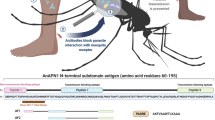
Abstract
Malaria begins when an infected mosquito injects saliva containing Plasmodium sporozoites into the skin of a vertebrate host. To prevent malaria, vaccination is the most effective strategy and there is an urgent need for new strategies to enhance current pathogen-based vaccines. Active or passive immunization against a mosquito saliva protein, AgTRIO, contributes to protection against Plasmodium infection of mice. In this study, we generated an AgTRIO mRNA-lipid nanoparticle (LNP) and assessed its potential usefulness as a vaccine against malaria. Immunization of mice with an AgTRIO mRNA-LNP generated a robust humoral response, including AgTRIO IgG2a isotype antibodies that have been associated with protection. AgTRIO mRNA-LNP immunized mice exposed to Plasmodium berghei-infected mosquitoes had markedly reduced initial Plasmodium hepatic infection levels and increased survival compared to control mice. In addition, as the humoral response to AgTRIO waned over 6 months, additional mosquito bites boosted the AgTRIO IgG titers, including IgG1 and IgG2a isotypes, which offers a unique advantage compared to pathogen-based vaccines. These data will aid in the generation of future malaria vaccines that may include both pathogen and vector antigens.
Similar content being viewed by others
Introduction
Human malaria can be caused by at least 5 different species of Plasmodium1. To prevent infection, vaccination is the most effective strategy. However, no highly effective malaria vaccine has been developed to date2,3,4. Several vaccine candidates are in clinical trials. One of the most advanced vaccines—just approved by the WHO in 2021—is a pathogen-based vaccine, RTS,S. RTS,S targets the Plasmodium falciparum circumsporozoite protein (PfCSP) in conjunction with immunostimulatory epitopes of the hepatitis B surface antigen and AS01 adjuvant5. The protection efficacy is moderate with a 30–50% reduction in infection in clinical trials, and the protection wanes over time5,6,7,8. In addition, the protection is limited to P. falciparum and there is still a risk of infection by other Plasmodium species—two weaknesses that limit the possibility of malaria eradication9. Furthermore, the widespread emergence of drug resistance in malaria parasites and insecticide resistance in mosquito vectors makes it imperative to develop strategies to identify vaccine targets that synergize with CSP, or that work independently of CSP.
Malaria is transmitted when an infected female Anopheles mosquito takes a bloodmeal and injects Plasmodium sporozoites along with saliva into the skin of the vertebrate host10,11. Following a mosquito bite, sporozoites travel within blood vessels to the liver, where they invade hepatocytes and establish infection12. Mosquito saliva contains biologically active molecules, which modulate the host immune and hemostatic responses13,14,15,16,17,18,19,20, and may also influence Plasmodium infection. There are reports of intimate interactions between proteins in the salivary glands of Anopheles mosquitoes and Plasmodium prior to, and after, migration of Plasmodium out of the mosquito21,22,23,24. It has also been shown that hyperimmune sera against mosquito salivary gland extracts may contain high titer antibodies against components in saliva that are not normally antigenic during a natural mosquito bite, and that such sera may provide some protection against Plasmodium transmission23. Specifically, we have identified at least one antigen, AgTRIO from Anopheles gambiae saliva, that elicits antibodies that decrease malaria infection in both a traditional mouse model and a humanized mouse model of malaria23. AgTRIO antibodies also offer synergistic protection when combined with a CSP monoclonal antibody23. These studies suggest that immunization against AgTRIO may contribute to protection against Plasmodium infection and could be useful in combination with pathogen immunogens.
mRNA vaccines are an important technological advance to prevent infection diseases, as demonstrated by the SARS-CoV2 pandemic25,26. Several studies using mRNA vaccines targeting PfCSP proved its usefulness, albeit without complete protection, in murine models of Plasmodium infection27,28,29. Furthermore, targeting of several antigens is readily feasible using mRNA vaccines29,30,31, which can potentially generate a more potent antimalarial response to enhance a PfCSP-based vaccine. We have produced a lipid nanoparticle (LNP) containing an mRNA encoding the mosquito saliva protein AgTRIO. Immunization of C57BL/6 mice with the AgTRIO mRNA-LNP vaccine decreased the initial Plasmodium burden in the liver and subsequent parasitemia, following exposure to Plasmodium-infected mosquitoes. Furthermore, the IgG response against AgTRIO can be boosted by mosquito bites, which could potentially solve an issue related to the duration of protection seen in pathogen-based vaccines. Based on these data, an AgTRIO mRNA-LNP vaccine may be useful as part of a multiantigen vaccine approach against malaria.
