Abstract
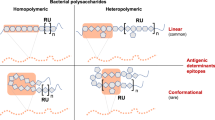
The Group A Carbohydrate (GAC) is a defining feature of Group A Streptococcus (Strep A) or Streptococcus pyogenes. It is a conserved and simple polysaccharide, comprising a rhamnose backbone and GlcNAc side chains, further decorated with glycerol phosphate on approximately 40% GlcNAc residues. Its conservation, surface exposure and antigenicity have made it an interesting focus on Strep A vaccine design. Glycoconjugates containing this conserved carbohydrate should be a key approach towards the successful mission to build a universal Strep A vaccine candidate. In this review, a brief introduction to GAC, the main carbohydrate component of Strep A bacteria, and a variety of published carrier proteins and conjugation technologies are discussed. Components and technologies should be chosen carefully for building affordable Strep A vaccine candidates, particularly for low- and middle-income countries (LMICs). Towards this, novel technologies are discussed, such as the prospective use of bioconjugation with PglB for rhamnose polymer conjugation and generalised modules for membrane antigens (GMMA), particularly as low-cost solutions to vaccine production. Rational design of “double-hit” conjugates encompassing species specific glycan and protein components would be beneficial and production of a conserved vaccine to target Strep A colonisation without invoking an autoimmune response would be ideal.
Similar content being viewed by others
Introduction
Vaccination is considered one of the most successful health interventions known to man1. Antibiotics and alternative therapeutics such as intravenous immunoglobulins are not suitable to control Streptococcus pyogenes or Group A Streptococcus (Strep A) infections at the population level or stop transmission within communities2. The emergence of antimicrobial resistance (AMR), along with the persistence of penicillin sensitivity, has led the WHO and CDC to both highlight vaccines as urgent safeguards against AMR3,4. Safe and efficacious Strep A specific vaccines are required for better control of Strep A related morbidity and mortality. Strep A is a major global pathogen, with disease manifestations ranging from Strep throat pharyngitis and impetigo to scarlet fever and invasive diseases such as toxic shock syndrome and necrotising fasciitis. These infections are often fast progressing and highly contagious, further emphasising the need for a vaccine. Invasive infections have high mortality and morbidity, and secondary diseases from autoimmune sequelae such as rheumatic heart disease (RHD) result in significant disease adjusted life years (DALYs), particularly in Low- and Middle-Income Countries (LMICs)5. In recent years there has further been a re-emergence of scarlet fever particularly noted in the UK and Europe, with particular concern in the current season with rising rates of sepsis associated with these infections6,7. There is therefore an urgent need globally to develop a vaccine against Strep A. With humans as the sole natural host there is also potential to eradicate the pathogen if transmission can be blocked.
Vaccine development against any pathogen is a complex and lengthy process, however Strep A vaccine development has had arguably a more complicated history with many challenges and hurdles. Strep A vaccine development has an official impeded status recognised by the WHO, due to many factors loosely relating to bacterial and host amongst others. First and foremost, Strep A is a complex pathogen with high antigen genomic heterogenicity due to recombination events, possessing different virulence factor expression profiles between strains, as well as complicated diverse global epidemiology of circulating Strep A serotypes. Strep A serotypes are based on variations in the major virulence factor, the M protein, with numerous variant serotypes globally and no consistent serotype observed in geographic regions or correlation with disease manifestations. At the genomic level there are even greater variations in the M-type, or emm type, with >200 variants. A recent genomic study showed that no single protein investigated during vaccine development has been 100% conserved between all the analysed Strep A isolates8. This has led to difficulty targeting a particular protein to cover all serotypes causing infections globally as a universal vaccine candidate. Gene exchange and single nucleotide polymorphisms (SNPs) causing protein sequence variation within vaccine candidates, such as within the most progressed NTD (N-terminal domain) M protein-based vaccines leads to the requirement of multicomponent inclusion to obtain acceptable levels of cross protection. Additionally broad spectrum of disease makes finding an effective long-term vaccine strategy challenging. Disproportionate Strep A disease burden and diverse serotype prevalence within LMICs, related to vaccine efficacy and protective coverage predication complicates testing when deciding on vaccine clinical endpoints generally and within these settings.
Strep A can be distinguished from other beta-haemolytic streptococci species by Lancefield serotyping. This technique is based on the identification of type-specific surface exposed carbohydrates that bind to specific antibodies9, with S. pyogenes being named Strep A due to the presence of the conserved group A carbohydrate (GAC) on its surface.
The group A carbohydrate (GAC)
Group A carbohydrate (GAC) is conserved across all Strep A strain cell surfaces8,10 and shown to be a key survival determinant10. GAC is abundant making up 40–60% of the total cell wall mass11, functioning to provide structural support as an environmental barrier, maintain cell morphology and enable cellular division12. In addition, GAC is also a major virulence determinant, providing resistance to zinc toxicity and resistance to neutrophil meditated killing10,13,14.
GAC polymers have an average molecular mass of 8.9 ± 1.0 kDa, corresponding to 18 repeating units14, though different purification methods result in varying average sizes15. GAC is made up of a linear polyrhamnose backbone with alternating GlcNAc sidechains, with a trisaccharide repeating unit of [3)α-l-Rhap(1→2)[β-d-GlcpNAc(1→3)]α-l-Rhap(1→3)]n (Fig. 1)14,16. GlcNAc is attached to the 2-hydroxyl linked rhamnose residue17 extending out to the periphery from the rhamnose helix18. Approximately 25–30% of the GlcNAc residues on GAC polymers contain glycerol phosphate (GroP) modifications, specifically on the C6-hydroxyl group. This polysaccharide modification has remained elusive for some time and the specific function has not been fully characterised. It may, however, contribute to the immune evasion activity through resistance to zinc toxicity19. A Strep A mutant devoid of GlcNAc, and therefore the GroP modification, was more susceptible to killing by human whole blood, as well as in the presence of purified human neutrophils, shown mechanistically to be due to greater binding of cationic human defence peptides, cathelicidin, LL-37 to the mutant polysaccharide. This loss of GlcNAc also seems to reduce the binding of cationic bactericidal enzyme human Group IIA secreted phospholipase A2 to mutant Strep A cell surfaces19,20.
