Abstract
Diagnostic assays currently used to monitor the efficacy of COVID-19 vaccines measure levels of antibodies to the receptor-binding domain of ancestral SARS-CoV-2 (RBDwt). However, the predictive value for protection against new variants of concern (VOCs) has not been firmly established. Here, we used bead-based arrays and flow cytometry to measure binding of antibodies to spike proteins and receptor-binding domains (RBDs) from VOCs in 12,000 serum samples. Effects of sera on RBD-ACE2 interactions were measured as a proxy for neutralizing antibodies. The samples were obtained from healthy individuals or patients on immunosuppressive therapy who had received two to four doses of COVID-19 vaccines and from COVID-19 convalescents. The results show that anti-RBDwt titers correlate with the levels of binding- and neutralizing antibodies against the Alpha, Beta, Gamma, Delta, Epsilon and Omicron variants. The benefit of multiplexed analysis lies in the ability to measure a wide range of anti-RBD titers using a single dilution of serum for each assay. The reactivity patterns also yield an internal reference for neutralizing activity and binding antibody units per milliliter (BAU/ml). Results obtained with sera from vaccinated healthy individuals and patients confirmed and extended results from previous studies on time-dependent waning of antibody levels and effects of immunosuppressive agents. We conclude that anti-RBDwt titers correlate with levels of neutralizing antibodies against VOCs and propose that our method may be implemented to enhance the precision and throughput of immunomonitoring.
Similar content being viewed by others
Introduction
Clinical trials for COVID-19 vaccines showed that protection against symptomatic infection with ancestral SARS-CoV-2 (hereafter referred to as SARS-CoV-2wt) correlated with the levels of antibodies binding to the spike protein (spike-wt) and the receptor-binding domain (RBDwt)1,2. There is also evidence that neutralizing titers for SARS-CoV-2wt are predictive of protection against other variants including Delta3,4. However, virus neutralization assays are poorly standardized and difficult to scale up3,5. Thus, the neutralization titers corresponding to 90% vaccine efficacy against symptomatic COVID-19 varied by more than ten-fold in two clinical trials1,2. Titers reported for binding antibodies (binding antibody units per milliliter, BAU/ml) after two doses of mRNA vaccines vary by seven-fold1,2,6,7,8. There is therefore an unmet need for standardized high-throughput assays that can be used to monitor binding- and neutralizing antibodies against different SARS-CoV-2 variants at the individual level.
Most neutralizing antibodies interfere with the binding of the RBD to the human receptor ACE29,10,11,12. Assays that measure inhibitory effects of serum on RBD-ACE2 interactions are therefore commonly used as a surrogate for virus neutralization assays8,10,13,14,15,16,17. An assay commercialized under the name of cPass has received US Food and Drug Administration authorization18. The optimal approach may be multiplexed measurement antibodies and of ACE2-binding to RBDs from SARS-CoV-2 variants of concern (VOCs)8,15,19,20. However, the methods are not standardized, and there is no consensus on the utility of RBD-ACE2 interaction assays in immunomonitoring.
Current diagnostic tests for humoral immunity against SARS-CoV-2 measure antibodies to RBDwt. Results from a recent study suggest that this may be adequate since anti-RBDwt titers were predictive of neutralizing activity of serum against the Delta and Omicron variants21. Other studies, however, show that there is extensive person-to-person variation in ratios between binding- and neutralizing antibodies and in how mutations affect antibody binding and neutralization10,11,20. It has also been suggested that the large increase in neutralization against the Omicron variant observed after a booster vaccine dose reflects an enhancement of antibody quality rather than in quantity22. The implication of person- to-person variation in antibody quality would be that anti-RBDwt titers have limited predictive value at the individual level. However, to this end, studies on the qualitative variation in COVID-19 vaccine responses are on small cohorts, and little is known about effects of immunosuppressive therapy.
The aim of the present study was to determine if anti-RBDwt titers correlate with neutralizing antibodies as measured by RBD-ACE2 interaction assays. Arrays containing spike proteins and RBDs from SARS-CoV-2wt and the Alpha, Beta, Gamma, Delta, Epsilon, and Omicron variants were incubated with 6693 sera (Fig. 1). In addition, more than 11.000 sera were analyzed with arrays containing proteins from all variants except Omicron. The arrays were labeled with fluorochrome-conjugated anti-human IgG to measure binding antibodies or with recombinant ACE2 to study effects of sera on RBD-ACE2 interactions (Fig. 1). In total, we analyzed 12,946 samples, and the cohort included COVID-19 convalescents, healthy individuals and patients on immunosuppressive therapy, who had received two to four doses of COVID-19 vaccines.
The figure shows an outline of the procedure. The starting point was the automated transfer of serum from blood collection tubes to two 384 well plates by use of a Tecan robot. Further processing was performed with liquid handling devices with 384 heads. Samples were serially diluted to 1:100 and 1:1000 in two pairs of 384 plates prefilled with assay buffer and bead-based arrays for measurement of RBD-ACE2 interactions and binding antibodies, respectively. Bead color codes corresponded to spike proteins and RBDs from SARS-CoV2 variants. After incubation with serum, the beads were washed and labeled with R-Phycoerythrin (PE)-conjugated anti-human IgG or successively with digoxigenin-conjugated ACE2 and anti-digoxigenin-PE. Eight additional barcodes were assigned to subarrays as addresses for positions A1-B2 in each of two 384 well plates. A robot with 96 heads was used to pool beads from one pair of 384 well plates into a single 96 deep well plate. Thus, each 96 well contained beads from eight samples for parallel analysis by flow cytometry.
Results
Antibodies in sera from COVID-19 convalescents and vaccinated individuals have broad and uniform coverage of RBDs from SARS-CoV-2 variants
We used bead-based arrays and flow cytometry to measure IgG antibodies to Nucleocapsid (wt) and RBDs from SARS-CoV-2 variants in 5145 sera diluted 1:1000 (Fig. 2). The samples were obtained in 2020 from COVID-19 convalescents (n = 318, red dots), or in 2021 or 2022 from vaccinees (green dots). The post-vaccine samples were from healthy individuals (n = 1060), immunocompetent individuals who had tested positive for the Delta variant (n = 43) and patients on immunosuppressive therapy (3703). We also included 23 samples collected from a cohort of double-vaccinated immunocompetent individuals with Omicron (BA1) infection (blue dots).
The dot plots show accumulated data from array-based measurement of 6693 samples. Each dot in the scatter plots correspond to a different sample. Bead-based arrays were incubated with 6693 sera diluted 1:1000 for 1 h prior to labeling with R-Phycoerythrin-conjugated anti-Human IgG. a–f The x-axes show median fluorescence intensity (MFI) of anti-human IgG binding to beads coupled with RBDwt divided by MFI measured for beads with no virus protein (relative MFI, rMFI). The y-axes show anti-human IgG rMFI measured for beads coupled with RBDs from indicated variants. g–l The y-axes are the same as in dot plots (a–f), while the x-axis shows rMFI for antibodies binding to beads coupled with nucleocapsid from SARS-CoV-2wt. Green dots: post-vaccine sera, red dots: sera obtained in 2020 from SARS-CoV-2 convalescents. Blue dots: sera obtained 10–20 days after symptom debut of Omicron BA.1 infection in double-vaccinated healthy individuals. Source data are provided as a Source Data file.
