Abstract
SARS-CoV-2 neutralizing antibodies have been suggested to reflect the efficacy of COVID-19 vaccines. This study reports the direct comparison of the SARS-CoV-2 neutralizing antibody response elicited by a protein- (NVX-CoV2373), an mRNA- (Comirnaty), and a vector-based (Vaxzevria) COVID-19 vaccine, calibrated against the WHO international SARS-CoV-2 antibody standard, and further supports the use of neutralizing antibody levels as a correlate of protection.
Similar content being viewed by others
Since late 2019, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread around the globe, and the resulting coronavirus disease 2019 (COVID-19) pandemic has had unprecedented impact on healthcare systems, economics, and social interactions. At similarly unprecedented speed, the development, clinical investigation, and regulatory assessment of COVID-19 vaccines have been pursued1, resulting in emergency use authorization (EUA) of several vaccines just about one year after the virus had initially been described. The first two formulations licensed in Western countries—Comirnaty (BioNTech-Pfizer) and Spikevax (Moderna)—employed an mRNA vaccine technology, while shortly thereafter, two vaccines based on recombinant adenoviral vectors—Vaxzevria (AstraZeneca) and Janssen COVID-19 Vaccine (Janssen)—received regulatory approvals in the European Union (EU) or the United States (US); all of these vaccines were characterized by high efficacy across gender, age groups, and ethnicities2,3,4,5.
Another large group of COVID-19 vaccine candidates employs the biotechnological production of immunogenic viral proteins or protein subunits, in case of SARS-CoV-2 the spike (S) protein or its receptor-binding domain1. One of these candidates is NVX-CoV2373 (TAK-019; Novavax), a recombinant nanoparticle vaccine for which safety and immunogenicity6 and subsequently high efficacy7 have been demonstrated. Compared to mRNA vaccines, long-term storage above freezing is a beneficial characteristic that is especially important for the supply of low- and middle-income countries. Correspondingly, as the first protein-based vaccine, NVX-CoV2373 has recently been granted EUA in Indonesia8 and the Philippines9, as well as a conditional marketing authorization in the EU10.
Large vaccination campaigns have meanwhile substantiated the effectiveness of the aforementioned mRNA- and vector-based vaccines, especially with respect to severe COVID-19 and thus COVID-19-related death. With respect to further vaccine candidates currently in the pipeline, there is a demand for estimating such performance indicators upfront, as the ultimately expected global reduction in case numbers (due to the already licensed vaccines) and ethical considerations (avoiding placebo groups when vaccines are already standard care) argue against large phase 3 efficacy studies11. Not unexpectedly, the levels of SARS-CoV-2 neutralizing antibodies (nAbs), and antibodies binding to the S protein, have recently been identified as promising correlates of protection12,13, i.e., measuring SARS-CoV-2 antibodies in serum samples of vaccinees enables to predict the risk of developing COVID-19. However, while the clinical evaluation of vaccine candidates regularly includes such antibody readouts, the heterogenous design of the underlying virus neutralization and binding assays limits the quantitative comparison of the primary result (e.g., the neutralization titer, or arbitrary binding units) across distinct studies. These technical hurdles can be alleviated by the incorporation of an international standard, which has recently been made available to study SARS-CoV-2 antibody-containing samples14. Indeed, this international standard has recently been employed for analyses of vaccinee sera from the two mRNA- and two vector-based vaccines mentioned above to predict an overall protective level of S protein-binding antibodies15. Further, using a pseudovirus neutralization assay, Gilbert et al. found that anti-SARS-CoV-2 potencies of 8 and 140 international units per milliliter (IU/mL) correspond to 70 and 90% efficacy of the Spikevax vaccine, respectively16. By additional comparison to a similar study on Vaxzevria recipients’ sera17, the authors also showed an encouragingly congruent quantitative relationship (4 and 83 IU/mL corresponding to 70 and 90% efficacy, respectively), despite the different vaccine platforms and pseudoviral assays.
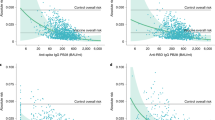
In the present study, we made use of the first international SARS-CoV-2 antibody standard and directly compared the nAb levels in response to a protein-, an mRNA- and a vector-based vaccine. All study subjects were confirmed to have been initially seronegative (no detectable SARS-CoV-2 neutralization for samples obtained at the day of first vaccination). Samples from individuals vaccinated either with NVX-CoV2373 (n = 30), Comirnaty (n = 35) or Vaxzevria (n = 12) were collected 15–32 days after complete immunization (2 doses of the respective vaccine; Table 1). Average post-vaccination anti-SARS-CoV-2 potency was 548 IU/ml, 557 IU/ml, and 202 IU/ml for recipients of NVX-CoV2373, Comirnaty, and Vaxzevria, respectively (Fig. 1). ANOVA (P = 0.004) and post-hoc pairwise comparisons confirmed that mean SARS-CoV-2 nAb levels were equivalent for NVX-CoV2373 and Comirnaty groups (adjusted P value: 0.998) and significantly lower for the Vaxzevria group (adjusted P values: NVX-CoV2373 vs. Vaxzevria: 0.007; Comirnaty vs. Vaxzevria: 0.005). SARS-CoV-2 nAb levels were insignificantly affected by donor age (P = 0.122) and gender (P = 0.768).