GAC is composed of a polyrhamnose backbone and GlcNAc side chains, decorated with glycerol phosphate. The wildtype repeating unit is [3)α-l-Rhap(1→ 2)[β-d-GlcpNAc(1 → 3)]α-l-Rhap] for 70% of the repeating units (i), and 30% modified on GlcNAc side chains with glycerol phosphate (ii) [3)-α-l-Rhap-(1 → 2)[β-d-GlcpNAc6P(S)Gro-(1 → 3)]-α-l-Rhap-(1 → 3)]. ΔgacI mutants and certain GAS strains passaged in mice have the repeating structure [3)-α-L-Rhap-(1 → 2)α-L-Rhap-(1 → 3)-α] deficient in GlcNAc sidechains (iii).
Branched polysaccharides composed of rhamnose and GlcNAc sidechains have been shown to be immunogenic, evidenced by rabbit and human antiserum21,22, computer simulations18,23,24 and by NMR25 techniques to demonstrate mAb and GAC interaction. The GlcNAc sidechain is predicted to play a role in human specific pathogenicity or immune evasion strategies. However, a consideration with using GAC as a vaccine component is the potential generation of autoimmune antibodies due to the presence of GlcNAc in many human glycan structures and similarities between GlcNAc and components of the extracellular matrix (ECM) in humans26,27. Clinically this has been observed with acute rheumatic fever (ARF) patients having 2- to 3- fold higher anti-GAC antibody titres at the point of infection compared to patients with pharyngitis28, as well as longer lived anti-GAC antibody populations in patients which have rheumatic heart disease (RHD)29. Isolated monoclonal antibodies from ARF patients appear to only recognise wildtype GAC, not cross reacting with mutated GAC which does not contain GlcNAc sidechains10, demonstrating the antibody specificity to GlcNAc epitopes. The role of GlcNAc is, however, controversial with the majority of autoimmune responses attributed instead to the M-protein in the manifestation of ARF and RHD30.
Antibody binding experiments have shown that GAC polymers are mainly localised to the outer surface of the cell wall11. This surface localisation, conservation between strains and protective properties makes GAC and derivatives of interest as a polysaccharide component of a glycoconjugate vaccine.
GAC immunogenicity and considerations for carrier proteins
Immunological studies have revealed that GAC is accessible to antibody binding14,31,32, with affinity-purified anti-GAC antibodies showing opsonic properties specifically against M3, M6, M14 and M28 serotypes32. Host infection with Strep A induces circulating GAC specific antibodies, which may be slow to generate initially, but are thought to gradually increase with age, peaking in adolescents33. Some studies suggest the GlcNAc sidechain portion of the polymer32, particularly the branch points within the trisaccharide repeat structure, to be important in recognition and generation of opsonophagocytic IgG antibodies22,32,34. However, more recent data has shown antibodies also recognise rhamnose epitopes within the polymer backbone10,35. The entire trisaccharide of Rha-Rha-GlcNAc has been demonstrated to bind to monoclonal antibodies by several techniques including 1D-TOCSY NMR25. Further, that the branch structure itself is important, but GlcNAc can be substituted with rhamnose for the same response36. Titres of anti-GAC antibodies are thought to correlate with reduced Strep A infection incidence in adolescents compared to young children, suggesting that anti-GAC antibodies may be important in long lasting immunity to Strep A infection. This justifies the argument that carbohydrate-based antibodies are a suitable approach towards protection from disease. This concept was highlighted in a study of Mexican children which showed high titres of GAC specific IgG antibodies correlated with protection against throat carriage31.
Polysaccharide antigens alone are T cell independent antigens, containing a repetitive structure which can be recognised and cross-linked by B cell receptors (BCR) on B cells, but provide no T cell epitopes. This leads to B cell differentiation into plasma cells which secrete antibodies directed against the polysaccharide epitopes but with reduced immunological memory particularly in infants37,38,39,40. Conjugation of polysaccharide to a carrier protein enables the protein moiety to provide such T cell epitopes through presentation on MHC Class II complexes to generate CD4+ T cell help41. This strengthens and improves immune response longevity by increasing polysaccharide specific antibody levels, affinity maturation and proliferation of polysaccharide-specific B cells from memory pools, resulting in IgM to IgG isotype switching leading to higher antibody avidity42.
Protein carriers selected for inclusion in glycoconjugate vaccines are ideally themselves immunogenic. Suitable protein carriers must therefore enable induction of effective anti-polysaccharide immune responses and be compatible with conjugation techniques, being safe and produced at high yields and low costs43,44,45. Traditionally, carrier proteins included in currently licenced glycoconjugate vaccines were heterologous to the organism against which you wish to vaccinate44,46. There are currently five carrier proteins in licenced glycoconjugate vaccines; Tetanus Toxoid (TT), Diphtheria Toxoid, CRM197 (a non-toxic mutant of diphtheria toxin), recombinant E. coli produced Haemophilus influenzae protein D and outer membrane protein complex of serogroup B meningococcus44,46. These traditional carriers have been successfully included in licenced vaccines due to their compatibility with well-characterised conjugation chemistries, and their ability to induce effective long lasting anti-polysaccharide immune responses45. Recently, however there has been a drive for new carriers to be investigated44, as data suggests that repeated immunisation with the same classical carrier for different glycoconjugate vaccines in some cases dampens immunological potency and efficacy47,48,49,50. This is mediated by inhibition of polysaccharide antibody responses by carrier-specific B cells and suppressor T cells due to pre-existing carrier protein immunity referred to as carrier-induced epitopic suppression49.
Currently, the potential benefit from protective antibodies directed against the protein component has not been fully exploited. In addition to avoiding carrier-specific epitopic suppression, vaccines containing both pathogen specific polysaccharide and protein antigens provides a “double-hit” approach45,51,52, which may achieve broader immunity44. There have been several studies on conserved Strep A protein antigens as vaccine candidates, often included in multicomponent vaccines and these have potential to be utilised as glycoconjugate carrier proteins35,53,54,55,56,57,58. Rational protein antigen design to improve pathogen specific immune responses, in addition to providing effective T helper cell function to improve polysaccharide responses, is a key consideration for a Strep A glycoconjugate vaccine.