The overall correlation (Pearson correlation coefficients) with levels of antibodies to RBDwt was 0.91 for RBD from Omicron and 0.94 or higher for antibodies to RBDs from all other variants tested (Fig. 2a–f). In samples obtained during 2020 from COVID-19 convalescents, the correlation between anti-RBDwt and anti-Nucleocapsid was 0.56 (Fig. 2, red dots). Reactivity with Omicron BA1 RBD was lower in convalescent sera, and the correlation with anti-RBDwt was 0.79 (Fig. 2f, red dots, Supplementary Fig. 1).
We identified 14 samples with stronger binding of IgG to RBD from Omicron than to RBDwt (Fig. 2, black dots). All were from 2022, and 9 contained antibodies to nucleocapsid (Fig. 2g–l). The samples are therefore likely to be from SARS-CoV-2/vaccine naive individuals infected with Omicron23. Overall, signals measured for binding of antibodies to RBD from Omicron BA.1 were weaker than those measured for RBDs from other variants (Fig. 2f, Supplementary Fig. 1). This was also observed in sera obtained from double-vaccinated individuals 12-20 days after symptom debut of Omicron BA1 infection (Fig. 2, blue dots).
To extend the dynamic range of antibody detection, we analyzed 438 sera at dilutions of 1:10,000 and 1:100,000. The correlations were similar to those observed after measurement at 1:1000 dilution (Supplementary Fig. 2). Collectively, these results show that most individuals generate antibodies with broad and similar coverage of RBDs from SARS-CoV-2 variants.
Validation and calibration of the RBD-ACE2 interaction assay
Effects of sera on RBD-ACE2 interactions were measured as a surrogate for neutralizing antibodies (Fig.1). To validate the assay, we analyzed sera that had been tested for neutralizing activity against SARS-CoV-2wt in two different laboratories (Fig. 3, lab1: n = 364, lab2: n = 138). The squared Pearson correlation coefficients between effects of sera on ACE2-binding to RBDwt and neutralization titers were 0.82 and 0.73, respectively (Fig. 3).
A total of 502 sera that had been tested for neutralizing activity against SARS-CoV-2 were analyzed with Multi-IgG-ACE2-RBD. Each dot in the scatter plots corresponds to a different sample. a, d: The x- and y-axes show binding of IgG- (x-axis) and ACE2 to beads that had been incubated with sera. b–c: the x-axis shows virus neutralization titers against SARS-CoV-2wt measured for 364 sera in Oslo. The y-axes show ACE2-binding to RBDwt (b) or spike-Beta (c). e–f: the x-axis shows virus neutralization titers against SARS-CoV-2wt measured for 138 sera in Bergen. Source data are provided as a Source Data file.
The WHO international standard (NIBSC code: 20/136) was analyzed to calibrate an in-house standard to international units (IU/ml). The in-house standard was serum from an individual who had received three doses of the Pfizer/BionTech COVID-19 vaccine. The serum was found to contain 50.000 BAU/ml using the Roche Elecsys anti-SARS-CoV-2 S assay, which has been calibrated to the WHO standard by the manufacturer.
Results obtained with serially diluted WHO standard showed that a 2-log inhibition of ACE2-binding to RBDwt corresponded to approximately 1000 IU (Fig. 4a, red dots). Complete inhibition of ACE2-binding to beta-spike observed at 10.000 IU (Fig.4b). For the post-vaccine serum, the units on the x-axis correspond to BAU/ml (Fig. 4, blue dots). The titration curves obtained with the two samples were highly similar. Thus, our results support the view that there is strong correlation between levels of binding- and neutralizing antibodies against SARS-CoV-2wt in sera from vaccinees1,2,8,21,24. For practical purposes, we hereafter consider BAU/ml and IU/ml as being equivalent for post-vaccine sera.
The dot plots show effects of serial dilutions of the WHO-international standard (NIBSC code: 20/136, red dots) or a post-vaccine serum (3x Pfizer/BionTech) on ACE2-binding to RBDwt and spike from the beta-variant. a, b The values on the x-axis correspond to WHO international units for neutralizing effects of the standard and WHO binding antibody units per ml for the post-vaccine serum measured by Roche Elecsys SARS-CoV-2 S. The units are equivalent for sera diluted 1:100. The results were cumulated from two experiments. The lowest dilution for the WHO-standard and the post-vaccine serum was 1:20 and 1:100, respectively. Source data are provided as a Source Data file.
The relative content of neutralizing antibodies against different SARS-CoV-2 variants is similar in COVID-19 convalescents and vaccinees
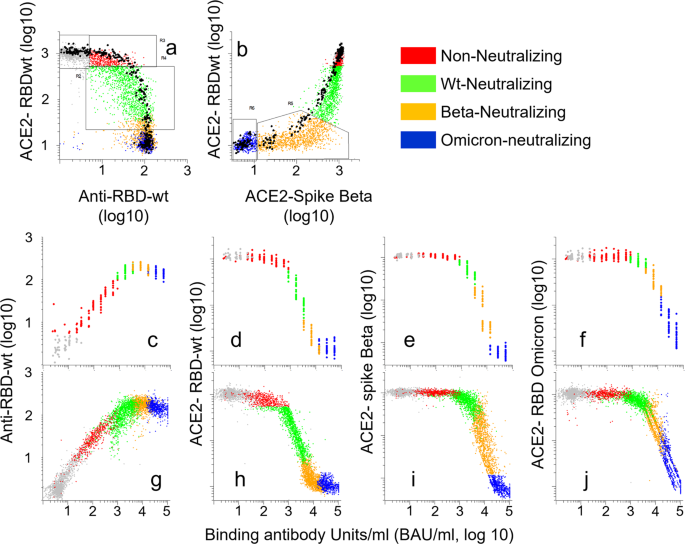
The 5145 sera that were analyzed for binding antibodies to RBDs from SARS-CoV-2 variants were subjected to multiplexed RBD-ACE2 interaction assays. We identified four groups of anti-RBDwt-positive sera (Fig. 5a, b). I) content of antibodies with minimal inhibition of ACE2-RBD interactions (red,17.6%), II) inhibition of ACE2-binding to RBDwt (green, 33%), III) near complete inhibition of ACE2-binding to RBDwt and partial inhibition of binding to RBD-Beta (orange, 27%), and IV) complete inhibition of ACE2 binding to RBD-Beta (blue, 22%). Sera with no detectable antibodies to RBDwt are shown in gray. Sera in group IV were also strongly inhibitory for ACE2-binding to RBD from Omicron BA1 (Fig. 5c). From here on, the four groups are referred to as non-neutralizing (red), wt-neutralizing (green), Beta-neutralizing (orange) and Omicron-neutralizing (blue).