Results
AgTRIO mRNA-LNP vaccine generates AgTRIO-specific antibodies in mice
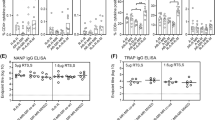
To determine if AgTRIO mRNA-LNP can generate AgTRIO specific antibodies, C57BL/6 mice were immunized three times with 10 μg of AgTRIO mRNA-LNP or control, Luciferase (Luc) mRNA-LNP (Fig. 1a). Two weeks after each injection, blood was collected and analyzed by an enzyme-linked immunosorbent assay (ELISA) using a purified E. coli derived AgTRIO (Fig. 1b). Immunization with AgTRIO mRNA-LNP generated significant IgG responses after the first booster immunization. To determine if immunization resulted in antibodies that recognized native AgTRIO, we collected salivary gland extracts (SGE) from female Anopheles gambiae and then coated plates with SGE for ELISA. Immunization with AgTRIO mRNA-LNP generated significant antibodies against SGE (Fig. 1c). In our recent study, we found that the IgG subclass of AgTRIO monoclonal antibody (mAb) may influence the protective effect against Plasmodium infection32. In particular, a murine IgG2a mAb against AgTRIO offered the most potent protection32. By ELISA, we found that the AgTRIO mRNA-LNP generated diverse antibody isotypes against AgTRIO, including IgG2a antibodies (Fig. 1d).
a Experiment scheme showing groups of C57BL/6 female mice injected with 10 μg of AgTRIO mRNA-LNP or control mRNA (Luc mRNA-LNP), and boosted twice, at two-week intervals. b Two weeks after each immunization, mice were bled. 1:2,500 and 1:12,500 dilutions of sera were examined for AgTRIO-specific IgG antibodies by ELISA using recombinant AgTRIO as the antigen. c 1:2,500 dilution of sera collected after the final were examined for AgTRIO-specific IgG antibodies against salivary gland extracts by ELISA. (Median ± IQR, p < 0.05 using the Mann Whitney U-test) d 1:2,500 dilution of sera were used to determine AgTRIO-specific IgG1, IgG2a and IgG2b. 1:500 dilution of sera were examined for AgTRIO specific IgG3 antibodies.
Immunization with AgTRIO mRNA-LNP alters mosquito-borne Plasmodium infection in mice
In our previous study, active immunization with AgTRIO or passive transfer with AgTRIO antisera offered partial protection against mosquito-borne Plasmodium infection in mice23.To determine whether immunization with AgTRIO mRNA-LNP can influence Plasmodium infection, we immunized C57BL/6 mice with 10 μg AgTRIO mRNA-LNP three times at two-week intervals. The control group received 10 μg Luc RNA-LNP. Three weeks after the last immunization, we challenged the mice using 3 P. berghei-infected A gambiae mosquitoes each. Mice immunized with AgTRIO mRNA-LNP had median hepatic levels of P. berghei reduced by 68% compared to those immunized with Luc mRNA-LNP (Fig. 2a, p = 0.048). To further examine the effects of this AgTRIO mRNA-LNP on the development of the later stages of malaria infection, such as parasitemia, we immunized an additional group of mice with either 10 μg AgTRIO mRNA-LNP or Luc mRNA-LNP thrice and then exposed the animals to three P. berghei-infected mosquitoes. 5 days after exposure to infected mosquito bites, there was significantly less parasitemia in the group received the AgTRIO mRNA-LNP vaccine (Fig. 2b, p = 0.012). Then we used Kaplan-Meier survival curves to demonstrate the kinetics of 0.01% parasitemia as the evidence of infection (Fig. 2c). Consistent with liver burden and Day 5 parasitemia results, there were less infected mice in the group given the AgTRIO mRNA-LNP vaccine (p = 0.036 by Log rank test). All these data demonstrate that immunization with AgTRIO mRNA-LNP can markedly reduce the hepatic Plasmodium burden in the murine model and contribute to protection against malaria.
