
Photocatalysis
Photocatalysts—semiconducting materials, typically metal oxides, such as titanium oxide and zinc oxide—rely on their ability to absorb light and generate reactive oxygen species (ROS), which are responsible for the degradation of pollutants.106,107 Composites consisting of metal doped semiconductors, as well as adsorbent materials combined with semiconductor materials can be used for photocatalytic processes. Table 3 presents recent photocatalysts shown to degrade EPCs, some of which have been modified with graphene-based materials to increase the adsorptive and degradative properties of the composites.
As Fig. 2 depicts, once light hits the surface of the photocatalyst, if the light is equivalent to or greater than the material’s bandgap, electrons in the material’s valence band can be excited and can then jump to the conduction band creating an electron–hole pair. The generated electron–hole pair is responsible for the subsequent redox reactions that ultimately degrade pollutants. The method by which they degrade EPCs can be complex. Briefly, the electron is responsible for the reduction of dissolved oxygen to form the superoxide anion (⦁O2−), and the hole is responsible for the oxidation of water forming hydrogen gas and the hydroxyl radical (⦁OH). The superoxide anion and hydroxyl radical are oxidative agents, which are capable of degrading a variety of different compounds. Photodegradation can follow complex pathways depending on the contaminant structure, contaminant concentration, water chemistry, experimental conditions and nanomaterial loading. For example, sulfa drug (e.g. sulfachlopyridaxine, sulfapyridine, sulfisoxazole) degradation has been found to be dominated by hydroxyl radicals and holes created during photocatalysis.108 Holes are thought to initiate the reaction by breaking the sulfur-nitrogen bond of the drug followed by hydroxyl radical incorporation in the sulfa drug structure, which ultimately dominates the breakdown of the drug.108 In paracetamol photocatalytic degradation, the hydroxyl radical is also the predominant reactant causing the hydroxylation and breakdown of the aromatic rings.29,45 The compounds formed due to the hydroxylation of paracetamol (ex. hydroquinone) are further oxidized producing unstable structures, which break down in aqueous solutions.29 Furthermore, it has been found that when the concentration of the superoxide anion is greater than the hydroxyl radical, the superoxide anion is also capable of degrading paracetamol by acting as a Lewis acid.29
Recent advancements in photocatalysts have effectively enabled the degradation of numerous EPCs as shown in Table 3. However, photocatalysts present several limitations that need to be overcome to increase their effectiveness. Inherently, they require energy to overcome the bandgap energy required for electron excitation. However, they may require additional energy due to insubstantial light penetration and absorption, which effectively increase cost requirements due to the increased power needed for UV lamps.109,110 Furthermore, recombination rate, charge carrier transfer rate, and charge carrier travel time can further limit the photocatalytic efficiency of the material.111 To improve efficiency, material alterations, such as structural changes or doping can be performed.109 These methods can make the bandgap smaller and may also decrease recombination rates. In addition to bandgap engineering, use of plasmonic material can further lower energy requirements.109,110
Another limitation of photocatalytic material is their potential impact to the environment. The possible transformation products are of great concern especially if released in the environment. In some cases, as in the case of diclofenac degradation, the degradation can result in harmful constituents such as phenol derivatives.28 Pharmaceuticals commonly have aromatic rings which, if not degraded, can form phenolic compounds that are known for their toxicity.28 Additionally, they could form acids (as in the case of paracetamol degradation29) which could alter environmental conditions causing harm to local organisms. Another concern with photocatalyst release in the environment arises due to their instability in water. The ions released during their dissolution in water can have harmful effects to the environment.112 Thus, the photocatalysts' degradation mechanisms as they pertain to EPCs and photocatalyst stability in water need to be understood prior to their use in water treatment facilities. Additionally, generating composites and using stabilizing agents, whether natural or chemical in nature, can improve photocatalyst stability and efficiency.113 Furthermore, by improving their stability their harmful effects in the environment can be reduced.
Despite their limitations, photocatalysts offer great possibilities in commercial applications. For example, titanium dioxide, a UV activated photocatalyst, has been introduced in commercially available water purification products and could be potentially applied to the AOPs to help degrade a variety of contaminants. With advancements in photocatalytic materials, photocatalysts are becoming increasingly more cost effective and their large-scale use more feasible. For example, a number of the reported photocatalysts are capable of utilizing low power UV or visible light to degrade EPCs. Direct comparison of many of these photocatalysts is limited due to the complexity of the reactions, such as structural and chemical properties of the photocatalyst, type and amount of the EPC, light source parameters, stirring rate, among others, which can affect the results. For instance, when TiO2-rGO loaded on optical fibers was used for degradation of different pharmaceuticals (ibuprofen, sulfamethoxazole, and carbamazepine) under the same concentrations (5 ppm) and same conditions (high pressure UV light of 140 W), the results were vastly different and with different reaction rates (8.98, 12.6, and 4.3 × 10−3/min, respectively).114 Furthermore, use of different light sources also yielded different results in the degradation of ibuprofen (160 W high pressure UV yielded a reaction rate of 8.89 × 10−3/min, 39 W low-pressure UV yielded a reaction rate of 3.32 × 10−3/min, and 40 W visible light yielded a reaction rate of 1.33 × 10−3/min). With this in mind, we can safely say that the ability of a multiwall CNT with titanium dioxide photocatalyst to fully remove 10 ppm of tetracycline from water at a high reaction rate (64.2 × 10−3/min) utilizing a 12 W UV lamp is notable.115 Other materials tested on 10 ppm tetracycline either required more energy to fully degrade the EPC, or did not perform as well.
While photocatalysis effectiveness is increased when UV light is utilized, numerous materials have been shown to be capable of visible light photodegradation of pharmaceuticals.109,114,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134 Although power utilization of many visible light lamps is equivalent to that of low-pressure UV lamps, the lifetime of UV lamps is significantly lower. Furthermore, with the increased availability of LED lighting options, the power of using visible light photoreactors can be greatly reduced.