The dot plots show accumulated data from array-based measurement of 6693 samples. Each dot in the scatter plots correspond to a different sample. a, b Binding of serum IgG or recombinant ACE2 to beads coupled with RBDwt or RBD from the Beta variant as indicated. The beads were incubated with serum diluted 1:1000 or 1:100 prior to labeling with anti-human IgG or recombinant ACE2, respectively. c Binding of ACE2 to beads coupled with RBDs and spike proteins from indicated SARS-CoV-2 variant. The colors correspond to groups within the regions shown in (a) and (b). Gray dots anti-RBDwt negative sera. Red dots: anti-RBD-positive sera with no or minimal inhibition of ACE2-binding to RBDwt. Green dots: sera with selective and partial inhibition of ACE2-binding to RBDwt. Orange dots: sera with complete inhibition of ACE2-binding to RBDwt and partial inhibition of binding to RBD-Beta. Blue dots. Complete inhibition of ACE2-binding to RBDwt and RBD-beta and strong inhibition of binding to RBD-Omicron BA.1. Source data are provided as a Source Data file.
The inhibitory effects of sera on ACE2-binding to RBDs and spike proteins from SARS-CoV-2 variants followed a stringent and uniform pattern in the four cohorts studied here (Fig. 5c). Effects on ACE2-binding to RBDwt and RBD-Delta were similar, while the resistance against serum inhibition for other variants increased from Alpha, Gamma, Beta to Omicron (Fig. 5c). The differences between the cohorts were in the distribution of samples in each of the groups identified in Fig. 5a, b. Thus, there was an increase in the frequency of sera with neutralizing antibodies against all variants starting from samples obtained in 2020 from COVID-19 convalescents to those obtained from double-vaccinated individuals with acute Omicron infection (Fig. 5c). In separate analysis of 540 post-vaccine sera, we established that the Pearson correlation between the inhibitory effects on ACE2-binding to RBD from BA.1 and BA.2 was 0.98 (r2 = 0.96) (Supplementary Fig. 3). Collectively, these results show that the relative neutralizing activity of post-vaccine sera against SARS-CoV-2 variants is highly similar between individuals. Thus, the polyclonal antibody response to vaccination appears to be highly convergent.
Anti-RBDwt titers are predictive for the levels of neutralizing antibodies to all SARS-CoV-2 variants
We aligned results from RBD-ACE2 interaction assays with those obtained by measuring anti-RBDwt in sera diluted 1:100,000 (Fig. 6). The plots in Fig. 6c–g show that there was an inverse correlation between ACE2-binding and anti-RBDwt titers. However, there were variant-specific thresholds for the inhibitory effect. Thus, anti-RBDwt signals showing linearity with ACE2-binding to RBD from Omicron BA.1 (y-axis) were right-shifted by approximately one log compared those with co-linearity with ACE2 binding to RBDwt (Fig. 6c–g).
a, b See legend to Fig. 5a, b. Each dot in the scatter plots correspond to a different sample. c–g The x-axes show binding of human IgG to RBDwt after incubation with serum diluted 1:100,000. The y-axes show binding of ACE2 to beads coupled with RBDs and spike proteins from indicated SARS-CoV-2 variant after incubation with serum diluted 1:100. The dot plots show accumulated data from array-based analysis of 550 sera. Each dot corresponds to a different sample. Pearson correlation coefficients were determined for log-10 transformed data. Source data are provided as a Source Data file.
Conversion of signals from Multi-IgG-ACE2-RBD to binding antibody units per milliter (BAU/ml)
The assay plates used to generate the results shown in Figs. 2–7 contained a standard series that was calibrated to BAU/ml using the Roche Elecsys anti-SARS-CoV-2 S assay (see “Methods”). Results obtained with the standard series in 26 consecutive 384 well plates are highlighted as black dots in Fig. 7a, b and colored according to the parent groups in Fig. 7c–f. Signal values measured for standards were subjected to regression in Excel to generate formulae for conversion of signals from test samples to BAU/ml (Fig. 7g–j)).
A total of 6693 sera were analyzed with multi-IgG-ACE2-RBD together with a standard series with pre-defined BAU/ml. Each dot in the scatter plots correspond to a different sample. a, b See in the legend to Fig. 5a, b, except that the black dots correspond to a standard series prepared by serial dilution of a serum with an anti-RBDwt titer of 53.000 BAU/ml. c–f Results obtained with the standard series in 26 consecutive 384 well plates. The x-axes show BAU/ml calculated from serial dilutions of the standard series. The y-axes show binding of IgG to beads coupled with RBDwt (c-g,j), or ACE2 binding to beads coupled with RBDs or spike proteins as indicated. g–j: Results obtained with the standard series were used as input in Excel regression functions to generate formulae for conversion of signals measured for the 6693 sera into BAU/ml (). Source data are provided as a Source Data file.
The results in Fig. 7 show that non-neutralizing sera contained 30-500 BAU/ml (Fig. 7c–f, red dots), SARS-CoV-2wt-neutralizing corresponded to 500–3000 BAU/ml (Fig. 7c-f, green dots), beta-neutralizing to 3000-11.000 BAU/ml while Omicron BA.1-neutralizing sera contained more than 11.000 BAU/ml (Fig. 7d–f). Reactivity patterns obtained by Multi-IgG-ACE2-RBD therefore yield an internal reference for anti-RBDwt titers.
To exclude bias from a single standard sample and a single reference assay, we analyzed 528 samples in parallel with Multi-IgG-ACE2-RBD and the Abbott SARS-CoV-2 IgG Quant II assay (Supplementary Fig. 4). The samples were obtained in the context of population-based screening in Norway in November/December) 2021 (i.e. prior to the Omicron wave). The assay used for these experiments also contained the RBD protein from the BA.4/5 variants (the two are identical). For anti-RBDwt levels in the range of 1000-20.000 AU (Abbot assay) the squared correlation with inhibitory effects of sera on ACE2-binding to RBDwt was 0.86 (Supplementary Fig. 4a). The corresponding squared correlations for RBDwt levels in the range of 3000–80000 AU and ACE2-binding to RBD-beta and Omicron variants BA.1, BA.2 and BA.4/5 were 0.78, 0.64, 0.67 and 0.61, respectively (Supplementary Fig. 4b–e). These results confirm those shown in Fig. 6 and extend the predictive value of anti-RBDwt levels to neutralizing activity against Omicron BA.4/5.
Results from Multi-IgG-ACE2-RBD analysis recapitulate published knowledge about time-dependent waning of antibodies and effects of immunosuppressive therapy
During 2021, we used Multi-IgG-ACE2-RBD to monitor the effects of COVID-19 vaccination of healthy individuals and patients on immunosuppressive therapy. At that time, we did not have access to the Omicron BA.1 RBD. The upper dynamic range of the assay was therefore approximately 20.000 BAU/ml.