C57BL/6 mice were immunized with 10 µg of AgTRIO mRNA-LNP or control mRNA (Luc) three times. 3 weeks after last boost, the immunized mice were exposed to 3 P. berghei-infected mosquitoes. a After 40 h, livers were dissected, and the Plasmodium infection level was determined by RT-PCR. (Median ± IQR, p < 0.05 using the Mann Whitney U-test). b In a separate experiment with mice vaccinated in the same way and exposed to infected mosquito bites, the parasitemia levels were determined at day 5 post infection. (Median ± IQR, p < 0.05 using the Mann Whitney U-test). c Kaplan–Meier survival curves were used to present the percent of uninfected mice. Infection was determined as 0.01% of parasitemia by flow cytometry. p = 0.036 by Log rank test between the two groups.
Humoral responses elicited by the AgTRIO mRNA-LNP can be enhanced by mosquito bites
Since AgTRIO is highly expressed in mosquito saliva23, we then examined whether the declining IgG responses that naturally occurs following any vaccination can—in the case of AgTRIO—be boosted by mosquito bites. We immunized C57BL/6 mice with three doses of AgTRIO mRNA-LNP and determined IgG titers at regular intervals. By 18 weeks after the beginning of the experiments (14 weeks after the final booster immunization), the IgG titers against AgTRIO (median: 0.45) had decreased by approximately 40% compared to 10 weeks after the beginning of the experiments (median: 0.716, Fig. 3a). Then, one group of mice was exposed to 10 uninfected mosquito bites weekly for 3 weeks and the control group of mice was not exposed to AgTRIO. When feeding on mice, there were no significant adverse effects noted during repeated mosquito bites. The anti-AgTRIO IgG concentration increased markedly in the group exposed to mosquito bites while the IgG levels continued to decrease in the control group (Fig. 3a, p = 0.03 by the Wilcoxon matched-pairs signed rank test). Next, we determined which IgG subclasses of AgTRIO specific antibody were boosted by mosquito bites. By ELISA, AgTRIO-specific IgG1 and IgG2a were significantly increased after mosquito bites (Fig. 3b). These results show that mosquito bites can maintain the IgG response against AgTRIO following immunization, including IgG isotypes that have been associated with protection32.
A group of mice was injected with 10 μg of AgTRIO mRNA-LNP or control mRNA (Gluc mRNA-LNP) and boosted twice over 4 weeks. Sera were collected at 10, 18, and 25 weeks after the beginning of the experiments. a 1:2,500 dilution of sera were examined for total AgTRIO-specific IgG antibodies by ELISA. b 1:2,500 dilution of sera were used to determine AgTRIO specific IgG1, IgG2a, and IgG2b. 1:500 dilution of sera were examined for AgTRIO specific IgG3 antibodies. (Median ± IQR, p < 0.05 using the Wilcoxon matched-pairs signed rank test). Red bar: exposed to 10 mosquitoes weekly after 18 weeks for three times. Blue bar: non-exposure.
Discussion
When probing for blood meal, mosquitoes secrete saliva to facilitate feeding, and sporozoites are simultaneously released into the skin10,11. Therefore, components of saliva can directly or indirectly affect the transmission of Plasmodium at the bite site and are potential targets for disease prevention23,33. In our previous studies, we demonstrated that passive or active immunization against one of the salivary proteins, AgTRIO, offers partial protection against P. berghei and P. falciparum transmitted by A. gambiae or A. stephensi mosquito bites23. In addition, we demonstrated that AgTRIO is highly secreted in mosquito saliva, and mosquitoes in which AgTRIO has been silenced by RNAi do not efficiently transmit sporozoites to murine skin compared with control mosquitoes22,23. This makes AgTRIO a suitable candidate for inclusion in a multi-component vaccine. In this study, we generated an AgTRIO mRNA-LNP that induces AgTRIO-specific humoral responses, including IgG2a isotype antibodies that have been associated with protection32. The AgTRIO mRNA-LNP significantly reduced Plasmodium infection in mice. Our finding indicates that immunization against AgTRIO, using an mRNA approach, can contribute to protection against Plasmodium transmission from the arthropod to a vertebrate host.