Chemical conjugation for glycoconjugates
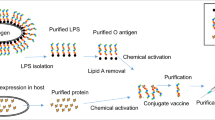
Glycoconjugate vaccines can be synthesised through several conjugation methodologies, and the method choice is also an important consideration. This has traditionally been through chemical conjugation, which requires extraction and purification of polysaccharide directly from the organism’s cell wall or capsule against which you wish to protect. It also requires purification of recombinantly expressed protein carriers before covalent attachment of the two components can occur (Fig. 2a–c). Conjugation chemistries can utilise naturally present reactive groups or alternatively add in either cross-linking reagents to artificially introduce compatible reactive groups or chemically modify components through activation for attachment. Both these modifying reactions can improve conjugation efficiency but can also damage confirmational epitopes in the process. Chemical modification can be through reducing end selective activation, or random multiple activation along polysaccharide chains59 (Fig. 2d). The choice of chemical conjugation approach is often governed by structure, size, and composition of vaccine components60. For example, larger polysaccharides are usually randomly activated, and smaller polysaccharides activated at the reducing end to preserve protective epitopes59. Some selectivity can be achieved by modulating component stoichiometry61, targeting or introducing specific non-natural amino acids on the protein facilitating site directed attachment62. Such selective or milder approaches can be important for carrier proteins with a dual purpose to maintain protective B cell epitopes.
Polysaccharide (PS) extracted from the native organism, e.g., GAC from Strep A cells (a), and recombinant protein carriers expressed and purified from E. coli cells (b). Extracted PS and recombinant protein carriers containing compatible reactive groups are conjugated together using a compatible cross-linking chemical reagent (c) yielding heterogenous glycoconjugate vaccine candidates depending on the selected approach (d). Schematic shows different approaches yielding different glycoconjugate species, with reducing end chemistries leading to terminal single ended glycoconjugate products (sun-like structures), and random activation chemistries yielding cross-linked mesh-like structures of higher molecular weights with several attached protein - polysaccharide molecules.
Progress towards a GAC glycoconjugate vaccine
There have been several studies investigating GAC or components of GAC as vaccine candidates. In this section we briefly summarise key studies and advancements from selected studies, while a list of all published studies on this topic is provided (Table 1). Early studies focussed on GAC immunogenicity when conjugated to classical carrier proteins to determine the anti-polysaccharide response and the immunological epitopes for protection. These included synthetic polymers, native GAC and GAC mutated to contain no GlcNAc sidechains (GACPR). The move towards modified GAC aimed to mitigate the potential risks of generating an autoimmune response26,27. There has been a renewed interest in GAC as a vaccine component, with studies in the last five years building on incorporation of GAC into multicomponent formulations or “double-hit” glycoconjugates. To date, these studies have all made use of chemical methods for conjugation (Table 1).
An early study by Sabharwal et al., utilised native GAC conjugated to TT and showed protection of mice from intranasal colonisation and intraperitoneal lethal challenge with two different M Strep A serotypes31. This study was built on by Kabanova et al. where synthetic polymers of varying lengths were conjugated to CRM197 to investigate size dependent immunogenicity14. These were also able to generate protective antibodies and demonstrated that a hexasaccharide (composed of four rhamnose sugars, two of which were decorated with a GlcNAc sidechain) was the minimal epitope able to invoke a robust immune response. This reiterated earlier observations that a hexasaccharide was key to the natural immune response to GAC22 and was further validated by a study that demonstrated equivalent immunogenicity from a hexasaccharide hapten as native GAC when conjugated to TT63.
To address potential vaccine safety concerns, van Sorge et al., used modified GAC devoid of GlcNAc (GACPR) extracted from a genetically modified Strep A strain and conjugated to S. pneumoniae protein SP043510. They showed that antibodies generated from the vaccine promoted phagocytic killing of multiple Strep A serotypes and protected mice against systemic infection following passive immunisation with rabbit anti-GAC antisera10. The conjugation methodology was unique using a Multiple Antigen Presenting System (MAPS)64, incorporating chemical methods for biotinylation of GAC to allow for a non-covalent bond between GAC and SP0435 containing a biotin-binding domain. This genetically mutated GAC was also able to show disease reduction with partial protection from bacteraemia and skin infections in further studies56, without any cross-reactivity with human heart or brain tissue lysates35. Further justification for modified GAC has been provided by a recent study using self-adjuvanting lipopeptides36. Using synthetic repeating unit epitopes this study demonstrated that the GlcNAc residue was not essential for a robust immune response to GAC. Instead, replacement with a third rhamnose could substitute for this structurally related immunogenicity, with a higher anti-GAC IgG response and equivalent or higher killing activity on Strep A strains than the wildtype repeating unit. These studies laid the foundation for interest in GAC as a viable broadly protective vaccine antigen.
The first study with a “double-hit” approach to a GAC glycoconjugate demonstrated that conjugation of modified GAC to Strep A protein ADI (arginine deiminase protein) gave a robust immune response to both components, without a loss of immunogenicity to the protein component compared with a protein alone response56. Following this, conjugation of synthetic polysaccharides (tri-, hexa- and nonasaccharides) to C5a peptidase (ScpA) demonstrated superior functional immunity of antibody binding to Strep A and opsonophagocytic killing over conjugation to CRM197 or TT with hexasaccharide haptens, and equivalent immunogenicity between carriers with a nonasaccharide65,66.