Regardless of the type of lighting used, inclusion of photocatalysts in water decontamination can decrease energy requirements. Conventional techniques for removal of organic chemicals such as EPCs may require the use of AOPs, which not only are energy-intensive processes but may be unable to fully remove EPCs. Use of photocatalysts, on the other hand, has been shown to remove non-biodegradable EPCs such as carbamazepine, iopromide, and norfloxacin.114,119,125,134,135,136 However, as with any new technology additional research needs to be conducted examining their safety to humans and the environment before large-scale utilization. While they are highly suitable for use in indirect potable water treatment, without a full examination of their properties and environmental impact, measures should be taken to ensure they are not released in the environment.
Advanced applications of photocatalysts
In an effort to increase usability of photocatalysts, a variety of materials have been developed, namely magnetic nanocomposites and optical fiber coated materials. Magnetic composites can make the removal of the photocatalysts from water easier and more effective reducing the chance that they may unintentionally end up in the environment. Furthermore, their degradative properties have been shown to increase with the introduction of magnetic materials in the composite.137,138 Recently, magnetic FeNi3/SiO2/CuS has been synthesized for tetracycline removal,139 while magnetic fluorinated mesoporous graphitic carbon nitride140 and a magnetic TiO2-GO-Fe3O4137 have been synthesized for amoxicillin removal.
In addition to magnetic material, photocatalytic materials loaded on optical fibers have been developed. Immobilization of photocatalysts on optical fibers can allow light to better reach the nanoparticles as less light is absorbed by other particles present in the solution. Furthermore, the nanoparticles do not require specialized methods for recovery. TiO2 has been successfully coated on optical fibers, which has resulted in the development of a compact, easy-to-use reactor utilizing light-emitting diodes for photocatalytic water treatment.141 Furthermore, TiO2-rGO composites have also been used to coat optical fibers and have been shown to be capable of degrading pharmaceutical compounds such as sulfamethoxazole and ibuprofen.142
Use of magnetic materials in photocatalyst composites and coating photocatalysts on optical fibers can be promising in potable water treatment. However, without modification of the existing potable water treatment plant equipment or processes, their use may not be as feasible. Inclusion of photocatalysts in membrane technologies, similar to the introduction of graphene-based material in membranes, can greatly improve the functionality of the membranes and can be easily introduced in water treatment plants. While photocatalysts can reduce fouling and degrade contaminants, they can also degrade membrane materials reducing the lifetime of the membranes. For instance, use of TiO2 in polyacrylonitrile membranes has been determined to be unsuitable for long-term use.143 Thus, additional research is needed to explore potential use of photocatalysts in membrane technologies.
Conclusions and perspectives
This review presents recent studies related to pharmaceutical removal with nanoparticles involving two different processes, adsorption and photocatalysis. We presented studies where nanomaterial demonstrated superior adsorptive or photocatalytic properties in the removal of EPCs. We also included photocatalysts modified with graphene in order to combine both properties, adsorption and degradation of organic molecules. These studies quantify EPC removal in simple solutions or in wastewater. However, most of these studies do not examine material use in actual water treatment systems. To fill the gap between fundamental research and practical applications there needs to be a focus on the potential practical applications of the different EPC removal techniques in indirect potable reuse water systems.
While EPC removal efficiencies are important, it is important to investigate at what concentration these EPCs pose a threat to humans, animals, and the ecosystem regardless of the fact that many recent research articles demonstrate high EPC removal efficiencies. Little is known about nanoparticle stability in solution, and the effects of ingestion of the particles or their solutes is largely unknown. Furthermore, the production of toxic byproducts from EPC degradation should be of concern since disinfection byproducts account for a different class of regulated contaminants. Intermediate degradation products can exhibit increased solubility as compared to that of the original contaminant, and higher toxicity values. Thus, it is important to thoroughly evaluate nanoparticle toxicity (toxic amount and maximum exposure time) and the risks associated with the employment of nanoparticles in water treatment.
Currently, AOPs are the best strategy to remove EPCs from water. However, associated costs are a major concern in communities with limited financial support. Scaling techniques to match industrial levels will be required. As seen in recent studies, inclusion of different nanomaterials in membranes or on optical fibers and use of magnetic photocatalysts result in significant EPC removal. Use of such technologies can help meet safe drinking water demands while reducing EPCs entering the environment. However, when used in indirect potable water cycles, these techniques need to break down a great variety of EPCs.
To guarantee water safety, the indirect potable reuse process requires understanding environmental and health standards. As such, the employment of recent technologies needs thorough risk assessments and health and safety evaluations performed to mitigate potential risks of the technology itself. While no legislation pertaining to EPC maximum allowable concentrations in water has been established, legislations regulating drinking water processes tend to be very strict to ensure human health and environmental safety. For instance, in an ongoing effort to maintain the safety of drinking water and lessen the effect of EPCs, the European Union has added additional requirements for pharmaceuticals whereby more extensive environmental risk assessments need to be conducted for each pharmaceutical’s use to be allowed.144 Furthermore, pharmaceutical contaminants in the environment are to be potentially monitored more extensively in order to be able to better evaluate their risk and environmental effects. Still, maximum EPC removal may be necessary, and the employment of nanotechnology in water treatment can be critical when it comes to human health and EPC persistence in environmental systems.
References
He, S. et al. Occurrence and ecological risk assessment of 22 emerging contaminants in the Jilin Songhua River (Northeast China). Environ. Sci. Pollut. Res. 25, 24003–24012 (2018).
Kapelewska, J. et al. Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchem. J. 137, 292–301 (2018).
Peng, F.-J. et al. Occurrence and ecological risk assessment of emerging organic chemicals in urban rivers: Guangzhou as a case study in China. Sci. Total Environ. 589, 46–55 (2017).
Miraji, H., Othman, O. C., Ngassapa, F. N. & Mureithi, E. W. Research trends in emerging contaminants on the aquatic environments of Tanzania. Sci. (Cairo). 2016, 3769690 (2016).
Riva, F. et al. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 221, 451–457 (2018).
Riva, F., Zuccato, E., Davoli, E., Fattore, E. & Castiglioni, S. Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in Italy. J. Hazard. Mater. 361, 103–110 (2019).
Lapworth, D. J. et al. Deep urban groundwater vulnerability in India revealed through the use of emerging organic contaminants and residence time tracers. Environ. Pollut. 240, 938–949 (2018).