In our previous study, mice immunized with AgTRIO protein in complete Freund’s adjuvant and then boosted with AgTRIO protein with incomplete Freund’s adjuvant had a 50% lower liver burden compared to the control group23. In the same study, 66% percent of the AgTRIO-immunized mice had detectable parasitemia compared to 95% of the control mice23. While Freund’s adjuvant is useful for demonstrating proof of principle as it is highly immunostimulatory, and it cannot be given to humans because of its toxic side effects. Using the AgTRIO mRNA approach, 44.4% (4/9) of mice that received AgTRIO mRNA-LNP reached a 0.01% parasitemia level after challenge compared to 88.8% (8/9) of the control group. In our previous study, an IgG2a AgTRIO monoclonal antibody offered substantial protection against Plasmodium infection in mice compared to the other isotype monoclonal antibodies, and the protection depended on the Fc region32. In our unpublished results, there were AgTRIO specific IgG1 and IgG2b responses after immunization with AgTRIO protein and Alum adjuvant, but there was no significant IgG2a response. AgTRIO-mRNA LNP provides a robust IgG isotype response, including the generation of IgG2a antibodies, which may offer more effective protection against Plasmodium infection. All these results demonstrate that the AgTRIO mRNA-LNP approach may offer advantages compared with protein-based vaccines.
In addition, following an infection or vaccination, waning of protective immune responses occurs over time and is the major concern. As an example, for most COVID-19 vaccines, the humoral response diminishes more that 50% after 6 months34,35,36,37,38. To maintain protective humoral responses, a boosting vaccine is required. Similar to other vaccines, PfCSP-based vaccines exhibit waning protection over time6,7. While the humoral response towards AgTRIO declined after 6 months of immunization, antibodies could be boosted by mosquito bites. On the other hand, there is no significant humoral immune response against AgTRIO after repeat mosquito bites in either human or mice23, which indicates the boosting effect only occurs following priming by active immunization with AgTRIO. There were no significant adverse effects, including redness, swelling or an allergic reaction, noted during repeated mosquito bites after immunization with AgTRIO mRNA-LNP vaccine in our study. However, it is important to examine safety directly in human as results in mice do not always correlate directly with humans. This naturally acquired boosting effect gives AgTRIO-based immunization a unique advantage over traditional pathogen-based vaccine. In malaria endemic areas, persistent exposure to the vector could readily increase and maintain AgTRIO antibodies in AgTRIO mRNA-LNP vaccinated individuals. Overall, our study demonstrates that an mRNA vaccine strategy targeting a mosquito saliva protein can aid in the development of the next generation of vaccines against malaria, and may be used in conjunction with traditional, Plasmodium-based vaccines.
Methods
Animals
A. gambiae (4arr strain) mosquitoes were raised at 27 °C, 80% humidity, under a 12/12-h light/dark cycle and maintained with 10% sucrose under standard laboratory conditions in the insectary at Yale University. Swiss Webster and C57BL/6 mice were purchased from Charles River Laboratories. All animal experiment protocols were approved by the Yale University Institutional Animal Care & Use Committee (Protocol Number: 2022–07941). All Plasmodium infections were performed in biosafety level 2 animal facilities. All animal experiments followed the Guide for the Care and Use of Laboratory Animals by the National Research Council. In all experiments, mice were housed and cared for in the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited animal facilities in Yale University. For retro-orbital blood collection or mosquito feeding, mice were anesthetized with intraperitoneal injection of Ketamine/Xylazine (100 mg/10 mg per kg body weight). When the whole experiments were finished, mice were euthanized using a CO2 chamber in a manner consistent with AVMA guidelines for euthanasia. Following death, mice were subject to cervical dislocation as a secondary means to ensure death.