Recent studies have highlighted the need to preserve protein epitopes when using species specific protein carriers. This was demonstrated by di Benedetto et al., where the benefits and disadvantages of different conjugation chemistries was investigated with native GAC conjugated to CRM197. This revealed that random conjugation demonstrated an equivalent anti-GAC IgG response to selective conjugation but affected the anti-protein response57. Despite high anti-GAC IgG titres from random conjugation of GAC to three Strep A proteins SLO, SpyAD and SpyCEP, the anti-protein responses were significantly impacted and resulted in a loss of functional immunity, such as neutralisation of SpyCEP IL-8 cleavage activity. Random conjugation produces a mesh-like structure, which has been demonstrated to be effective for anti-polysaccharide responses, but likely will mask immunologically relevant protein epitopes. Selective conjugation, meanwhile, is likely to be the most effective method for “double-hit” glycoconjugates, as demonstrated with a site-specific click-chemistry approach to produce a SpyAD-GACPR conjugate35. In this study, four lysine residues were replaced with a non-native amino acid, p-azidomethyl phenylalanine (pAMF) in a cell-free expression system, as target sites for dibenzocyclooctyne (DMCO) derivatised modified GAC (conjugated through 1-Cyano-4-dimethyl aminopyridinium tetrafluoroborate, CDAP, chemical conjugation). IgG titres against SpyAD were high for both conjugate and protein alone, and improved shifts in antibody binding by flow cytometry for SpyAD-GACPR compared with SpyAD alone. Combination of SpyAD-GACPR with SLO and C5a peptidase resulted in improved protection in both passive and active immunisation murine challenge models compared with components alone, with no cross-reactivity observed to human heart lysates. This site-directed click chemistry approach was further investigated with SLO as a carrier protein for GACPR62. Conjugates showed high antibody titres for GAC in all conjugates produced, and similar antibody titres for SLO in all conjugates produced compared with SLO variants alone. A specific SLO neutralising assay was not conducted to demonstrate retained functional neutralising antibodies, but an in vivo model with intraperitoneal challenge showed significant protection with the SLO-GACPR conjugate compared with immunisation with SLO and CRM197-GACPR62. This was a successful demonstration that site-specific conjugation of GAC to a Strep A carrier protein provides effective antibody responses to both components and superior protection in vivo.
Though promising, the conjugates here relied on costly chemical conjugation, which has limitations such as technical challenges, low product yields, and batch to batch variation67. Alternative conjugation methods and vaccine structures are therefore of benefit to Strep A vaccinology to reduce costs and improve manufacturing consistency.
Protein Glycan Coupling Technology
In recent years the development of Protein Glycan Coupling Technology (PGCT) or bioconjugation has provided an alternative and in some cases superior method to chemical conjugation68,69. PGCT has the potential to simplify glycoconjugate production, and several vaccines using this technology are in clinical trials70,71,72,73.
PGCT relies on the innate capability of certain prokaryotic cells to synthesise polysaccharides and attach them to proteins as a post-translational modification. Using PGCT the polysaccharide attachment on the carrier protein can be an asparagine (N-linked) or serine/threonine (O-linked) amino acid glycosylation74,75. N-linked glycosylation, specifically using oligosaccharyltransferase (OST) PglB from Campylobacter jejuni (CjPglB), is the most applied bioconjugation approach75. The key experiment for glycoengineering and vaccine development was the demonstration that the pglB operon could be cloned and fully expressed in E. coli cells for functional recombinant glycosylation76.
Polysaccharides for exploitation of this system, are first synthesised onto an undecaprenol-pyrophosphate (Und-PP) lipid linker attached to the cytoplasmic leaflet of the inner membrane. A flippase enzyme is then responsible for the translocation of the fully synthesised polysaccharide across to the periplasm, where it can subsequently be recognised by PglB or other OST and attached onto a recognition sequon on a given acceptor protein (Fig. 3)77,78. The protein recognition sequon for N-linked glycosylation is D/E-X-N-X-S/T, where X represents any amino acid except proline, and positive D/E and S/T amino acids are at the ±2 positions, pivotal in locating asparagine (N) as the acceptor amino acid79. Unlike eukaryotic glycosylation, bacterial N-linked glycosylation occurs after protein folding, therefore to be accessible to PglB engineered Glycotags of the D/E-X-N-X-S/T sequon can be added to the N- and C- termini of carrier proteins allowing enhanced glyco-modification in vaccine design80,81.
a Plasmids containing genes encoding polysaccharide (PS) biosynthesis (green), the protein acceptor (blue) containing a glycotag (yellow star), and the oligosaccharyltransferase (OST) PglB (red) are co-transformed into E. coli host cells. b Bioconjugates are produced as follows; (1) The PS biosynthesis locus is expressed and built onto undecaprenol-pyrophosphate (Und-PP) lipid linkers within the inner membrane. (2) The PS is flipped from the cytoplasm to the periplasm by a specific flippase enzyme. (3) Synthesised carrier proteins are exported to the periplasm through the Sec secretion system. (4) In the periplasm both the PS, and the carrier protein containing a specific glycotag can be recognised by the PglB OST enzyme. PglB transfers the PS from Und-PP onto the asparagine residue within the glycotag D/E-X-N-X-S/T motif on the fully folded carrier protein, resulting in protein glycosylation. (5) An inexhaustible supply of glycoproteins can be subsequently purified from E. coli cells.
PGCT is a feasible alternative to chemical conjugation78, with benefits such as E. coli systems producing inexhaustible fully synthesised recombinant polysaccharide resources, and readily purified glycoproteins at reduced costs and improved yields82. This great promise is currently limited by PglB specificity, such that only polysaccharides with a reducing end containing an acetamido group at the C2 position are permissive for wildtype enzyme transfer79,83. However, this limitation can be mitigated by modification of the PglB enzyme by directed evolutionary mutagenesis to improve transfer compatibility and efficiency84,85,86.
Alternative OST enzymes are also widely used for N-linked glycosylation74,77,87,88, and O-linked OST enzymes, such as PglL and PglS for glycans with galactose89,90 and glucose end groups91,92. As the discovery and understanding of bacterial OSTs increases there will be further opportunities to develop PGCT for custom designed glycoconjugate vaccines such that theoretically almost any glycan could be coupled to any protein. In addition, PGCT provides the opportunity to couple multiple glycans at precise positions on a given carrier protein, which has been demonstrated to increase glycoconjugate vaccine efficacy93.
Furthermore, PGCT offers benefits such as minimal alteration to the protein carrier and target recombinant polysaccharide, dissimilar to chemical conjugation approaches which are often harsher in their attachment method.