Sharma, B. M. et al. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 646, 1459–1467 (2019).
Lindim, C. et al. Exposure and ecotoxicological risk assessment of mixtures of top prescribed pharmaceuticals in Swedish freshwaters. Chemosphere 220, 344–352 (2018).
Scheurer, M., Brauch, H.-J. & Lange, F. T. in Transformation Products of Emerging Contaminants in the Environment 525–544 (John Wiley and Sons Ltd, 2014). https://doi.org/10.1002/9781118339558.ch17.
Mauter, M. S. et al. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 1, 166–175 (2018).
Richardson, S. D. & Ternes, T. A. Water analysis: emerging contaminants and current issues. Anal. Chem. 90, 398–428 (2018).
Bennett, R. et al. White paper: aquatic life criteria for contaminants on emerging concern. Part I General challenges and recommendations. US EPA 46 (2008).
David, A., Lange, A., Tyler, C. R. & Hill, E. M. Concentrating mixtures of neuroactive pharmaceuticals and altered neurotransmitter levels in the brain of fish exposed to a wastewater effluent. Sci. Total Environ. 621, 782–790 (2018).
Xiang, J. et al. The fate and risk assessment of psychiatric pharmaceuticals from psychiatric hospital effluent. Ecotoxicol. Environ. Saf. 150, 289–296 (2018).
Mirzaei, R., Mesdaghinia, A., Hoseini, S. S. & Yunesian, M. Antibiotics in urban wastewater and rivers of Tehran, Iran: consumption, mass load, occurrence, and ecological risk. Chemosphere. https://doi.org/10.1016/J.CHEMOSPHERE.2018.12.187 (2018).
Sorenson, S. B., Morssink, C. & Campos, P. A. Safe access to safe water in low income countries: water fetching in current times. Soc. Sci. Med. 72, 1522–1526 (2011).
Ahmed, Y., Huang, Y.-L., Martin, A. & Otten, B. Assessment of the relation between water quality and water quantity for international metropolitan cities. In World Environmental and Water Resources Congress 2015 669–684 (American Society of Civil Engineers, 2015). https://doi.org/10.1061/9780784479162.062.
Wilcox, J., Nasiri, F., Bell, S. & Rahaman, M. S. Urban water reuse: a triple bottom line assessment framework and review. Sustain. Cities Soc. 27, 448–456 (2016).
Wintgens, T., Salehi, F., Hochstrat, R. & Melin, T. Emerging contaminants and treatment options in water recycling for indirect potable use. Water Sci. Technol. 57, 99–107 (2008).
Huerta-Fontela, M., Galceran, M. T. & Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 45, 1432–1442 (2011).
Krzeminski, P. et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: a review. Sci. Total Environ. 648, 1052–1081 (2019).
Roccaro, P. Treatment processes for municipal wastewater reclamation: the challenges of emerging contaminants and direct potable reuse. Curr. Opin. Environ. Sci. Heal. 2, 46–54 (2018).
Rome D. R A.-M. I. M. Handbook of Wastewater Reclamation and Reuse. (Lewis Publishers, 1995).
Foureaux, A. F. S. in Separation and Purification Technology Vol. 212 (Elsevier 2018).
Rojas, M. R. et al. Assessment of the effectiveness of secondary wastewater treatment technologies to remove trace chemicals of emerging concern. Crit. Rev. Environ. Sci. Technol. 43, 1281–1314 (2013).
Heberer, T., Reddersen, K. & Mechlinski, A. From municipal sewage to drinking water: fate and removal of pharmaceutical residues in the aquatic environment in urban areas. Water Sci. Technol. 46, 81–88 (2002).
Banaschik, R., Jablonowski, H., Bednarski, P. J. & Kolb, J. F. Degradation and intermediates of diclofenac as instructive example for decomposition of recalcitrant pharmaceuticals by hydroxyl radicals generated with pulsed corona plasma in water. J. Hazard. Mater. 342, 651–660 (2018).
Yang, L., Yu, L. E. & Ray, M. B. Photocatalytic oxidation of paracetamol: dominant reactants, intermediates, and reaction mechanisms. Environ. Sci. Technol. 43, 460–465 (2009).
Drewes, J. E. & Khan, S. J. Contemporary design, operation, and monitoring of potable reuse systems. J. Water Reuse Desalin. 5, 1–7 (2015).
Sgroi, M. & Vagliasindi, F. G. A. Feasibility, sustainability and circular economy concepts in water reuse. Curr. Opin. Environ. Sci. Heal. 2, 20–25 (2018).
Rodriguez, C. et al. Indirect potable reuse: a sustainable water supply alternative. Int. J. Environ. Res. Public Health 6, 1174–209 (2009).
Muntau, M. et al. Evaluation of the short-term fate and transport of chemicals of emerging concern during soil-aquifer treatment using select transformation products as intrinsic redox-sensitive tracers. Sci. Total Environ. 583, 10–18 (2017).
Laws, B. V., Dickenson, E. R. V., Johnson, T. A., Snyder, S. A. & Drewes, J. E. Attenuation of contaminants of emerging concern during surface-spreading aquifer recharge. Sci. Total Environ. 409, 1087–1094 (2011).
Regnery, J. et al. Integration of artificial recharge and recovery systems for impaired water sources in urban settings: overcoming current limitations and engineering challenges. Environ. Eng. Sci. 30, 409–420 (2013).
Adapa, S. Factors influencing consumption and anti-consumption of recycled water: evidence from Australia. J. Clean. Prod. 201, 624–635 (2018).
Herman, J. G., Scruggs, C. E. & Thomson, B. M. The costs of direct and indirect potable water reuse in a medium-sized arid inland community. J. Water Process Eng. 19, 239–247 (2017).
Venkatesan, A. K., Ahmad, S., Johnson, W. & Batista, J. R. Salinity reduction and energy conservation in direct and indirect potable water reuse. Desalination 272, 120–127 (2011).
Khan, A. et al. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: a review. Environ. Sci. Pollut. Res. 24, 7938–7958 (2017).
Awfa, D., Ateia, M., Fujii, M., Johnson, M. S. & Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: a critical review of recent literature. Water Res. 142, 26–45 (2018).