Among sera obtained from healthy individuals 10-50 days after the second vaccine dose, 98% were classified as neutralizing (i.e. >500 BAU/ml), and 70% as Beta-neutralizing (Fig. 8c, green, orange, and blue dots, respectively, see also pie charts in Supplementary Fig. 3). The median titer was 6425 BAU/ml. Our assay was calibrated against the Roche Elecsys anti-SARS-CoV-2 S assay, and the titers measured here were comparable to those reported for the reference assay earlier7. The time-dependent waning of antibody levels was also in line with results reported earlier25. Thus, four months after vaccination, the median titer was reduced by approximately one log (746). The frequencies of wt-neutralizing and Beta-neutralizing sera were 58% and 12%, respectively (Fig. 8c). After a booster dose, 80% of sera from healthy donors were classified as Beta-neutralizing, and 31% as Omicron BA.1-neutralizing (Fig. 8d). The enhancement in responses after dose 3 was underestimated since the assay did not contain RBD from Omicron BA.1.
Indicated numbers of post-vaccine sera obtained from healthy individuals and patients on immunosuppressive therapy were analyzed with Multi-IgG-ACE2-RBD. Each dot corresponds to a different sample. a, b See legend to Fig. 5a-b. c–o: The x- and y-axes show days after vaccination and antibody levels in BAU/ml (log 10), respectively. MS: multiple sclerosis, IBD: Inflammatory bowel disease. “Other”: IBD: alpha 4 beta 7 antagonist, IL12_IL23 antagonist, MS: Cladribine, Leflunomide, glatirameracetate, interferon beta, Alpha 4 beta 1 antagonist. Tacrolimus was used in combination with other agents, mainly mycophenolic acid. Source data are provided as a Source Data file.
After two doses, 29% of 465 patients treated with anti-CD20 antibodies for multiple sclerosis (MS) had detectable antibodies, and 12% of sera were classified as “neutralizing” (Fig. 8e). Seroconversion increased to 44% after the 3rd dose, but there was only a modest increase in the frequency of sera classified as wt-neutralizing (Fig. 8e, f). MS patients treated with Natalizumab, Cladribine, Glatirameracetate, or Leflunomide (Fig. 8g-h, “other”) had responses that were comparable to those observed in healthy individuals.
Treatment of inflammatory bowel disease with TNF-alpha antagonists (n = 548) was associated with a shorter duration of the vaccine response (Fig. 8i-j). Thus, 80% of sera obtained earlier than 50 days after the 2nd vaccine were classified as neutralizing, whereas the frequency fell to 15% after four months (Fig. 8i-j). At this time 13% had no detectable antibodies. This is in agreement with results from earlier studies26. By comparison, patients who were treated for inflammatory bowel disease with antagonists for IL12/IL23 or α4β7 integrin (Fig. 8k-l, “other”) had responses that were comparable to those observed in healthy individuals.
Only 26% of 1769 kidney transplant recipients treated with Tacrolimus had detectable antibodies to RBDwt after two vaccines, and 6.5% of sera were classified as “wt-neutralizing” (Fig. 8m). However, a booster dose was quite effective in this group. More than half of the patients had detectable antibodies after dose 3, and nearly a third of sera were classified as neutralizing while 10% were Beta-neutralizing (Fig. 8n). The responses observed in sera from liver transplant recipients (n = 430) reflect the lower doses of Tacrolimus used in this patient cohort (Fig. 8o). Thus, 78% had detectable antibodies after two doses, and 40% were classified as “wt-neutralizing”. These results are in good agreement with those in earlier reports27. Sera obtained after three doses were not available.
Discussion
We have used bead-based arrays to measure binding- and neutralizing antibodies to RBDs and Spike proteins from SARS-CoV-2wt and VOCs in 12,000 sera. The most important finding is that anti-RBDwt titers correlate with the inhibitory effects of sera on ACE2 binding to RBDs from all VOCs tested (Figs. 4, 5). The correlations between levels of binding antibodies could indicate that the most immunodominant epitopes are conserved in all variants (Fig. 2). RBD-ACE2 interaction assays, however, detect antibodies that bind to epitopes that undergo mutations driven by immune escape9,10,11,12. Our results recapitulate those in earlier studies on differences in inhibitory effects of sera on ACE2 binding to RBDs and spike proteins from VOCs. An important new finding is that the effects followed a stringent pattern that was conserved in all cohorts (Fig. 3c). The narrow distribution indicates that there is little inter-individual variation. We therefore conclude that anti-RBDwt titers have high predictive value for neutralizing activity against VOCs at the individual level.
Earlier studies have shown that neutralizing activity against the Omicron BA.1 variant is almost exclusive to sera from individuals who have received a booster dose of COVID-19 vaccination21,28,29,30,31. However, it has not been clear if the effect of a third vaccine dose on cross-protection is primarily qualitative or quantitative. Vaccination leads to time-dependent affinity maturation of B-cells and broadening of the epitope coverage8,22,32. The ratio of serum antibodies that are capable of cross-neutralization may therefore increase after boosting22. Here, we show that a quantitative difference in the antibody response is sufficient to explain the large increase in neutralization titers for Omicron BA.1 observed after a booster dose. The results in Fig. 8 predict that sera containing at least 11.000 BAU/ml have strong neutralizing effect against this VOC. We also show that anti-RBDwt titers are predictive for the inhibitory effect of sera on ACE2-binding to RBD from BA.2 and BA.4/BA.5 (Supplementary Fig. 4).
A simplistic interpretation of our results is that multiplexed assays are not needed since they yield essentially the same information as anti-RBDwt titers. However, there are several good reasons to measure RBD-ACE2 interactions in parallel with anti-RBDwt titers. The two assays are orthogonal and yield independent evidence for the presence of RBD-binding antibodies. The methods are also complementary to the extent that they yield high resolution for high and low titers, respectively. A single dilution for each assay was sufficient to obtain a dynamic range of four logs. A high dynamic range usually comes at the cost of reduced throughput. For example, the ELISA protocol used to generate the results shown in Fig. 5 involved duplicate measurement of each sample at eight serial dilutions. Multi-IgG-ACE2-RBD therefore yields a unique combination of high precision and throughput.
Multi-IgG-ACE2-RBD also opens the door to more standardized serology. Current diagnostic assays for anti-RBDwt titers are not interchangeable, even when results are converted to BAU/ml33. Thus, the median titers reported for sera collected from healthy individuals after two doses of mRNA vaccines vary by more than seven-fold (959 to 7812 BAU/ml)1,2,6,7. Variation in samples used as standards in different assays and laboratories is likely to be a contributing factor. Multi-IgG-ACE2-RBD may eliminate the need for a standard since the relative binding of ACE2 to RBDs and spike proteins from VOCs serves as an internal reference. One may argue that membership in the groups identified in Fig. 5a-b only yields a rough estimate of anti-RBD titers. However, it is worth noting that the correlate for an increase in vaccine efficacy of from 80% to 90% was a 10-fold increase in titers of neutralizing antibodies1,2 }. In this perspective, the classification of sera on the basis of membership in the groups identified here appears as a more robust alternative to numerical titers. The classification also seems more intuitive since there is a direct correlate to neutralizing activity.