GAC biosynthesis and exploitation of recombinant polyrhamnose structures
GAC polymers are encoded by a conserved 12-gene cluster termed gacA-L8,10 (Fig. 4a). The operon is highly conserved with one study finding that 2017 of 2083 tested Strep A genomes had >70% DNA sequence similarity for the entire 12-gene cluster8 supporting observations from a smaller dataset10. Within the operon the first seven genes (gacA-G) encode biosynthesis of the polyrhamnose backbone, conserved across other streptococci groups, specifically A, B, C and G94, whilst 3 genes (gacI-K) are known to be implicated in GlcNAc sidechain attachment and modification10. To date not all genes have been fully characterised, and some are believed not to be essential to survival due to frameshift mutations in some Strep A genomes8,13. However, the rhamnose encoding genes10 and the availability of l-rhamnose substrates12 have been shown to be essential to Strep A survival.
a Schematic representation of the gac operon (gacA-L) in Strep A. Horizontal arrows represent each gene designation with colour denoting predicted gene function. Green, polyrhamnose biosynthesis; blue, GlcNAc biosynthesis. b Schematic diagram of GAC biosynthesis. GAC biosynthesis is initiated on lipid linked GlcNAc attached to the inner leaflet of the periplasmic membrane for polyrhamnose synthesis catalysed by rhamnosyltransferase enzymes (GacBCFG). After polymerisation, the polyrhamnose backbone is flipped to the outer leaflet by an ABC transporter (GacDE complex) before GlcNAc (GacL) and glycerol phosphates (GacH) are transferred to the polyrhamnose backbone as a sidechain modification. A LytR-CpsS-Psr (LCP) phosphotransferase protein is hypothesised to attach GAC to peptidoglycan via a phophodiester bond17.
There is a strong case for the exploitation of GAC with PGCT. A rhamnose backbone structure related to GAC in Streptococcus mutans has previously been expressed and exported to the surface of E. coli cells, altering the LPS profiles, with similarity shown between native rhamnose biosynthesis pathways in S. mutans and the reconstituted pathways expressed and synthesised in E. coli host cells95. Recently, Castro et al followed a similar recombinant approach, where E. coli cells encoded the genes for GAC rhamnose backbone biosynthesis and produced Outer Membrane Vesicles (OMVs) loaded with the carbohydrate96. Their work has shown that the GAC rhamnose backbone can be built successfully in E. coli with the correct chemical structure and produces antibodies in mice and rabbits that can target Strep A serotypes. This system can now serve as a foundation for PGCT with Strep A antigens.
GAC has a GlcNAc at the reducing end, suggesting it would be suitably recognised by CjPglB, however the following sugar in the chain, rhamnose, is attached to the GlcNAc by a β-1,4 linkage by GacB94. This particular linkage may not be recognised or transferred by CjPglB efficiently, as demonstrated by studies assessing CjPglB transfer capability of a GlcNAc β-1,4 GlcNAc disaccharide attached to either a non-native eukaryotic isoprene lipid linker97, or a native Und-PP lipid linker98,99. Therefore, alternative strategies may be required to transfer Strep A rhamnose polymers by PGCT.
Concluding remarks
The global imperative to develop a Strep A vaccine has resulted in a burgeoning field of GAS targeted glycoconjugate vaccines. Following initial studies demonstrating a robust immune response to GAC, focus has shifted to “double-hit” glycoconjugates incorporating species specific carrier proteins along with GAC, either native or modified to remove GlcNAc side chains. Studies have suggested that milder selective approaches are more appropriate for “double-hit” glycoconjugates to prevent shielding of protein immune epitopes, as they are less likely to disrupt the protein structure and conformation, ensuring its role as a T cell epitope carrier, as well as a protective antigen itself. However, such approaches may reduce overall polysaccharide attachment due to limiting the region of the chain which is activated and available for attachment100. This in some respects is similar to bioconjugation where protein carriers are engineered with glycotags at the N- and C- termini, which is more likely to preserve the protein’s stability and structure, and therefore B and T cell epitopes81. Investigation of the use of bioconjugation for generation of a Strep A glycoconjugate would help lead the way to a cost-effective Strep A vaccine.
Studies with Generalised Modules for Membrane Antigens (GMMA) showed that different mechanisms of presentation, specifically the structure and chain length of the polysaccharide antigen, dictates the dominance of T cell responses. Based on a S. typhi capsule glycoconjugate, shorter polysaccharide antigens appear to require more T cell help for robust immune stimulation compared to glycoconjugates containing longer polysaccharide antigens101. Therefore, carrier proteins which can strongly stimulate T cell help responses may be necessary for future vaccines with GAC. Such T cell activity is important in the magnitude as well as response duration, as without T cell activity apoptosis of polysaccharide-specific B cells in the spleen and depletion of polysaccharide-specific B cells in the bone marrow can occur leading to hypo-responsiveness101. Such impact of polysaccharide size on B and T cell interactions is known to be antigen-specific41,100, requiring future studies to focus on deciphering the best protein carrier to induce T cell activity and enable GAC directed memory responses.
There has also been increasing interest in novel non-protein carriers for GAC. Gold nanoparticles have been shown to successfully present GAC for recognition by immune sera, though its own intrinsic immunogenicity was not assessed102. Self-adjuvating lipopeptides have shown great promise with GAS peptides and have also shown a robust immune response against GAC36, with recent studies taking this further showing strong immune responses to conjugated GMMA103 and recombinant OMVs96. Such technologies will help to build up an understanding of a protective immune response to GAC and derivatives, and push for a low-cost solution to the global need for a vaccine. Recombinant technology, particularly PGCT, may answer this need with its low-cost, inexhaustible and renewable supply along with its ability for rational design82. Carrier proteins and glycans can easily be manipulated such that glycosylation sites can be precise, glycan number increased as needed and chain lengths potentially controlled.
In summary, GAC is a conserved antigen, found in all isolated Strep A strains, a property which recommends it for a universal global vaccine against Strep A infection. Investigation into cost-effective solutions for glycoconjugate production is imperative for a global vaccine, particularly to target low- and middle-income countries. Newer technologies such as PGCT and GMMA/OMVs show great promise towards this aim and examination with GAC is warranted. Impressive progress has been made towards a Strep A glycoconjugate vaccine, and further investigation into production of “double-hit” conjugates and their protection from infection would be a sweet reward.
References
Bonanni, P. Demographic impact of vaccination. Vaccine 17, 120–125 (1999).
Vekemans, J. et al. The path to Group A Streptococcus vaccines: WHO research and development technology roadmap and preferred product characteristics. Clin. Infect. Dis. 16, 877–883 (2019).