AgTRIO LNP mRNA synthesis
AgTRIO (AGAP001374) without the signal peptide were codon optimized between the 22th and 389th amino acids and then AgTRIO or luciferase (control) mRNAs were transcribed to contain 101 nucleotide-long poly(A) tails. N-1-methylpseudouridine (m1Ψ-5′)-triphosphate instead of UTP was used to generate modified nucleoside-containing mRNA. Capping of the in vitro transcribed mRNAs was performed co-transcriptionally using the trinucleotide cap1 analog, CleanCap. mRNA was purified by cellulose purification39. The mRNA was then encapsulated in LNPs using an aqueous solution of mRNA at acidic pH 4.0 and mixed with a solution of lipids40,41, consisting of an ionizable cationic lipid/phosphatidylcholine/cholesterol/PEG-lipid (50:10:38.5:1.5 mol/mol)28,29,30,31. For encapsulation, RNA was mixed with the lipids at a ratio of ∼0.05 (wt/wt). The LNP had a diameter of ∼80 nm as measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK) instrument, and is stored at −80 °C.
Active immunization
Five-week-old female C57BL/6 mice were immunized with either 10 µg of AgTRIO mRNA-LNP or control (Luc mRNA-LNP) and then given boosts with the same amount of vaccine 2 and 4 weeks later. Two weeks after each immunization, serum was collected from each mouse, and the IgG responses were tested using an ELISA to confirm antigen-specific antibodies. To determine if mosquito bite can boost the IgG, after the AgTRIO IgG titers were decayed, we let 10 uninfected mosquitoes feed on the mice. The control mice were not exposed to mosquitoes.
Plasmodium burden
The burden of Plasmodium in livers after sporozoite infection was determined by assessing the expression level of P. berghei 18 S rRNA, normalized to M. musculus hepatocyte nuclear factor 4 alpha, hnf4α, which has been used as housekeeping gene for liver tissue23,33. These data were presented as the copy number of the target gene per 10,000 copies of the housekeeping gene. TRIzol Reagent (Thermo Fisher Scientific) was used to purify total RNA from murine livers. All extractions followed the manufacturer’s protocols. The iScript RT-qPCR kit (Bio-Rad) was used to generate cDNA from RNA. Using iTaq SYBR Green Supermix (Bio-Rad), real time PCR was performed on a CFX96 real time platform (Bio-Rad). PCR involved an initial denaturation at 95 °C for 2 min, 50 cycles of 15 s at 95 °C, 15 s at 60 °C, and 20 s at 72 °C. Fluorescence readings were taken at 72 °C after each cycle. At the end of each reaction, a melting curve (60–95 °C) was checked to confirm the identity of the PCR product. The primers used for the expression of sporozoite genes are listed in Table 1. To assess parasitemia, 20 μl of blood was collected from all mice by retro-orbital bleeding. The percentage of parasitemia were determined by comparing the GFP-positive RBCs to the total RBC count by flow cytometry (CytoFLEX, ThermoFisher).
AgTRIO protein expression and purification
The AgTRIO genetic sequence without the region corresponding to the signal peptide has been cloned to the bacterial expression vector, pET21b (GE Healthcare)23. The AgTRIO-expressing plasmid will be transformed into BL21 chemically competent cells (Thermo Fisher Scientific). Expression of the AgTRIO protein will be induced with 0.1 mM IPTG at 17 °C for 24 h. The cells will be sonicated in PBS with complete EDTA-free Protease Inhibitors tablets (Roche). Soluble AgTRIO will be purified from the supernatant by a combination of sizing, ion-exchange and affinity chromatography. The expression of recombinant AgTRIO will be confirmed by immunoblots, which will be probed with the anti-AgTRIO serum raised against AgTRIO from our previous study23. Protein purity will be determined by SDS-PAGE and the concentration will be determined by the BCA Protein assay kit (Pierce, IL).
Enzyme-linked immunosorbent assays (ELISA)
Antigen-specific antibody responses were measured by ELISA42,43. To prepare the salivary gland extract (SGE), 20 salivary glands were collected in 100 μl PBS and the concentration of SGE protein was 0.2 mg/ml. The 96-well microplates were coated with purified AgTRIO (1 µg/ml) or SGE overnight. After blocking with a blocking buffer (PBS, 0.05% tween-20, and 1% bovine serum albumin), different dilution of mouse antisera were diluted in PBS and added to the wells and incubated at room temperature for 2 hours. After washing with washing buffer (PBS, 0.1% tween-20), horseradish peroxidase-conjugated goat anti-mouse antibody (Invitrogen, Cat. No. 62–6520) with 1:10000 dilution was used to detect total IgG. For the detection of IgG subclasses, horseradish peroxidase-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 heavy chain antibodies (Abcam, Cat. No. ab97240, ab97245, ab97250, ab97260) with 1:10,000 dilution was used to detect specific IgG subclass.