The present study has limitations. First, we only used SARS-CoV-2wt in virus neutralization assays. Further studies are needed to determine the exact predictive values of Multi-IgG-RBD-ACE2 for protection against each VOC. Second, RBD-ACE2 interaction assays do not measure neutralizing antibodies binding outside of the RBD or factors that promote infectivity, such as virus replication rates. This may explain why the results shown in Fig. 3c did not recapitulate published differences in neutralizing titers for Delta and SARS-CoV-2wt (Fig. 3c)31,34. The implication is that neutralization assays are still needed to establish if titers for new variants correlate with anti-RBDwt levels3. It should also be noted that multiplexed bead-based assays cannot be implemented in all parts of the world due to a relatively high cost. Finally, it is worth noting that cellular immunity may be equally important as antibodies in protection against severe COVID-19.
In conclusion, we show that anti-RBDwt titers in post-vaccine sera are broadly predictive for levels of neutralizing antibodies to VOCs. Our results also demonstrate the feasibility and utility of Multi-IgG-ACE2-RBD in large-scale immunomonitoring.
Methods
Serum samples
All participants gave written informed consent before taking part in the study. Samples from Bergen were from studies approved by the Northern and Western Norway Regional Ethical Committees (approval numbers 218629, 118664). Samples from Oslo were obtained from the COVID-19 biobank at Oslo University Hospital. The biobank was approved by the Norwegian Regional Ethical Committee (reference number 135924). Sera from healthy volunteers were from participants in the Norwegian Coronavirus study, the Mother and Child study and the NorFlu study (approval numbers: 124170, 127798, 18403). Sera from kidney transplant recipients, patients with multiple sclerosis (MS), and inflammatory bowel disease (IBD) and other autoimmune diseases were obtained in context of ongoing studies on the immune responses to COVID-19 vaccination in the respective cohorts35,36,37. A total of 520 sera collected in September 2021 were obtained from the Trøndelag Health Study (HUNT), which is a population-based study including individuals 18 years of age and older. All studies were approved by the Norwegian Regional Ethical Committee (reference numbers: 200631, 127798, 2021/8504, 135924, 204104). The samples shown in each figure are listed in the Source Data file. The file also contains information about vaccine status and medications for organ transplant recipients and patients with MS and IBD. Information about age and sex is provided for a total of 11693 samples. More than 96% of samples were from individuals between the age of 20 and 80, 53% and 47% were from females and males, respectively.
Standards
The WHO international standard was used as a reference for neutralizing activity (NIBSC code: 20/136)38,39. As an internal reference in all assays we used a serum sample from a healthy individual (author JTV) who had received three doses of the Pfizer/BionTech mRNA anti-Covid-19 vaccine. The serum was measured with the Roche Elecsys anti-SARS-CoV-2 spike assay and determined to contain 53.000 Binding Antibody Units per milliliter (BAU/ml). Aliquots were stored at −70 °C and serially diluted two- three-fold.
Expression and hapten-conjugation of recombinant ACE2
cDNA encoding a truncated human ACE2 fused to human albumin was sub-cloned into pFUSE2ss-CLIg-hk (InvivoGen). The vector was transiently transfected into Expi293F cells in suspension (Thermo Fisher Scientific) using the ExpiFectamine 293 transfection kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Cells were cultured for 7 days at 37 °C with 80% humidity and 8% CO2 on an orbital shaker platform set to 125 rpm before the medium was collected. The secreted fusion protein was purified on a CaptureSelect™ human albumin affinity matrix (Life Technologies), and protein eluted by adding 20 mM Tris and 2.0 M MgCl2, pH 7.0 before up-concentration using Amicon® Ultra-15 50K Centrifugal Filter Units (Merck Millipore). Buffer exchange to PBS was performed before size exclusion chromatography (Äkta Avant, GE Healthcare) with a SuperdexTM 200 Increase 10/300 GL (Cytiva) prior to up-concentration using Amicon® Ultra-0.5 Centrifugal Filter Units (Merck Millipore). The protein eluted as a dimer. For hapten-conjugation, digoxigenin-NHS (Sigma Aldrich, cat. No 11333054001, 40μg/mg protein) was added to protein solublized in PBS. After 30 min of incubation at 22 °C, free digoxigenin was removed using Amicon® Ultra-0.5 Centrifugal Filter Units.
Expression and biotinylation of virus proteins
Except for Nucleocapsid (Prospec-Tany-TechnoGene, Israel) and RBD from Omicron (BA.1, BA.2, BA.4/5 Sino Biologicals, China), all proteins were produced in-house. Plasmids encoding SARS-CoV2 RBD and full-length spike were obtained from Florian Krammer and Ian McLellan, respectively40,41. The sequences were used as the basis for custom-made constructs encoding RBDs and Spike proteins from the Alpha, Beta, Gamma, Delta, and Epsilon variants (ordered from Genscript). cDNA encoding His-tagged Spike and RBD variants of SARS-CoV-2 were sub-cloned into pFUSE2ss-CLIg-hk (InvivoGen). The vectors were transfected as described above for the ACE2-albumin fusion protein, and secreted His-tagged proteins were purified on HisTrap™ HP 1 mL columns (Cytiva), eluted with 250 mM imidazole diluted in PBS followed by up-concentration and buffer-exchange to PBS using Amicon® Ultra-15 10K Centrifugal Filter Units (Merck Millipore). Monomeric fractions were isolated by size exclusion chromatography as described above for the ACE2-albumin fusion protein. Purified recombinant viral proteins were solubilized in PBS and biotinylated chemically with sulfo-NHS-LC-biotin (sulfo-NHS-LC-biotin, Proteochem, USA) at a molar ratio of 1:1. Free biotin was removed with spin filters with a 10 kDa cutoff (Merck, Millipore).
Bead-based arrays with virus proteins
Bead-based arrays were produced by surface labeling of amine-functionalized poly meta-acrylate (PMMA) microspheres (Bangs Laboratories, IN, USA)42,43. The microspheres were suspended at 10% solids in PBS with 1% Tween 20 (PBT) in PCR-plates (Axygen). During all modification steps, the beads were agitated at 1800 rpm on an Eppendorf MixMate at 22 °C. Each modification step was followed by five wash steps which include centrifugation of beads at 600 × g for 1 min and resuspension of beads in PBT. Liquid handling was performed using CyBio SELMA robots with 96 heads (Jena Analytika, Germany). Neutravidin coupling; Beads were reacted successively with biotin-LC-NHS (sulfo-NHS-LC-biotin, 100μg/ml, Proteochem, USA) and neutravidin (Thermo Fisher, 100μg/ml). Each incubation lasted 30 min. Fluorescent barcoding; Beads were dyed successively with serially diluted Cy5-NHS (Lumiprobe), Bodipy-NHS (Lumiprobe), and Pacific Blue-NHS (Thermo Fisher) to generate a 108- plex (6 × 6 × 3). The starting concentrations were 1–2 μg/ml, dilutions were 1:2.2, and the incubation time was 10–15 min. A 0.2μl aliquot of the suspension was monitored by flow cytometry every 5 min to determine the optimal time of incubation. Coupling of virus proteins; Dyed Neutravidin-coupled beads were incubated for 30 min with biotinylated virus proteins solubilized in PBT (100 μg/ml). Preparation and storage of bead-based arrays; Beads with different color codes and proteins were washed and then pooled in an assay buffer composed of PBT containing of 1% Bovine serum albumin (BSA), 0.1% sodium azide, 10 μg/ml D-Biotin, and 10 μg/ml Neutravidin. A production lot yields eight subarrays, each with 12 different barcodes and the same content of proteins. Ten color codes corresponded to different virus proteins, while two were used as a reference for background binding of IgG to neutravidin beads. The eight subarrays have bar codes that can be discriminated by flow cytometry to allow sample multiplexing. They were distributed into positions A1, A2, B1, B2 in two 384 well plates prefilled with assay buffer. These served as stock plates and were kept at 4–8 °C. The resolution of bead subsets and the gating strategy is shown in Supplementary Fig. 6.