Fay, K. et al. Patterns of antibiotic nonsusceptibility among invasive group A Streptococcus Infections—United States, 2006–2017. Clin. Infect. Dis. 73, 1957–1964 (2021).
WHO. The Current Evidence for the Burden of Group A Streptococcal Diseases. https://apps.who.int/iris/handle/10665/69063 (2005).
Watkins, D. A. et al. Global, regional, and national burden of rheumatic heart disease 1990–2015. N. Engl. J. Med. 377, 713–722 (2017).
Herdman, M. T. et al. Clinical management and impact of scarlet fever in the modern era: findings from a cross-sectional study of cases in London, 2018-2019. BMJ Open 11, e057772 (2021).
Rümke, L. W. et al. Dominance of M1UK clade among Dutch M1 Streptococcus pyogenes. Lancet Infect. Dis. 20, 539–540 (2020).
Davies, M. R. et al. Atlas of Group A Streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat. Genet. 51, 1035–1043 (2019).
Lancefield, R. A serological differentiation of human and other groups of hemolytic Streptococci. J. Exp. Med. 57, 571–595 (1933).
van Sorge, N. M. et al. The classical lancefield antigen of group a streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 15, 729–740 (2014).
Mccarty, M. The lysis of Group A hemolytic Streptococci by extracellular enzymes of Streptomyces albus. Nature of the cellular substrate attacked by the lytic enzymes. J. Exp. Med. 96, 569–580 (1952).
van der Beek, S. L. et al. GacA is essential for Group A Streptococcus and defines a new class of monomeric dTDP-4-dehydrorhamnose reductases (RmlD). Mol. Microbiol. 98, 946–962 (2015).
Henningham, A. et al. Virulence role of the GlcNAc side chain of the Lancefield cell wall carbohydrate antigen in non-M1-serotype Group A. Streptococcus. MBio 9, 1–12 (2018).
Kabanova, A. et al. Evaluation of a group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine 29, 104–114 (2010).
Hamada, S. et al. Isolation and immunochemical characterization of carbohydrate antigens prepared from Group A Streptococcus pyogenes. Zentralbl Bakteriol. Mikrobiol. Hyg. A 256, 37–48 (1983).
Rush, J. S. et al. The molecular mechanism of N-acetylglucosamine side-chain attachment to the Lancefield Group A carbohydrate in Streptococcus pyogenes. J. Biol. Chem. 292, 19441–19457 (2017).
Rush, J. S. et al. PplD is a de-N-acetylase of the cell wall linkage unit of Streptococcal rhamnopolysaccharides. Nat. Commun. 13, 590 (2022).
Kreis, U. C., Varma, V. & Pinto, B. M. Application of two-dimensional NMR spectroscopy and molecular dynamics simulations to the conformational analysis of oligosaccharides corresponding to the cell-wall polysaccharide of Streptococcus Group A. Int. J. Biol. Macromol. 17, 117–130 (1995).
Edgar, R. J. et al. Discovery of glycerol phosphate modification on Streptococcal rhamnose polysaccharides. Nat. Chem. Biol. 15, 463–471 (2019).
van Hensbergen, V. P. et al. Streptococcal Lancefield polysaccharides are critical cell wall determinants for human Group IIA secreted phospholipase A2 to exert its bactericidal effects. PLoS Pathog. 14, e1007348 (2018).
Reimer, K. B., Gidney, M. A. J., Bundle, D. R. & Mario Pinto, B. Immunochemical characterization of polyclonal and monoclonal Streptococcus Group A antibodies by chemically defined glycoconjugates and synthetic oligosaccharides. Carbohydr. Res. 232, 131–142 (1992).
Michon, F. et al. Doubly branched hexasaccharide epitope on the cell wall polysaccharide of Group A Streptococci recognized by human and rabbit antisera. Infect. Immun. 73, 6383–6389 (2005).
Weimar, T., Harris, S. L., Pitner, J. B., Bock, K. & Pinto, B. M. Transferred nuclear overhauser enhancement experiments show that the monoclonal antibody strep 9 selects a local minimum conformation of a Streptococcus Group A trisaccharide-hapten. Biochemistry 34, 13672–13681 (1995).
Stuike-Prill, R. & Pinto, B. M. Conformational analysis of oligosaccharides corresponding to the cell-wall polysaccharide of the Streptococcus Group A by Metropolis Monte Carlo simulations. Carbohydr. Res. 279, 59–73 (1995).
Johnson, M. A. & Pinto, B. M. Saturation transfer difference 1D-TOCSY experiments to map the topography of oligosaccharides recognized by a monoclonal antibody directed against the cell-wall polysaccharide of Group A Streptococcus. J. Am. Chem. Soc. 124, 15368–15374 (2002).
Adderson, E. E., Shikhman, A. R., Ward, K. E. & Cunningham, M. W. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetylglucosamine / anti-myosin antibody V region genes. J. Immunol. 161, 2020–2031 (1998).
Shikhman, A. R., Cunningham, M. W., Hildebrand, W., Fischetti, V. A. & Cunningham, M. W. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J. Immunol. 152, 4375–4387 (1994).
Martins, T. B. et al. Comprehensive analysis of antibody responses to Streptococcal and tissue antigens in patients with acute rheumatic fever. Int. Immunol. 20, 445–452 (2008).
Dudding, B. A. & Ayoub, E. M. Persistence of Streptococcal Group A antibody in patients with rheumatic valvular disease. J. Exp. Med. 128, 1081–1098 (1968).
Faé, K. C. et al. How an autoimmune reaction triggered by molecular mimicry between Streptococcal M protein and cardiac tissue proteins leads to heart lesions in rheumatic heart disease. J. Autoimmun. 24, 101–109 (2005).
Sabharwal, H. et al. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. 193, 129–135 (2006).
Salvadori, L. G., Blake, M. S. & Mccarty, M. Group A Streptococcus-liposome ELISA antibody titers to group a polysaccharide and opsonophagocytic capabilities of the antibodies. J. Immunol. 171, 593–600 (1995).
Zimmerman, R. A., Auernheimer, A. H. & Taranta, A. Precipitating antibody to Group A Streptococcal polysaccharide in humans. J. Immunol. 107, 832–83241 (1971).