P. berghei infection
P. berghei (ANKA GFPcon, ATCC) were maintained by serial passage in 6–8 week old female Swiss Webster or C57BL/6 mice as previously described23. Briefly, Swiss Webster or C57BL/6 mice were challenged with P. berghei-infected red blood cells by intraperitoneal injection. A. gambiae mosquitoes then took a blood meal from the infected mice, when the parasitemia was approximately 5%. 17–24 days after P. berghei infection, the mosquitoes were sorted using the fluorescent signal of the salivary glands.
Statistical analysis
Data from at least three biological replicates were used to calculate medians for graphing purposes. Statistical analyses employed the Mann-Whitney test for unrelated groups and the one-sided Wilcoxon matched-pairs signed rank test for the same group with serial sampling to determine if the mosquito bites increased AgTRIO specific IgGs by ELISA. The difference and the data were presented as median with interquartile range (IQR). A p-value of <0.05 was considered statistically significant. The analysis, graphs, and statistics of all data were performed using Prism 9.0 software (GraphPad Software).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data associated with this study are present in the paper. Requests for resources, data, and reagents should be directed to the corresponding author, Dr. Y.-M.C. (yu-min.chuang@yale.edu). All unique reagents described in this study are available upon request to the corresponding author with a completed Materials Transfer Agreement.
References
World Health Organization. (World Health Organization, 2019).
Beeson, J. G. et al. The RTS,S malaria vaccine: current impact and foundation for the future. Sci. Transl. Med. 14, eabo6646 (2022).
Duffy, P. E. Current approaches to malaria vaccines. Curr. Opin. Microbiol. 70, 102227 (2022).
Sinnis, P. & Fidock, D. A. The RTS,S vaccine-a chance to regain the upper hand against malaria? Cell 185, 750–754 (2022).
Rts, S. C. T. P. et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365, 1863–1875 (2011).
Rts, S. C. T. P. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015).
Rts, S. C. T. P. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 11, e1001685 (2014).
Rts, S. C. T. P. et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 (2012).
Mitran, C. J. & Yanow, S. K. The case for exploiting cross-species epitopes in malaria vaccine design. Front Immunol. 11, 335 (2020).
Vanderberg, J. P. & Frevert, U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34, 991–996 (2004).
Yamauchi, L. M., Coppi, A., Snounou, G. & Sinnis, P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 9, 1215–1222 (2007).
Healer, J., Cowman, A. F., Kaslow, D. C. & Birkett, A. J. Vaccines to accelerate malaria elimination and eventual eradication. Cold Spring Harbor Perspect. Med. 7 https://doi.org/10.1101/cshperspect.a025627 (2017).
Stark, K. R. & James, A. A. Salivary gland anticoagulants in culicine and anopheline mosquitoes (Diptera:Culicidae). J. Med. Entomol. 33, 645–650 (1996).
Figueiredo, A. C. et al. Unique thrombin inhibition mechanism by anophelin, an anticoagulant from the malaria vector. Proc. Natl Acad. Sci. USA 109, E3649–3658 (2012).
Ribeiro, J. M., Rossignol, P. A. & Spielman, A. Role of mosquito saliva in blood vessel location. J. Exp. Biol. 108, 1–7 (1984).
Yoshida, S. et al. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood 111, 2007–2014 (2008).
Hayashi, H. et al. Anopheline anti-platelet protein from a malaria vector mosquito has anti-thrombotic effects in vivo without compromising hemostasis. Thrombosis Res. 129, 169–175 (2012).
Ronca, R. et al. The Anopheles gambiae cE5, a tight- and fast-binding thrombin inhibitor with post-transcriptionally regulated salivary-restricted expression. Insect Biochem. Mol. Biol. 42, 610–620 (2012).
Ribeiro, J. M., Nussenzveig, R. H. & Tortorella, G. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae. J. Med. Entomol. 31, 747–753 (1994).