Zhao, L. et al. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: systematic review and bibliometric analysis. Sci. Total Environ. 627, 1253–1263 (2018).
Lee, C. M., Palaniandy, P. & Dahlan, I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: a review. Environ. Earth Sci. 76. https://doi.org/10.1007/s12665-017-6924-y (2017).
Reinert, L. et al. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon N. Y. 54, 432–443 (2012).
Ruthven, D. M. Principles of Adsorption and Adsorption Processes (Wiley, 1984).
Yang, L., Yu, L. E. & Ray, M. B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 42, 3480–3488 (2008).
Zhu, S. et al. Adsorption of emerging contaminant metformin using graphene oxide. Chemosphere 179, 20–28 (2017).
Xu, J., Wang, L. & Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 28, 8418–8425 (2012).
Zhu, D. & Pignatello, J. J. Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ. Sci. Technol. 39, 2033–2041 (2005).
Cortés Arriagada, D., Sanhueza, L. & Wrighton, K. Removal of 4-chlorophenol using graphene, graphene oxide, and a-doped graphene (A = N, B): a computational study. Int. J. Quantum Chem. 113, 1931–1939 (2013).
Gao, Y. et al. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 368, 540–546 (2012).
Swathi, R. S. & Sebastian, K. L. Long range resonance energy transfer from a dye molecule to graphene has (distance)-4 dependence. J. Chem. Phys. 130, 086101 (2009).
Zhu, Y. et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924 (2010).
Yui, Y. Purchases of condominiums by single women and their backgrounds in Tokyo: housing problems for women. Geogr. Rev. Jpn. 79, 165–179 (2006).
Soldano, C., Mahmood, A. & Dujardin, E. Production, properties and potential of graphene. Carbon N.Y. 48, 2127–2150 (2010).
Kyzas, G. Z., Deliyanni, E. A. & Matis, K. A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 89, 196–205 (2014).
Yusuf, M., Elfghi, F. M., Zaidi, S. A., Abdullah, E. C. & Khan, M. A. Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv. 5, 50392–50420 (2015).
Ren, X., Li, J., Tan, X. & Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalt. Trans. 42, 5266–5274 (2013).
Al-Khateeb, L. A., Almotiry, S. & Salam, M. A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 248, 191–199 (2014).
Tang, Y. et al. Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 424, 74–80 (2013).
Umbreen, N., Sohni, S., Ahmad, I., Khattak, N. U. & Gul, K. Self-assembled three-dimensional reduced graphene oxide-based hydrogel for highly efficient and facile removal of pharmaceutical compounds from aqueous solution. J. Colloid Interface Sci. 527, 356–367 (2018).
Jauris, I. M. et al. Adsorption of sodium diclofenac on graphene: a combined experimental and theoretical study. Phys. Chem. Chem. Phys. 18, 1526–1536 (2016).
Yang, G. C. C. & Tang, P. L. Removal of phthalates and pharmaceuticals from municipal wastewater by graphene adsorption process. Water Sci. Technol. 73, 2268–2274 (2016).
Wang, F. et al. Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq. 222, 188–194 (2016).
Yu, F., Ma, J. & Bi, D. Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene. Environ. Sci. Pollut. Res. 22, 4715–4724 (2015).
Ma, J., Yang, M., Yu, F. & Zheng, J. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel. Sci. Rep. 5, 1–10 (2015).
Lv, M. et al. Non-covalent functionalized graphene oxide (GO) adsorbent with an organic gelator for co-adsorption of dye, endocrine-disruptor, pharmaceutical and metal ion. Chem. Eng. J. 349, 791–799 (2018).
Sarker, M., Song, J. Y. & Jhung, S. H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 335, 74–81 (2018).
Kyzas, G. Z., Deliyanni, E. A., Bikiaris, D. N. & Mitropoulos, A. C. Graphene composites as dye adsorbents: review. Chem. Eng. Res. Des. 129, 75–88 (2018).
Sharifi-Bonab, M., Arjomandi Rad, F. & Talat Mehrabad, J. Preparation of laccase-graphene oxide nanosheet/alginate composite: application for the removal of cetirizine from aqueous solution. J. Environ. Chem. Eng. 4, 3013–3020 (2016).
Kyzas, G. Z., Bikiaris, D. N., Seredych, M., Bandosz, T. J. & Deliyanni, E. A. Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite. Bioresour. Technol. 152, 399–406 (2014).
Wang, J. et al. Reduced graphene oxide/ZnO composite: reusable adsorbent for pollutant management. ACS Appl. Mater. Interfaces 4, 3084–3090 (2012).
Santos, C. M. et al. Antimicrobial graphene polymer (PVK-GO) nanocomposite films. Chem. Commun. 47, 8892–8894 (2011).
Nguyen, H. N., Nadres, E. T., Alamani, B. G. & Rodrigues, D. F. Designing polymeric adhesives for antimicrobial materials: poly(ethylene imine) polymer{,} graphene{,} graphene oxide and molybdenum trioxide - a biomimetic approach. J. Mater. Chem. B 5, 6616–6628 (2017).
Mejías Carpio, I. E., Santos, C. M., Wei, X. & Rodrigues, D. F. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 4, 4746 (2012).
Santos, C. M. et al. Graphene nanocomposite for biomedical applications: fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 23, 395101 (2012).
Perreault, F., De Faria, A. F., Nejati, S. & Elimelech, M. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9, 7226–7236 (2015).
Janković, A. et al. Graphene-based antibacterial composite coatings electrodeposited on titanium for biomedical applications. Prog. Org. Coat. 83, 1–10 (2015).
Peña-Bahamonde, J. et al. Functionalization of reduced graphene oxide with polysulfone brushes enhance antibacterial properties and reduce human cytotoxicity. Carbon N. Y. 111, 258–268 (2017).
Crock, Ca, Rogensues, A. R., Shan, W. & Tarabara, V. V. Polymer nanocomposites with graphene-based hierarchical fillers as materials for multifunctional water treatment membranes. Water Res. 47, 3984–3996 (2013).
Li, C. et al. The antifungal activity of graphene oxide-silver nanocomposites. Biomaterials 34, 3882–3890 (2013).