Zabriskie, J. B., Poon-King, T., Blake, M. S., Michon, F. & Yoshinaga, M. Phagocytic, serological, and protective properties of Streptococcal Group A carbohydrate antibodies. Adv. Exp. Med. Biol. 418, 917–919 (1997).
Gao, N. J. et al. Site-specific conjugation of cell wall polyrhamnose to protein SpyAD envisioning a safe universal group a streptococcal vaccine. Infect. Microbes Dis. 3, 87–100 (2021).
Khatun, F. et al. Immunogenicity assessment of cell wall carbohydrates of group A Streptococcus via self-adjuvanted glyco-lipopeptides. ACS Infect. Dis. 7, 390–405 (2021).
Barrett, D. J. Human immune responses to polysaccharide antigens: an analysis of bacterial polysaccharide vaccines in infants. Adv. Pediatr. 32, 139–158 (1985).
Weintraub, A. Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 338, 2539–2547 (2003).
Kelly, D. F., Pollard, A. J. & Moxon, E. R. Immunological memorythe role of b cells in long-term protection against invasive bacterial pathogens. JAMA 294, 3019–3023 (2005).
Pollard, A. J., Perrett, K. P. & Beverley, P. C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9, 213–220 (2009).
Costantino, P., Rappuoli, R. & Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 6, 1045–1066 (2011).
Avci, F. Y., Li, X., Tsuji, M. & Kasper, D. L. A novel mechanism for glycoconjugate vaccine activation of the adaptive immune system. Nat. Med. 17, 1602–1609 (2012).
Dagan, R., Poolman, J. & Siegrist, C. A. Glycoconjugate vaccines and immune interference: A review. Vaccine 28, 5513–5523 (2010).
Micoli, F., Adamo, R. & Costantino, P. Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and new trends. Molecules 23, 1–18 (2018).
Bröker, M., Berti, F., Schneider, J. & Vojtek, I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency” – Potential and limitations. Vaccine 35, 3286–3294 (2017).
Pichichero, M. E. Characteristics, development, and clinical trials. Hum. Vaccin. Immunother. 9, 2505–2523 (2013).
Schutze, M. P., Leclerc, C., Jolivet, M., Audibert, F. & Chedid, L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J. Immunol. 135, 2319–2322 (1985).
Schutze, M.-P., Leclerc, C., Vogel, F. R. & Chedid, L. Epitopic suppression in synthetic vaccine models: Analysis of the effector mechanisms. Cell. Immunol. 104, 79–90 (1987).
Etlinger, H. M. et al. Use of prior vaccinations for the development of new vaccines. Science 249, 423–425 (1990).
Falugi, F. et al. Rationally designed strings of promiscuous CD4+ T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: a model for new conjugate vaccines. Eur. J. Immunol. 31, 3816–3824 (2001).
Reglinski, M. et al. A recombinant conjugated Pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. npj Vaccines 3, 1–11 (2018).
Pozzi, C. et al. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS ONE 7, e46648 (2012).
Bensi, G. et al. Multi high-throughput approach for highly selective identification of vaccine candidates: the group A Streptococcus Case. Mol. Cell. Proteom. 11, M111.015693 (2012).
Rivera-Hernandez, T. et al. An experimental group A Streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. MBio 10, 1–10 (2019).
Rivera-Hernandez, T. et al. Vaccine-induced Th1-type response protects against invasive Group A Streptococcus infection in the absence of opsonizing antibodies. MBio 11, 1–11 (2020).
Rivera-Hernandez, T. et al. Differing efficacies of lead group a streptococcal vaccine candidates and full-length M protein in cutaneous and invasive disease models. MBio 7, 1–9 (2016).
Di Benedetto, R. et al. Rational design of a glycoconjugate vaccine against Group A Streptococcus. Int. J. Mol. Sci. 21, 1–21 (2020).
Castro, S. A. & Dorfmueller, H. C. A brief review on Group A Streptococcus pathogenesis and vaccine development. R. Soc. Open Sci. 8, 1–17 (2021).
Kuberan, B. & Linhardt, R. J. Carbohydrate Based Vaccines. Curr. Org. Chem. 4, 653–677 (2000).
Zarei, A. E., Almehdar, H. A. & Redwan, E. M. Hib vaccines: past, present, and future perspectives. J. Immunol. Res. 2016, 1–18 (2016).
Crotti, S. et al. Defined conjugation of glycans to the lysines of CRM197 guided by their reactivity mapping. ChemBioChem 15, 836–843 (2014).
Kapoor, N. et al. Non-native amino acid click chemistry-based technology for site-specific polysaccharide conjugation to a bacterial protein serving as both carrier and vaccine antigen. ACS Omega 7, 24111–24120 (2022).
Auzanneau, F. I., Borrelli, S. & Pinto, B. M. Synthesis and immunological activity of an oligosaccharide-conjugate as a vaccine candidate against Group A Streptococcus. Bioorg. Med. Chem. Lett. 23, 6038–6042 (2013).
Zhang, F., Lu, Y.-J. & Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl Acad. Sci. USA 110, 13564–13569 (2013).
Zhao, Y. et al. Synthesis and immunological studies of Group A Streptococcus cell-wall oligosaccharide–Streptococcal C5a peptidase conjugates as bivalent vaccines. Org. Chem. Front. 6, 3589–3596 (2019).
Wang, S. et al. Group A Streptococcus cell wall oligosaccharide-streptococcal C5a peptidase conjugates as effective antibacterial vaccines. ACS Infect. Dis. 6, 281–290 (2020).
Frasch, C. E. Preparation of bacterial polysaccharide-protein conjugates: analytical and manufacturing challenges. Vaccine 27, 6468–6470 (2009).
Langdon, R. H., Cuccui, J. & Wren, B. W. N-linked glycosylation in bacteria: an unexpected application. Future Microbiol. 4, 401–412 (2009).
Cuccui, J. & Wren, B. Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins. J. Pharm. Pharmacol. 67, 338–350 (2015).
Riddle, M. S. et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study. Clin. Vaccine Immunol. 23, 908–917 (2016).
Huttner, A. et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 17, 528–537 (2017).
Hatz, C. F. R. et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: a single blind, partially randomized Phase I study. Vaccine 33, 4594–4601 (2015).