Preparation of serum for analysis
Sample processing is outlined in Fig. 1. Serum (100 μl) was transferred from blood sampling tubes into 384 well serum stock plates using a Tecan Robot. A 384-head CyBio SELMA robot was used to transfer 10μl of serum into a 384 well plate prefilled with 90 μl serum dilution buffer. The buffer composition is the same as the assay buffer described above except that the neutravidin concentration is ten-fold higher (100 μg/ml) to neutralize neutravidin-reactive antibodies. The plates were kept overnight at 4–8 °C before use. Serum remaining in the original 384 plates was stored at −20 °C.
Array-based measurement
The steps in the assay are illustrated in Fig. 1. A 384 head SELMA robot (Jena Analytica, Germany) was used to transfer beads (3 μl) from stock plates into two pairs of 384 well plates prefilled with assay buffer. To one pair, we added 11 μl of diluted serum (1:10). The plate was subjected to mixing for 1 min on an Eppendorf MixMate before the beads were pelleted. Diluted serum (10 μl of 1:100 dilution) was next transferred to the second pair. The two plate pairs were agitated on an Eppendorf MixMate for 1 h. At this point, the beads were pelleted and the supernatant was removed. For detection of IgG, the beads were washed three times in PBT and labeled for 30 min with R-Phycoerythrin-conjugated Goat-anti-Human IgG Fc (Jackson Immunoresearch, 30μl of a 1:600 dilution of stock in assay buffer). Beads used for ACE2-Spike interaction measurement were not washed. Digoxigenin-labeled recombinant ACE2 (30 μl, 300 ng/ml) was added to the beads, and the plate was agitated for 40 min. The beads were washed twice in PBT and labeled with monoclonal anti-Digoxin (Jackson Immunoresearch, 1μg/ml) conjugated in-house to R-Phycoerythrin for 30 min at constant agitation. After labeling with secondary antibodies, the beads were washed twice. The bead-based arrays in positions A1, A2, B1, B2 in each 384 well plate have different barcodes. Thus, the contents of the two 384 well plates were pooled into a single 96 deep well plate prior to flow cytometric analysis. The beads were next analyzed with an Attune Next Flow cytometer equipped with four lasers (violet, blue, yellow, and red) and a harvesting unit for microwell plates). Instrument usage averaged 60 min per 96 well plate.
Data analysis and visualization
Raw flow cytometry data were analyzed using WinList 3D version 10. The median R-Phycoerythrin fluorescence intensity (MFI) for each bead subset was exported to a spreadsheet. Further analysis was performed in Microsoft Excel. The MFI values measured for binding of anti-human IgG and ACE2/anti-digoxigenin to beads with viral proteins were divided by those of beads with neutravidin only (hereafter referred to as relative MFI, rMFI). To visualize results as colored dot plots, we exported data from Excel to WinList 3D. In all dot plots in Figs. 2–8 and supplementary figures, each dot corresponds to a different sample. Colors correspond to subgroups of samples as indicated in the text and figure legends.
Calibration of signals to binding antibody units per milliliter (BAU/ml)
Serum was obtained from a healthy individual two weeks after receiving an mRNA vaccine booster dose. The serum was serially diluted and analyzed using the Roche Elecsys anti-SARS-CoV-2 S assay. The end titer was 53.000 BAU/ml. The serum stock was diluted serially two- and three-fold to generate a standard series in the range of 3-50.000 BAU/ml, and the standard series was added to separate wells in all 384 plates analyzed. Numerical values for rMFI measured for anti-RBDwt and RBD-ACE2 interactions were subjected to regression in Excel (Supplementary Fig. 7). The best curve-fit was obtained with the power function (i.e. calculation based on log-transformed data). The signals measured with study samples were used as input in regression functions to convert rMFI to BAU/ml. The algorithm calculates BAU/ml on the basis of four signals/impact values: anti-RBDwt, RBD-ACE2wt, Spike-ACE2Beta and RBD-ACE2 Omicron and select the highest value provided that the sample is anti-RBD positive (value above 5).
The samples in Fig. 8 in the article were analyzed in the period of mid-July to mid-December 2021. At that time, the serum was diluted 1:100 for detection of anti-RBDwt IgG as well as for measurement of RBD-ACE2 interactions. This dilution was chosen to optimize sensitivity in samples obtained from patients on immunosuppressive therapy. In the same period, we used a plasma standard selected to be similar to the WHO international standard (i.e. titer = 1000 BAU/ml). However, plasma yielded low resolution for anti-RBDwt IgG due to an extensive “hook effect” (not shown). The reactivity patterns in RBD-ACE2 interaction assays also deviated somewhat from those observed with serum (not shown). We therefore analyzed the serum standard obtained in November 2021 in seven experiments during November and December 2021 at a dilution of 1:100. The results from all experiments were used as input to generate an “average” regression formula for conversion of signals measured for anti-RBDwt sera in 2021 to BAU/ml. To generate formulas for RBD-ACE2 interactions we used results from 11 experiments performed in late December 2021 to mid-January 2022. The values reported for BAU/ml in Fig. 8 in the article are therefore an approximation. However, minor variations in absolute titers should be interpreted in view of results from clinical trials showing that a ten-fold increase in neutralizing titers is required for a 10% absolute increase in vaccine efficacy. The classification on basis of neutralizing activity used in Fig. 8 is independent of the standard and therefore more robust.
Statistics
Pearson correlations for log-transformed data were determined using Excel.
Virus neutralization assay
The virus neutralization assay has been described previously5. Vero E6 cells were plated out into 96-well cell culture plates at 1 × 104 cells/well. The next day, 100xTCID of SARS-CoV-2 virus were mixed with 2-fold titrations of sera assayed in quadruplicates. Following 1 h of incubation at 37 °C in a 5% CO2 humidified atmosphere, the mixture was added to the plated cells. Plates were next incubated for 50 h at 37 °C in a 5% CO2 humidified atmosphere. Next, monolayers were washed with PBS and fixed in cold 80% acetone for 20 min.