Francischetti, I. M., Valenzuela, J. G. & Ribeiro, J. M. Anophelin: kinetics and mechanism of thrombin inhibition. Biochemistry 38, 16678–16685 (1999).
Arora, G. et al. Immunomodulation by mosquito salivary protein AgSAP contributes to early host infection by plasmodium. mBio 12, e0309121 (2021).
Chuang, Y. M. et al. Anopheles gambiae lacking AgTRIO inefficiently transmits Plasmodium berghei to mice. Infect. Immunity 87, https://doi.org/10.1128/IAI.00326-19 (2019).
Dragovic, S. M. et al. Immunization with AgTRIO, a protein in Anopheles saliva, contributes to protection against Plasmodium infection in mice. Cell Host Microbe 23, 523–535.e525 (2018).
Schleicher, T. R. et al. A mosquito salivary gland protein partially inhibits Plasmodium sporozoite cell traversal and transmission. Nat. Commun. 9, 2908 (2018).
Bettini, E. & Locci, M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines (Basel) 9, https://doi.org/10.3390/vaccines9020147 (2021).
Kremsner, P. G. et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 22, 329–340 (2022).
Kratochvil, S. et al. Vaccination in a humanized mouse model elicits highly protective PfCSP-targeting anti-malarial antibodies. Immunity 54, 2859–2876.e2857 (2021).
Mallory, K. L. et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines 6, 84 (2021).
Hayashi, C. T. H. et al. mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. NPJ Vaccines 7, 155 (2022).
Sajid, A. et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 13, eabj9827 (2021).
Arevalo, C. P. et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 378, 899–904 (2022).
Chuang, Y. M., Tang, X. D. & Fikrig, E. A mosquito AgTRIO monoclonal antibody reduces early Plasmodium infection of mice. Infect. Immun. 90, e0035921 (2022).
Chuang, Y. M. et al. The effects of a mosquito salivary protein on sporozoite traversal of host cells. J. Infect. Dis. 224, 544–553 (2021).
Falsey, A. R. et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 385, 1627–1629 (2021).
Pegu, A. et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373, 1372–1377 (2021).
Brunner, W. M. et al. Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers. Int J. Infect. Dis. 123, 183–191 (2022).
Chivese, T. et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery - a comprehensive systematic review and meta-analysis. Pathog. Glob. Health 116, 269–281 (2022).
Pooley, N. et al. Durability of vaccine-induced and natural immunity against COVID-19: a narrative review. Infect Dis. Ther. 1–21 https://doi.org/10.1007/s40121-022-00753-2 (2023).
Baiersdorfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int Ed. Engl. 51, 8529–8533 (2012).
Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).
Kisalu, N. K. et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 24, 408–416 (2018).
Chuang, Y. M. et al. Stringent response factors PPX1 and PPK2 play an important role in Mycobacterium tuberculosis metabolism, biofilm formation, and sensitivity to isoniazid in vivo. Antimicrob. Agents Chemother. 60, 6460–6470 (2016).
Acknowledgements
We thank Kathleen DePonte and Ming-Jie Wu for their technical assistance. These studies were supported by grants AI158615, AI142708, and AI145779 from the National Institute of Allergy and Infectious Diseases and the Howard Hughes Medical Institute Emerging Pathogens Initiative.
Author information
Authors and Affiliations
Contributions
Y.-M.C. and E.F. conceptualized and designed the study. Y.-M.C., S.A., and H.R. planned and performed animal immunizations, including bleeding and challenge for protection. Y-M.C and S.A. performed ELISA assays. M.-G.A. and D.W. produced mRNA, produced empty LNP, encapsulated mRNA into LNP, and performed physicochemical characterization of LNPs. Y.-M.C., M.L., D.W., and E.F. wrote the paper with the help of co-authors.
Corresponding author
Ethics declarations
Competing interests
E.F. has an equity interest and serves as a consultant for L2 Diagnostics. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chuang, YM., Alameh, MG., Abouneameh, S. et al. A mosquito AgTRIO mRNA vaccine contributes to immunity against malaria. npj Vaccines 8, 88 (2023). https://doi.org/10.1038/s41541-023-00679-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-023-00679-x