Micoli, F. et al. Glycoconjugate vaccines: current approaches towards faster vaccine design. Expert Rev. Vaccines 18, 881–895 (2019).
Nothaft, H. & Szymanski, C. M. Protein glycosylation in bacteria: Sweeter than ever. Nat. Rev. Microbiol. 8, 765–778 (2010).
Iwashkiw, J. A., Vozza, N. F., Kinsella, R. L. & Feldman, M. F. Pour some sugar on it: the expanding world of bacterial protein O-linked glycosylation. Mol. Microbiol. 89, 14–28 (2013).
Young, N. M. et al. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J. Biol. Chem. 277, 42530–42539 (2002).
Kowarik, M. et al. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 25, 1957–1966 (2006).
Feldman, M. F. et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl Acad. Sci. USA 102, 3016–3021 (2005).
Lizak, C., Gerber, S., Numao, S., Aebi, M. & Locher, K. P. X-ray structure of a bacterial oligosaccharyltransferase. Nature 474, 350–356 (2011).
Kowarik, M. et al. N-linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase. Science 314, 1148–1150 (2006).
Fisher, A. C. et al. Production of secretory and extracellular N-linked glycoproteins in Escherichia coli. Appl. Environ. Microbiol. 77, 871–881 (2011).
Dow, J. M., Mauri, M., Scott, T. A. & Wren, B. W. Improving protein glycan coupling technology (PGCT) for glycoconjugate vaccine production. Expert Rev. Vaccines 19, 507–527 (2020).
Wacker, M. et al. Substrate specificity of bacterial oligosaccharyltransferase suggests a common transfer mechanism for the bacterial and eukaryotic systems. Proc. Natl Acad. Sci. USA 103, 7088–7093 (2006).
Ihssen, J. et al. Increased efficiency of Campylobacter jejuni N -oligosaccharyltransferase PglB by structure-guided engineering. Open Biol. 5, 140227 (2015).
Ollis, A. A. et al. Substitute sweeteners: diverse bacterial oligosaccharyltransferases with unique N-glycosylation site preferences. Sci. Rep. 5, 1–13 (2015).
Ihnken, L. A. et al. Mutated PglB Oligosaccharyltransferase Enzymes. 1–107 (2020).
Jervis, A. J. et al. Characterization of N-linked protein glycosylation in Helicobacter pullorum. J. Bacteriol. 192, 5228–5236 (2010).
Mills, D. C. et al. Functional analysis of N-linking oligosaccharyl transferase enzymes encoded by deep-sea vent proteobacteria. Glycobiology 26, 398–409 (2016).
Pan, C. et al. Biosynthesis of conjugate vaccines using an O-linked glycosylation system. MBio 7, 1–11 (2016).
Sun, P. et al. Design and production of conjugate vaccines against S. paratyphi A using an O-linked glycosylation system in vivo. npj Vaccines 3, 1–9 (2018).
Harding, C. M. et al. A platform for glycoengineering a polyvalent Pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 10, 1–11 (2019).
Feldman, M. F. et al. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl Acad. Sci. USA 116, 18655–18663 (2019).
Marshall, L. E. et al. An O-antigen glycoconjugate vaccine produced using protein glycan coupling technology is protective in an inhalational rat model of tularemia. J. Immunol. Res. 2018, 1–12 (2018).
Zorzoli, A. et al. Group A, B, C, and G Streptococcus Lancefield antigen biosynthesis is initiated by a conserved α-D-GlcNAc-β-1,4-L-rhamnosyltransferase. J. Biol. Chem. 294, 15237–15256 (2019).
Shibata, Y., Yamashita, Y., Ozaki, K., Nakano, Y. & Koga, T. Expression and characterization of Streptococcal rgp genes required for rhamnan synthesis in Escherichia coli. Infect. Immun. 70, 2891–2898 (2002).
Castro, S. A. et al. Recombinant Group A Carbohydrate backbone embedded into Outer Membrane Vesicles is a potent vaccine candidate targeting Group A Streptococcus from Streptococcus pyogenes and Streptococcus dysgalactiae subsp. bioRxiv. p. 1–21 Preprint at https://www.biorxiv.org/content/10.1101/2021.11.12.468441v1 (2021).
Chen, M. M., Glover, K. J. & Imperiali, B. From peptide to protein: Comparative analysis of the substrate specificity of N-linked glycosylation in C. jejuni. Biochemistry 46, 5579–5585 (2007).
Valderrama-Rincon, J. D. et al. An engineered eukaryotic protein glycosylation pathway in Escherichia coli. Nat. Chem. Biol. 8, 434–436 (2012).
Glasscock, C. J. et al. A flow cytometric approach to engineering Escherichia coli for improved eukaryotic protein glycosylation. Metab. Eng. 47, 488–495 (2018).
Jones, C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An. Acad. Bras. Cienc. 77, 293–324 (2005).
Micoli, F. et al. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc. Natl Acad. Sci. USA 117, 24443–24449 (2020).
Pitirollo, O. et al. Gold nanoparticles morphology does not affect the multivalent presentation and antibody recognition of Group A Streptococcus synthetic oligorhamnans. Bioorg. Chem. 99, 1–8 (2020).
Micoli, F. et al. Generalized modules for membrane antigens as carrier for polysaccharides: impact of sugar length, density, and attachment site on the immune response elicited in animal models. Front. Immunol. 12, 1–12 (2021).
Acknowledgements
All images created with BioRender.com.
Author information
Authors and Affiliations
Contributions
K.B. and H.A.S. wrote the manuscript, H.D., B.W. and F.M. edited and provided feedback.
Corresponding author
Ethics declarations
Competing interests
H.C.D. holds a patent on the rhamnose polysaccharide platform technology (WO2020249737A1). B.W.W. holds a patent for an E. coli bioconjugation strain (US20150344928A1).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burns, K., Dorfmueller, H.C., Wren, B.W. et al. Progress towards a glycoconjugate vaccine against Group A Streptococcus. npj Vaccines 8, 48 (2023). https://doi.org/10.1038/s41541-023-00639-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-023-00639-5
This article is cited by
-
Update on the development of Group A Streptococcus vaccines
npj Vaccines (2023)