Serum samples were obtained 15 to 32 days after complete SARS-CoV-2 immunization, i.e., 2 doses of either NVX-CoV2373 (n = 30), Comirnaty (n = 35), or Vaxzevria (n = 12), and analyzed by live virus neutralization assay. Anti-SARS-CoV-2 potency is given as IU/ml, i.e., relative to the first WHO international SARS-CoV-2 antibody standard (NIBSC code 20/136). Individual samples are shown as grey dots and overlaid by geometric mean ± 95% confidence interval. Statistical analysis (one-way ANOVA with Tukey’s multiple comparisons test) was conducted on ln-transformed data.
Previously, the explanatory power of SARS-CoV-2 nAbs with respect to vaccine efficacy has been investigated by an indirect approach, i.e., via normalization of neutralization titers to cohorts of convalescent (post-COVID-19) individuals analyzed in parallel12,13. However, such cohorts are subject to considerable variation (e.g., due to different parameters that define convalescence, due to diverging fractions of individuals that had suffered from severe versus mild disease, or sample size), as is the sequence of mathematical operations for normalization. Thus, while Khoury et al. found the average SARS-CoV-2 nAb levels induced by Vaxzevria to be lower than average post-COVID-19 levels12, Earle et al. found slightly higher mean anti-SARS-CoV-2 potency of the vaccine13. The latter result is similar to our own data, as the mean vaccine-induced SARS-CoV-2 nAb level of 202 IU/ml (Table 1) is above the mean (140 IU/ml) of a post-COVID-19 group analyzed in one of our earlier studies18. The accuracy of the earlier used comparison to convalescent plasma for normalization must therefore be considered limited.
In contrast, the results of the present study are derived from the same assay and provide a first direct comparison of the SARS-CoV-2 nAb response between three COVID-19 vaccines based on different immunogenic principles. Comparable anti-SARS-CoV-2 potency in sera of NVX-CoV2373 and Comirnaty recipients and the slightly lower levels in response to Vaxzevria are in line with previously published levels of vaccine efficacy2,4,7, directionally confirm the previous, more indirect approaches12,13 and lend further support to the notion that neutralizing antibody responses represent a suitable correlate of protection. Between the three vaccination groups, there is a somewhat diverging mean time between second vaccination and sample collection (Table 1), which is a limitation of our study. However, it should be noted that the peak immune response (and hence the achievement of a ‘fully vaccinated’ status) is generally considered to commence two weeks after the second shot (i.e., when serum samples from the NVX-CoV2373 group were obtained) and antibody waning occurs over several weeks to months rather than days (the latter being the difference in sampling between our three groups). Further, while distinct ethnicities might be a confounding factor of the present study, it should be noted that diverging COVID-19 vaccine efficacy between Asian and White vaccine recipients has not been reported2,4. Most importantly, the calibration of results against the first international SARS-CoV-2 antibody standard14 allows for an objective comparison beyond the present study population.
Methods
SARS-CoV-2 neutralization assay
Detection of SARS-CoV-2 neutralizing antibodies employed live SARS-CoV-2 (strain BetaCoV/Germany/BavPat1/2020) and was based on microscopic readout of cytopathic effects on Vero cells19. Briefly, human serum samples were prediluted 1:5–1:40 with cell culture medium followed by serial dilution in twofold steps. Sample dilutions were combined with an equal volume of virus stock at 103.0 tissue culture infectious doses 50% per milliliter (TCID50/mL) and incubated for 150 ± 15 min before titration on Vero cells (Cat. no. 84113001, ECACC, Porton Down, Salisbury, UK) in eight-fold replicates per dilution. The virus-induced cytopathic effect was determined after 5–7 days of incubation (36 °C; humidified atmosphere with 5% CO2). The 50% neutralization titer, i.e., the reciprocal sample dilution resulting in 50% virus neutralization (NT50) was determined using the Spearman-Kaerber formula. The neutralization assay included several validity criteria, i.e., confirmatory titration of input virus infectivity, cell viability, and neutralization testing of an internal reference standard, all of which had to comply with defined ranges. Further, the internal standard was calibrated against the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (human; NIBSC code: 20/136)14 to enable the quantification of anti-SARS-CoV-2 potency in international units per milliliter (IU/ml). Data from 28 subjects of the Comirnaty group has been included in a previous study20.
Ethics
The study was performed in accordance with applicable regulations, policies, and procedures, and the authors’ institutions required informed consent that was obtained from all study subjects. For Comirnaty and Vaxzevria groups, all subjects received two vaccinations according to EMA approval. For NVX-CoV2373, samples from 30 participants who received two vaccinations of NVX-CoV2373/TAK-019 (5 µg of a recombinant nanoparticle spike protein plus 50 µg of Matrix-M adjuvant; with 21 days apart) of an ongoing phase 1/2 clinical trial in Japan (TAK-019-1501 study, ClinicalTrials.gov Identifier: NCT04712110) were randomly selected.