Sawangphruk, M., Srimuk, P. & Chiochan, P. Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon N. Y. 50, 5156–5161 (2012).
Liao, K. H., Lin, Y. S., MacOsko, C. W. & Haynes, C. L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 3, 2607–2615 (2011).
Lammel, T., Boisseaux, P., Fernández-Cruz, M. L. & Navas, J. M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 10, 1–21 (2013).
Zhou, R. & Gao, H. Cytotoxicity of graphene: recent advances and future perspective. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnology 6, 452–474 (2014).
Hu, W. et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 5, 3693–3700 (2011).
Chandra, A., Deshpande, S., Shinde, D. B., Pillai, V. K. & Singh, N. Mitigating the cytotoxicity of graphene quantum dots and enhancing their applications in bioimaging and drug delivery. ACS Macro Lett. 3, 1064–1068 (2014).
Yang, G. C. C., Chen, Y. C., Yang, H. X. & Yen, C. H. Performance and mechanisms for the removal of phthalates and pharmaceuticals from aqueous solution by graphene-containing ceramic composite tubular membrane coupled with the simultaneous electrocoagulation and electrofiltration process. Chemosphere 155, 274–282 (2016).
Chu, K. H. et al. Evaluation of removal mechanisms in a graphene oxide-coated ceramic ultrafiltration membrane for retention of natural organic matter, pharmaceuticals, and inorganic salts. ACS Appl. Mater. Interfaces 9, 40369–40377 (2017).
Javier Benitez, F., Acero, J. L., Real, F. J., Roldán, G. & Rodriguez, E. Ultrafiltration and nanofiltration membranes applied to the removal of the pharmaceuticals amoxicillin, naproxen, metoprolol and phenacetin from water. J. Chem. Technol. Biotechnol. 86, 858–866 (2011).
Zambianchi, M. et al. Graphene oxide doped polysulfone membrane adsorbers for the removal of organic contaminants from water. Chem. Eng. J. 326, 130–140 (2017).
Qurie, M. et al. Stability and removal of naproxen and its metabolite by advanced membrane wastewater treatment plant and micelle-clay complex. Clean.—Soil, Air, Water 42, 594–600 (2014).
Nghiem, L. D. & Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (PhACs): mechanisms and role of membrane pore size. Sep. Purif. Technol. 57, 176–184 (2007).
Simon, A., Nghiem, L. D., Le-Clech, P., Khan, S. J. & Drewes, J. E. Effects of membrane degradation on the removal of pharmaceutically active compounds (PhACs) by NF/RO filtration processes. J. Memb. Sci. 340, 16–25 (2009).
Zazouli, M. A., Susanto, H., Nasseri, S. & Ulbricht, M. Influences of solution chemistry and polymeric natural organic matter on the removal of aquatic pharmaceutical residuals by nanofiltration. Water Res. 43, 3270–3280 (2009).
Košutić, K., Dolar, D., Ašperger, D. & Kunst, B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 53, 244–249 (2007).
Verliefde, A. R. D. et al. Influence of electrostatic interactions on the rejection with NF and assessment of the removal efficiency during NF/GAC treatment of pharmaceutically active compounds in surface water. Water Res. 41, 3227–3240 (2007).
Radjenović, J., Petrović, M., Ventura, F. & Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 42, 3601–3610 (2008).
Kang, Guodong & Cao, Yiming Development of antifouling reverse osmosis membranes for water treatment: a review. Water Res. 46, 584–600 (2012).
Musico, Y. L. F., Santos, C. M., Dalida, M. L. P. & Rodrigues, D. F. in ACS Sustainable Chemistry and Engineering. Vol. 2, 1559–1565 (American Chemical Society, 2014).
Hu, M. & Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 47, 3715–3723 (2013).
Cao, B., Ansari, A., Yi, X., Rodrigues, D. F. & Hu, Y. Gypsum scale formation on graphene oxide modified reverse osmosis membrane. J. Memb. Sci. 552, 132–143 (2018).
Peña-Bahamonde, J., San-Miguel, V., Cabanelas, J. C. & Rodrigues, D. F. Biological degradation and biostability of nanocomposites based on polysulfone with different concentrations of reduced graphene oxide. Macromol. Mater. Eng. 303, 1700359 (2018).
Smith, S. C. & Rodrigues, D. F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon N.Y. 91, 122–143 (2015).
Liu, M. K. et al. Effective removal of tetracycline antibiotics from water using hybrid carbon membranes. Sci. Rep. 7, 43717 (2017).
Perreault, F., Tousley, M. E. & Elimelech, M. Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ. Sci. Technol. Lett. 1, 71–76 (2013).
Fernández-Castro, P., Vallejo, M., San Román, M. F. & Ortiz, I. Insight on the fundamentals of advanced oxidation processes: role and review of the determination methods of reactive oxygen species. J. Chem. Technol. Biotechnol. 90, 796–820 (2015).
Nosaka, Y. & Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336 (2017).
Yang, H., Li, G., An, T., Gao, Y. & Fu, J. Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: a case of sulfa drugs. in. Catal. Today 153, 200–207 (2010).
Dong, H. et al. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 79, 128–146 (2015).
Alvarez, P. J. J., Chan, C. K., Elimelech, M., Halas, N. J. & Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 13, 634–641 (2018).
Zhang, T. C. et al. Nanotechnologies for Water Environment Applications (American Society of Civil Engineers (ASCE), 2009). https://doi.org/10.1061/9780784410301.
Misra, S. K., Dybowska, A., Berhanu, D., Luoma, S. N. & Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 438, 225–232 (2012).
Vadlapudi, V. Novel Capping Agents for nanomaterials. Eur. J. Appl. Sci. 7, 297–301 (2015).
Lin, L., Wang, H. & Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 310, 389–398 (2017).
Ahmadi, M. et al. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2nano-composite. J. Environ. Manag. 186, 55–63 (2017).
Shao, H. et al. Synergetic activation of peroxymonosulfate by Co3O4 modified g-C3N4 for enhanced degradation of diclofenac sodium under visible light irradiation. Appl. Catal. B Environ. 218, 810–818 (2017).