The SARS-CoV-2 virus Human 2019-nCoV strain 2019-nCoV/Italy-InMI1 (008V-03893) from the European Virus Archive (EVA) was detected in Vero E6 cell cultures by ELISA using a mAb against SARS-CoV-2 nucleocapsid (40143-R004, SinoBiological) and HRP-conjugated goat anti-rabbit IgG-Fc mAb (SSA003, SinoBiological). Plates were developed using TMB substrate buffer (sc-286967, Santa Cruz), and read with the Tecan reader. The neutralization assay was done at laboratory 1 in Oslo.
The microneutralization (MN) assay was done in laboratory 2 and performed in a certified Biosafety Level 3 Laboratory in Norway17,18,19 against a clinically isolated virus: SARS-CoV-2/Human/NOR/Bergen1/2020 (GISAID accession ID EPI_ISL_541970)44. Briefly, serum samples were heat-inactivated at 56 °C for 60 min, analyzed in serial dilutions (duplicated, starting from 1:20), and mixed with 100 TCID50viruses in 96-well plates and incubated for 1 h at 37 °C. Mixtures were transferred to 96-well plates seeded with Vero cells. The plates were incubated at 37 °C for 24 h. Cells were fixed and permeabilized with methanol and 0.6% H2O2 and incubated with rabbit monoclonal IgG against SARS-CoV2 NP (Sino Biological). Cells were further incubated with biotinylated goat anti-rabbit IgG (H + L) and HRP-streptavidin (Southern Biotech). The reactions were developed with o-Phenylenediamine di-hydrochloridec (OPD) (Sigma-aldrich). The MN titer was determined as the reciprocal of the serum dilution giving 50% inhibition of virus infectivity. Negative titers (<20) were assigned a value of 5 for calculation purposes.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Change history
25 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41541-023-00600-6
References
Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 27, 2032–2040 (2021).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 375, 43–50 (2022).
Cromer, D. et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 3, e52–e61 (2022).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Nguyen, D. et al. SARS-CoV-2 neutralising antibody testing in Europe: towards harmonisation of neutralising antibody titres for better use of convalescent plasma and comparability of trial data. Euro Surveill 26, https://doi.org/10.2807/1560-7917.ES.2021.26.27.2100568 (2021).
Wei, J. et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med., https://doi.org/10.1038/s41591-022-01721-6 (2022).
Brehm, T. T. et al. Low SARS-CoV-2 infection rates and high vaccine-induced immunity among German healthcare workers at the end of the third wave of the COVID-19 pandemic. Int J. Hyg. Environ. Health 238, 113851 (2021).
Roltgen, K. et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell, https://doi.org/10.1016/j.cell.2022.01.018 (2022).
Barnes, C. O. et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687 (2020).
Piccoli, L. et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 183, 1024–1042.e1021 (2020).
Greaney, A. J. et al. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe 29, 463–476.e466 (2021).
Harvey, W. T. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19, 409–424 (2021).
Tan, C. W. et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 38, 1073–1078 (2020).
Abe, K. T. et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 5, https://doi.org/10.1172/jci.insight.142362 (2020).
Andrews, N. et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 386, 1532–1546 (2022).
Starr, T. N. et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 597, 97–102 (2021).
Pegu, A. et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373, 1372–1377 (2021).
Zhu, F. et al. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe 3, e81–e82 (2022).
Becker, M. et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat. Commun. 12, 3109 (2021).
Junker, D. et al. COVID-19 patient serum less potently inhibits ACE2-RBD binding for various SARS-CoV-2 RBD mutants. Sci. Rep. 12, 7168 (2022).
Garcia-Beltran, W. F. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185, 457–466.e454 (2022).
Wesemann, D. R. Omicron’s message on vaccines: boosting begets breadth. Cell 185, 411–413 (2022).
Søraas, A. et al. Breakthrough infections with the omicron and delta variants of SARS-CoV-2 result in similar re-activation of vaccine-induced immunity. Front Immunol. 13, 964525 (2022).
Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021).
Levin, E. G. et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 385, e84 (2021).
Jena, A. et al. Effectiveness and durability of COVID-19 vaccination in 9447 patients with IBD: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol., https://doi.org/10.1016/j.cgh.2022.02.030 (2022).
Thuluvath, P. J., Robarts, P. & Chauhan, M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J. Hepatol. 75, 1434–1439 (2021).
Liu, L. et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 602, 676–681 (2022).
Schmidt, F. et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 386, 599–601 (2022).
Sokal, A. et al. Analysis of mRNA vaccination-elicited RBD-specific memory B cells reveals strong but incomplete immune escape of the SARS-CoV-2 Omicron variant. Immunity 55, 1096–1104.e1094 (2022).
Planas, D. et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596, 276–280 (2021).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591, 639–644 (2021).
Perkmann, T. et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol. Spectr. 9, e0024721 (2021).
Mlcochova, P. et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 599, 114–119 (2021).
Konig, M. et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J. Neurol. Neurosurg. Psychiatry, jnnp-2021-327612, https://doi.org/10.1136/jnnp-2021-327612 (2021).
Konig, M. et al. Immunogenicity and Safety of a Third SARS-CoV-2 Vaccine Dose in Patients With Multiple Sclerosis and Weak Immune Response After COVID-19 Vaccination. JAMA Neurol. 79, 307–309 (2022).
Midtvedt, K. et al. Low immunization rate in kidney transplant recipients also after dose 2 of the BNT162b2 vaccine: continue to keep your guard up! Transplantation 105, e80–e81 (2021).
Kristiansen, P. A. et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 397, 1347–1348 (2021).
Knezevic, I. et al. WHO International Standard for evaluation of the antibody response to COVID-19 vaccines: call for urgent action by the scientific community. Lancet Microbe 3, e235–e240 (2022).
Amanat, F. et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 26, 1033–1036 (2020).
Hsieh, C. L. et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 369, 1501–1505 (2020).
Sikorski, K. et al. A high-throughput pipeline for validation of antibodies. Nat. Methods 15, 909–912 (2018).
Wu, W. et al. Antibody array analysis with label-based detection and resolution of protein size. Mol. Cell Proteom. 8, 245–257 (2009).
Kuwelker, K. et al. Attack rates amongst household members of outpatients with confirmed COVID-19 in Bergen, Norway: a case-ascertained study. Lancet Reg. Health Eur. 3, 100014 (2021).