Data preparation and statistical analyses
Basic statistical calculations for normalization of SARS-CoV-2 neutralization titers and conversion into IU/ml were conducted using MS Excel (v2102; Microsoft, Redmont, WA, US). For hypothesis testing, SARS-CoV-2 nAb levels (IU/ml) were ln-transformed. Graphical illustration and statistical analyses for the influence of distinct vaccines were done using GraphPad Prism (v8.1.1; GraphPad Software, San Diego, CA, US; one-way ANOVA and Tukey’s multiple comparisons test). The influence of donor age and gender was assessed using Minitab (v17.3.1; Minitab, LLC, State College, PA, US; general linear model). All P values are 2-sided and an alpha level of 0.05 is used.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Krammer, F. SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020).
Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med 384, 403–416 (2021).
Falsey, A. R. et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 385, 2348−2360 (2021).
Sadoff, J. et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med 384, 2187–2201 (2021).
Keech, C. et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med 383, 2320–2332 (2020).
Heath, P. T. et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med 385, 1172–1183 (2021).
Novavax Inc. Novavax and Serum Institute of India Receive Emergency Use Authorization for COVID-19 Vaccine in Indonesia. last accessed: 09-FEB-2022, https://ir.novavax.com/2021-11-01-Novavax-and-Serum-Institute-of-India-Receive-Emergency-Use-Authorization-for-COVID-19-Vaccine-in-Indonesia (2021).
Novavax Inc. Novavax and Serum Institute of India Receive Emergency Use Authorization for COVID-19 Vaccine in the Philippines. last accessed: 09-FEB-2022, https://ir.novavax.com/2021-11-17-Novavax-and-Serum-Institute-of-India-Receive-Emergency-Use-Authorization-for-COVID-19-Vaccine-in-the-Philippines (2021).
European Medicines Agency. EMA recommends Nuvaxovid for authorisation in the EU. last accessed: 09-FEB-2022, https://www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu (2021).
Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 27, 1147–1148 (2021).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Earle, K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39, 4423–4428 (2021).
Kristiansen, P. A. et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 397, 1347–1348 (2021).
Goldblatt, D. et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine 40, 306–315 (2021).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 375, 43–50 (2022).
Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 27, 2032–2040 (2021).
Farcet, M. R. et al. Rapidly Increasing SARS-CoV-2 Neutralization by Intravenous Immunoglobulins Produced from Plasma Collected During the 2020 Pandemic. J. Infect. Dis. 16, jiab142 (2021).
Schwaiger, J. et al. Neutralization by Intravenous Immunoglobulins Produced From Plasma Collected Before the 2020 Pandemic. J. Infect. Dis. 222, 1960–1964 (2020).
Zollner, A. et al. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine 70, 103539 (2021).
Acknowledgements
We thank all study participants that consented to participate in this project. The contributions of the entire Global Pathogen Safety team, most notably Simone Knotzer, Jasmin de Silva, Stefan Pantic, Brigitte Kainz, Melanie Graf, Elisabeth List and Petra Gruber (neutralization assays), Veronika Sulzer, Sabrina Brandtner (cell culture), Eva Ha and Alexandra Schlapschy-Danzinger (virus culture) are gratefully acknowledged. SARS-CoV-2 was sourced via EVAg (supported by the European Community, agreement no. 871029) and kindly provided by Christian Drosten and Victor Corman (Charité Universitätsmedizin, Institute of Virology, Berlin, Germany). This study was supported by Takeda Pharmaceutical Company Limited, the Christian Doppler Research Association, and the Johannes Kepler University Linz.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design: M.K., M.R.F., and T.R.K.; Methodology: M.K. and M.R.F.; Data curation: M.K. and A.Z.; Writing-original draft preparation: M.K., M.R.F., and T.R.K.; Writing-review and editing: all authors. Final approval of the completed version: all authors. M.K. and M.R.F. serve as co-first authors, i.e., have contributed equally to the study.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing non-financial interests, but the following competing financial interests apply: MK, MRF and TRK are employees of Takeda Manufacturing Austria AG, Vienna, Austria, and have Takeda stock interest. TM and MM are employees of Takeda Pharmaceutical Limited. Takeda has an ongoing collaboration with Novavax to manufacture and distribute NVX-CoV2373 in Japan.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karbiener, M., Farcet, M.R., Zollner, A. et al. Calibrated comparison of SARS-CoV-2 neutralizing antibody levels in response to protein-, mRNA-, and vector-based COVID-19 vaccines. npj Vaccines 7, 22 (2022). https://doi.org/10.1038/s41541-022-00455-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-022-00455-3
This article is cited by
-
Progress with COVID vaccine development and implementation
npj Vaccines (2024)