Lu, X. et al. The facile fabrication of novel visible-light-driven Z-scheme CuInS2/Bi2WO6heterojunction with intimate interface contact by in situ hydrothermal growth strategy for extraordinary photocatalytic performance. Chem. Eng. J. 356, 819–829 (2019).
Sun, J. et al. H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at g-C3N4/BiVO4 photoanode under visible light irradiation. Chem. Eng. J. 332, 312–320 (2018).
Surenjan, A., Sambandam, B., Pradeep, T. & Philip, L. Synthesis, characterization and performance of visible light active C-TiO2 for pharmaceutical photodegradation. J. Environ. Chem. Eng. 5, 757–767 (2017).
Li, J. et al. Visible light driven photocatalytic decomposition of penicillin G by Ti3+ self-doped TiO2 nano-catalyst through response surface methodology. J. Taiwan Inst. Chem. Eng. 87, 174–181 (2018).
Guo, R. et al. Construction of Ag3PO4/TiO2 nano-tube arrays photoelectrode and its enhanced visible light driven photocatalytic decomposition of diclofenac. Sep. Purif. Technol. 200, 44–50 (2018).
Jo, W.-K., Kumar, S., Isaacs, M. A., Lee, A. F. & Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 201, 159–168 (2017).
Pastrana-Martínez, L. M. et al. Advanced nanostructured photocatalysts based on reduced graphene oxide-TiO2composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 123–124, 241–256 (2012).
Davari, N., Farhadian, M., Nazar, A. R. S. & Homayoonfal, M. Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: structural and operational comparison. J. Environ. Chem. Eng. 5, 5707–5720 (2017).
Cao, D., Wang, Y., Qiao, M. & Zhao, X. Enhanced photoelectrocatalytic degradation of norfloxacin by an Ag3PO4/BiVO4 electrode with low bias. J. Catal. 360, 240–249 (2018).
Zammouri, L. et al. Enhancement under UV–visible and visible light of the ZnO photocatalytic activity for the antibiotic removal from aqueous media using Ce-doped Lu3Al5O12 nanoparticles. Mater. Res. Bull. 106, 162–169 (2018).
de Luna, M. D. G., Lin, J. C., Te, Gotostos, M. J. N. & Lu, M. C. Photocatalytic oxidation of acetaminophen using carbon self-doped titanium dioxide. Sustain. Environ. Res. 26, 161–167 (2016).
Kaur, A. & Kansal, S. K. Bi2WO6 nanocuboids: an efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 302, 194–203 (2016).
Deng, F. et al. One-step hydrothermal fabrication of visible-light-responsive AgInS2/SnIn4S8 heterojunction for highly-efficient photocatalytic treatment of organic pollutants and real pharmaceutical industry wastewater. Appl. Catal. B Environ. 219, 163–172 (2017).
Kumar, A., Khan, M., Zeng, X. & Lo, I. M. C. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: a magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 353, 645–656 (2018).
Sheydaei, M., Shiadeh, H. R. K., Ayoubi-Feiz, B. & Ezzati, R. Preparation of nano N-TiO2/graphene oxide/titan grid sheets for visible light assisted photocatalytic ozonation of cefixime. Chem. Eng. J. 353, 138–146 (2018).
Hu, X.-Y., Zhou, K., Chen, B.-Y. & Chang, C.-T. Graphene/TiO2/ZSM-5 composites synthesized by mixture design were used for photocatalytic degradation of oxytetracycline under visible light: mechanism and biotoxicity. Appl. Surf. Sci. 362, 329–334 (2016).
Deng, F., Zhao, L., Luo, X., Luo, S. & Dionysiou, D. D. Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem. Eng. J. 333, 423–433 (2018).
Gao, X., Peng, W., Tang, G., Guo, Q. & Luo, Y. Highly efficient and visible-light-driven BiOCl for photocatalytic degradation of carbamazepine. J. Alloy. Compd. 757, 455–465 (2018).
An, Y. et al. Adsorption and photocatalytic degradation of pharmaceuticals and pesticides by carbon doped-TiO2 coated on zeolites under solar light irradiation. Water Sci. Technol. 73, 2868–2881 (2016).
Paredes, L. et al. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 240, 9–18 (2018).
Li, Q., Kong, H., Li, P., Shao, J. & He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 373, 437–446 (2019).
Sun, Q., Hong, Y., Liu, Q. & Dong, L. Synergistic operation of photocatalytic degradation and Fenton process by magnetic Fe3O4 loaded TiO2. Appl. Surf. Sci. 430, 399–406 (2018).
Nasseh, N., Taghavi, L., Barikbin, B. & Nasseri, M. A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 179, 42–54 (2018).
Mirzaei, A., Chen, Z., Haghighat, F. & Yerushalmi, L. Magnetic fluorinated mesoporous g-C3N4 for photocatalytic degradation of amoxicillin: transformation mechanism and toxicity assessment. Appl. Catal. B Environ. 242, 337–348 (2019).
O’Neal Tugaoen, H., Garcia-Segura, S., Hristovski, K. & Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total Environ. 613–614, 1331–1338 (2018).
Lin, L., Wang, H. & Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 310, 389–398 (2017).
Chin, S. S., Chiang, K. & Fane, A. G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Memb. Sci. 275, 202–211 (2006).
Bruce, G. M., Pleus, R. C. & Snyder, S. A. Toxicological relevance of pharmaceuticals in drinking water. Environ. Sci. Technol. 44, 5619–5626 (2010).
Jones, O. A., Lester, J. N. & Voulvoulis, N. Pharmaceuticals: a threat to drinking water? Trends Biotechnol. 23, 163–167 (2005).
Escher, B. I., Bramaz, N., Eggen, R. I. L. & Richter, M. In vitro assessment of modes of toxic action of pharmaceutical in aquatic life. Environ. Sci. Technol. 39, 3090–3100 (2005).
Baquero, F., Martínez, J.-L. & Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 19, 260–265 (2008).
Crider, K. S. et al. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch. Pediatr. Adolesc. Med. 163, 978–985 (2009).
WHO. IPCS international programme on chemical safety, Healthy and Safety Guide No. 96 (1989).