Acknowledgements
This study was funded by grants from the Coalition of Epidemic Preparedness and Innovation (CEPI) and from South-Eastern Norway Regional Health Authority. The authors thank Prof. George Georgiou, University of Texas at Austin, USA for helpful discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
T.T.T.: Main responsibility for assay development, preparation of figures, manuscript writing. E.B.V.: Assay development, production of arrays, data acquisition, data analysis, preparation of figures, manuscript writing. A.M.: Assay development, data acquisition, data analysis. A.C.: Production of arrays, data acquisition, data analysis. L.T.: Data acquisition, data analysis. A.K., Aina Anthi: Assay development: Production of and functional validation of proteins for arrays. Manuscript writing. J.T.A.: Conceptualization of RBD-ACE2 interaction assays. Design of DNA constructs for virus proteins and ACE2-fusion protein. Supervision of protein production and functional validation of proteins. Manuscript writing, funding via grant from South-Eastern Norway Regional Health Authority. M.K. and G.N.: Responsible for the trial on vaccine responses in MS patients. Data analysis. Manuscript writing. K.M. and Anders Åsberg: Responsible for the trial on vaccine responses in kidney transplant recipients. Data analysis. Manuscript writing. A.W.M., M.L.H., K.L.: Responsible for the trial on serology of patients with inflammatory bowel disease. Data analysis. E.M., T.L.A., K.W.: Responsible for the trial on vaccine responses in liver transplant recipients. A.B.-D., P.M., L.T., S.M., L.S.N.-M.: Responsible for trials on vaccine responses in healthy individuals. A.S.: Responsible for trials on vaccine responses in healthy individuals and in the cohort of Omicron BA.1-infected individuals. R.F.K., R.D., K.D., K.S.T., G.B.K.: Sample logistics, data management and quality control of all serology data. Reporting of results to study participants. A.L., F.M.: Responsible for calibration of assay to the Roche Elecsys anti-SARS-CoV-2 S assay, virus sequencing for the Omicron BA.1 cohort. Data analysis. G.G.: Responsible for virus neutralization experiments in Oslo. Data analysis, manuscript writing. R.J.C., F.Z., N.L.: responsible for virus neutralization tests, ELISA and data analysis in Bergen, manuscript writing. P.A., J.C.H.: Co-responsible for the cohort of Omicron BA.1-infected individuals, data analysis, manuscript writing. L.A.M.: Project conceptualization, design of vaccine trials, manuscript writing. Funding via grant from CEPI. J.T.V.: Project conceptualization, design and coordination of vaccine trials, main responsibility for sample logistics and data management. Funding via grants from Oslo University Hospital, CEPI and South-Eastern Norway Regional Health Authority. Manuscript writing. F.L.-J.: Project conceptualization, assay development, production of arrays, data analysis, manuscript writing. Funding via grants from CEPI and South-Eastern Norway Regional Health Authority.
Corresponding author
Ethics declarations
Competing interests
Trung The Tran has nothing to disclose. Eline Benno Vaage has nothing to disclose. Adi Mehta has nothing to disclose. Adity Chopra has nothing to disclose. Lisa Tietze has nothing to disclose. Anette Kolderup has received support from South-Eastern Norway Regional Health Authority (Grant no 10357 and 2021069. The payments were made to the institution, Oslo University Hospital, and covered salary and consumables). Aina Karen Anthi has received support from South-Eastern Norway Regional Health Authority (Grant no 2019084. The payments were made to the institution, Oslo University Hospital, and covered salary and consumables). M. Konig has received speaker honoraria from Novartis, Biogen, Sanofi, and Merck and support from Coalition for Epidemic Preparedness Innovations (CEPI) through Oslo University Hospital. Gro Nygard has received support from Coalition for Epidemic Preparedness Innovations (CEPI) through Oslo University Hospital. Andreas Lind has nothing to disclose. Fredrik Muller has nothing to disclose. Lise Sofie Haug Nissen-Meyer has nothing to disclose. Per Magnus has received support from Norwegian Institute of Public Health for collection of blood samples in NorFlu and MoBa since 2020. Lill-Iren Schou Trogstad has received support from Norwegian Institute of Public Health for collection of blood samples in NorFlu and MoBa since 2020. Siri Mjaaland has nothing to disclose. Arne Soraas has received support from Norwegian Cancer Association, Norwegian research Council, and Southeastern Norway Health Authority. Karsten Midtvedt has nothing to disclose. Anders Asberg has received payment to the institution from Sandos and Orifarm. Andreas Barratt-Due has nothing to disclose. Asle Wilhelm Medhus has received a grant from Takeda (Grant to a research-initiated project, payment to institution). Marte Lie Hoivik has received research grants to the institution from Pfizer, Janssen, Takeda, Ferring, Tillotts and has participated on a Data Safety Monitoring Board or Advisory Board at Takeda with personal fees. Knut Lundin has nothing to disclose. Randi Fuglaas Karlsen has nothing to disclose. Reidun Dahle has nothing to disclose. Karin Danielsson has nothing to disclose. Kristine Stien Thomassen has nothing to disclose. Grete Birkeland Kro has nothing to disclose. Rebecca Jane Cox has nothing to disclose. Fan Zhou has nothing to disclose. Nina Langeland has nothing to disclose. Pal Aukrust has received support from Klinbeforsk. grants for COVID-19 research 2020. Espen Melum has nothing to disclose. Tone Lise Åvitsland has nothing to disclose. Kristine Wiencke has nothing to disclose. Jan Cato Holter has received support from Oslo University hospital, Research Council of Norway (Grant no 312780), and Philantropic donation from Vivaldi Invest A/A owned by Jon Steohenson von Tetzchner. Ludvig Andre Munthe has received support from CEPI (Oslo University Hospital, University of Oslo), funding from University of Oslo, RCN, Norwegian Cancer Society, Health South East Health Research Funds (Stiftelsen KG Jebsen Grant 19), consulting fees in 20201: Astrazeneca for a panel discussing options for Long-Acting anti-SARS-CoV-2 Biologic AZD 7442, 4-time slots, < 1000USD, and payment for expert testimony in 20201 (Part of the expert panel for Norwegian Medicines Agency on vaccinology and associated topics, salary 1500USD and part of the expert panel for Norwegian Institute of Public Health for Norwegian Corona Vaccination Program. 8 meetings. Pro Bono). Gunnveig Grodeland has received support from South-Eastern Norway Regional Health Authority, grants from the Research Council of Norway and EU-Horizon 2020, consulting fees from the Norwegian System of Compensation to Patients, honoraria from Bayer, ThermoFisher, and Saofi. She has also participated on the Data Safety Monitoring Board or Advisory Board with AstraZeneca. Jan Terje Andersen has received support from South-Eastern Norway Regional Health Authority (Grant no 10357 and 2021069. The payments were made to the institution, Oslo University Hospital, and covered salary and consumables) and from Juni Andrell, Stockholm University, Sweden (cDNA encoding 8xHis-tagged HexaPro spike protein and 6xHis-tagged receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Wuhan, in the expression plasmids pαH and pCAGGS, respectively. He has collaborated with Roche, Tillotts Pharma, SJJ Solutions, Argenx, and Nordic Nanovector. Not directly related to the work of this manuscript. John Torgils Vaage has nothing to disclose. Fridtjof Lund-Johansen has received support from the South-Eastern Norway Regional Health Authority grant for developing COVID-19 serology. He has also received support from The Coalition for Epidemic Preparedness and Innovation (CEPI), a grant to monitor vaccine responses in patients on immunosuppressive therapy. The grants were paid to the institution.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tran, T.T., Vaage, E.B., Mehta, A. et al. Titers of antibodies against ancestral SARS-CoV-2 correlate with levels of neutralizing antibodies to multiple variants. npj Vaccines 7, 174 (2022). https://doi.org/10.1038/s41541-022-00586-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-022-00586-7