Bull, R. J., Crook, J., Whittaker, M. & Cotruvo, J. A. Therapeutic dose as the point of departure in assessing potential health hazards from drugs in drinking water and recycled municipal wastewater. Regul. Toxicol. Pharmacol. 60, 1–19 (2011).
Lopez, B., Ollivier, P., Togola, A., Baran, N. & Ghestem, J. P. Screening of French groundwater for regulated and emerging contaminants. Sci. Total Environ. 518–519, 562–573 (2015).
Du, B. et al. Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci. Total Environ. 466–467, 976–984 (2014).
Reichert, J. F., Souza, D. M. & Martins, A. F. Antipsychotic drugs in hospital wastewater and a preliminary risk assessment. Ecotoxicol. Environ. Saf. 170, 559–567 (2019).
Tan, C. et al. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 260, 1008–1016 (2013).
Fent, K., Weston, A. A. & Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 76, 122–159 (2006).
Steger-Hartmann, T., Länge, R. & Schweinfurth, H. Environmental risk assessment for the widely used iodinated X-ray contrast agent iopromide (ultravist). Ecotoxicol. Environ. Saf. 42, 274–281 (1999).
Matsushita, T. et al. Changes in mutagenicity and acute toxicity of solutions of iodinated X-ray contrast media during chlorination. Chemosphere 135, 101–107 (2015).
Peng, B. et al. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π–π interactions. Sci. Rep. 6, 31920 (2016).
Kerkez-Kuyumcu, Ö., Bayazit, Ş. S. & Salam, M. A. Antibiotic amoxicillin removal from aqueous solution using magnetically modified graphene nanoplatelets. J. Ind. Eng. Chem. 36, 198–205 (2016).
Yang, G. C. C., Tang, P. L. & Yen, C. H. Removal of micropollutants from municipal wastewater by graphene adsorption and simultaneous electrocoagulation/electrofiltration process. Water Sci. Technol. 75, 1882–1888 (2017).
Chen, H., Gao, B. & Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 282, 201–207 (2015).
Wu, S. et al. Adsorption of ciprofloxacin onto biocomposite fibers of graphene oxide/calcium alginate. Chem. Eng. J. 230, 389–395 (2013).
Tabrizian, P., Ma, W., Bakr, A. & Rahaman, M. S. pH-sensitive and magnetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high-efficiency removal of tetracyclines. J. Colloid Interface Sci. 534, 549–562 (2019).
Song, Z., Ma, Y. L. & Li, C. E. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total Environ. 651, 580–590 (2019).
Zhang, Y. et al. Removal of tetracycline and oxytetracycline from water by magnetic Fe3O4@graphene. Environ. Sci. Pollut. Res. 24, 2987–2995 (2017).
Tao, H., Liang, X., Zhang, Q. & Chang, C. T. Enhanced photoactivity of graphene/titanium dioxide nanotubes for removal of Acetaminophen. Appl. Surf. Sci. 324, 258–264 (2015).
Lee, G. et al. Fabrication of graphene-oxide/β-Bi2O3/TiO2/Bi2Ti2O7 heterojuncted nanocomposite and its sonocatalytic degradation for selected pharmaceuticals. Chemosphere 212, 723–733 (2018).
Rizzo, L., Fiorentino, A., Grassi, M., Attanasio, D. & Guida, M. Advanced treatment of urban wastewater by sand filtration and graphene adsorption for wastewater reuse: effect on a mixture of pharmaceuticals and toxicity. J. Environ. Chem. Eng. 3, 122–128 (2015).
Hiew, B. Y. Z. et al. Adsorptive removal of diclofenac by graphene oxide: optimization, equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 0, 1–13 (2018).
Seo, P. W., Bhadra, B. N., Ahmed, I., Khan, N. A. & Jhung, S. H. Adsorptive removal of pharmaceuticals and personal care products from water with functionalized metal-organic frameworks: remarkable adsorbents with hydrogen-bonding abilities. Sci. Rep. 6, 1–11 (2016).
Lee, X. J., Chemmangattuvalappil, N. & Lee, L. Y. Adsorptive removal of salicylic acid from aqueous solutions using new graphene-based nanosorbents. Chem. Eng. Trans. 45, 1387–1392 (2015).
Amalraj Appavoo, I., Hu, J., Huang, Y., Li, S. F. Y. & Ong, S. L. Response surface modeling of Carbamazepine (CBZ) removal by Graphene-P25 nanocomposites/UVA process using central composite design. Water Res. 57, 270–279 (2014).
Kurniawan, T. A., Yanyan, L., Ouyang, T., Albadarin, A. B. & Walker, G. BaTiO3/TiO2 composite-assisted photocatalytic degradation for removal of acetaminophen from synthetic wastewater under UV–vis irradiation. Mater. Sci. Semicond. Process. 73, 42–50 (2018).
Yanyan, L., Kurniawan, T. A., Ying, Z., Albadarin, A. B. & Walker, G. Enhanced photocatalytic degradation of acetaminophen from wastewater using WO3/TiO2/SiO2 composite under UV–VIS irradiation. J. Mol. Liq. 243, 761–770 (2017).
Akkari, M. et al. ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 156, 104–109 (2018).
Hassani, A., Khataee, A., Karaca, S. & Fathinia, M. Degradation of mixture of three pharmaceuticals by photocatalytic ozonation in the presence of TiO2/montmorillonite nanocomposite: simultaneous determination and intermediates identification. J. Environ. Chem. Eng. 5, 1964–1976 (2017).
Zhu, J. et al. Calcined layered double hydroxides/reduced graphene oxide composites with improved photocatalytic degradation of paracetamol and efficient oxidation-adsorption of As(III). Appl. Catal. B: Environ. 225, 550–562 (2018).
Jallouli, N., Elghniji, K., Trabelsi, H. & Ksibi, M. Photocatalytic degradation of paracetamol on TiO2nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem. 10, S3640–S3645 (2017).
Kanakaraju, D., Motti, C. A., Glass, B. D. & Oelgemöller, M. Solar photolysis versus TiO2-mediated solar photocatalysis: a kinetic study of the degradation of naproxen and diclofenac in various water matrices. Environ. Sci. Pollut. Res. 23, 17437–17448 (2016).
Arthur, R. B. et al. Photocatalytic degradation of ibuprofen over BiOCl nanosheets with identification of intermediates. J. Hazard. Mater. 358, 1–9 (2018).
Di, G. et al. Simultaneous removal of several pharmaceuticals and arsenic on Zn-Fe mixed metal oxides: combination of photocatalysis and adsorption. Chem. Eng. J. 328, 141–151 (2017).
Jallouli, N. et al. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 334, 976–984 (2018).
Huyen, T., Chi, T., Dung, N., Kosslick, H. & Liem, N. Enhanced photocatalytic activity of {110}-faceted TiO2 rutile nanorods in the photodegradation of hazardous pharmaceuticals. Nanomaterials 8, 276 (2018).
Bastami, T. R. & Ahmadpour, A. Preparation of magnetic photocatalyst nanohybrid decorated by polyoxometalate for the degradation of a pharmaceutical pollutant under solar light. Environ. Sci. Pollut. Res. 23, 8849–8860 (2016).
Štrbac, D. et al. Photocatalytic degradation of Naproxen and methylene blue: comparison between ZnO, TiO2 and their mixture. Process Saf. Environ. Prot. 113, 174–183 (2018).
Shargh, M. & Behnajady, M. A. A high-efficient batch-recirculated photoreactor packed with immobilized TiO2-P25 nanoparticles onto glass beads for photocatalytic degradation of phenazopyridine as a pharmaceutical contaminant: artificial neural network modeling. Water Sci. Technol. 73, 2804–2814 (2016).
Kumar, A. et al. Magnetically recoverable ZrO2/Fe3O4/chitosan nanomaterials for enhanced sunlight driven photoreduction of carcinogenic Cr(VI) and dechlorination & mineralization of 4-chlorophenol from simulated waste water. RSC Adv. 6, 13251–13263 (2016).
Shooshtari, N. M. & Ghazi, M. M. An investigation of the photocatalytic activity of nano α-Fe2O3/ZnO on the photodegradation of cefixime trihydrate. Chem. Eng. J. 315, 527–536 (2017).
Zheng, X. et al. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO 2 nanocomposite: strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 527, 202–213 (2018).
Derikvandi, H. & Nezamzadeh-Ejhieh, A. A comprehensive study on electrochemical and photocatalytic activity of SnO2-ZnO/clinoptilolite nanoparticles. J. Mol. Catal. A Chem. 426, 158–169 (2017).
Derikvandi, H. & Nezamzadeh-Ejhieh, A. Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 321, 629–638 (2017).
Yadav, M. S. P., Neghi, N., Kumar, M. & Varghese, G. K. Photocatalytic-oxidation and photo-persulfate-oxidation of sulfadiazine in a laboratory-scale reactor: analysis of catalyst support, oxidant dosage, removal-rate and degradation pathway. J. Environ. Manag. 222, 164–173 (2018).
Liu, X., Liu, Y., Lu, S., Guo, W. & Xi, B. Performance and mechanism into TiO2/Zeolite composites for sulfadiazine adsorption and photodegradation. Chem. Eng. J. 350, 131–147 (2018).
Wang, X., Jia, J. & Wang, Y. Combination of photocatalysis with hydrodynamic cavitation for degradation of tetracycline. Chem. Eng. J. 315, 274–282 (2017).
Khodadadi, M., Ehrampoush, M. H., Ghaneian, M. T., Allahresani, A. & Mahvi, A. H. Synthesis and characterizations of FeNi3@SiO2@TiO2 nanocomposite and its application in photo-catalytic degradation of tetracycline in simulated wastewater. J. Mol. Liq. 255, 224–232 (2018).
Gar Alalm, M., Ookawara, S., Fukushi, D., Sato, A. & Tawfik, A. Improved WO3 photocatalytic efficiency using ZrO2 and Ru for the degradation of carbofuran and ampicillin. J. Hazard. Mater. 302, 225–231 (2016).
Jakub, T., Robert, S. & Paweł, S. Investigation of the photolysis and TiO2, SrTiO3, H2O2-mediated photocatalysis of an antipsychotic drug loxapine—evaluation of kinetics, identification of photoproducts, and in silico estimation of properties. Chemosphere 204, 1–10 (2018).
Deng, F. et al. One-step in situ hydrothermal fabrication of octahedral CdS/SnIn4S8 nano-heterojunction for highly efficient photocatalytic treatment of nitrophenol and real pharmaceutical wastewater. J. Hazard. Mater. 340, 85–95 (2017).
Bansal, P., Verma, A. & Talwar, S. Detoxification of real pharmaceutical wastewater by integrating photocatalysis and photo-Fenton in fixed-mode. Chem. Eng. J. 349, 838–848 (2018).
Acknowledgements
This work was supported by the following funds: NSF BEINM Grant Number: 1705511; NSF CHE Grant Number: 1904472; the USDA National Institute of Food and Agriculture, AFRI Project No. 2018-67022-27969, the Welch foundation award number (E-2011-20190330) and the US Department of Interior, Bureau of Reclamation under the Desalination and Water Purification Research and Development Program (Agreement No. R16AC00123); NPRP grant no. [9-318-1-064] from the Qatar National Research Fund (a member of Qatar Foundation). The findings achieved herein are solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fanourakis, S.K., Peña-Bahamonde, J., Bandara, P.C. et al. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. npj Clean Water 3, 1 (2020). https://doi.org/10.1038/s41545-019-0048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-019-0048-8
This article is cited by
-
A hydrate-based zero liquid discharge method for high-concentration organic wastewater: resource recovery and water reclamation
npj Clean Water (2023)
-
Highly efficient visible light active ZnO/Cu-DPA composite photocatalysts for the treatment of wastewater contaminated with organic dye
Scientific Reports (2023)
-
An overview on the prevalence and potential impact of antimicrobials and antimicrobial resistance in the aquatic environment of India
Environmental Monitoring and Assessment (2023)
-
Synthesis and application of novel microporous framework of nanocomposite from trona for photocatalysed degradation of methyl orange dye
Environmental Monitoring and Assessment (2023)
-
Low-cost Posidonia oceanica bio-adsorbent for efficient removal of antibiotic oxytetracycline from water
Environmental Science and Pollution Research (2022)