Abstract
Obesity and cirrhosis are associated with poor hepatitis B virus (HBV) vaccine responses, but vaccine efficacy has not been assessed in nonalcoholic fatty liver disease (NAFLD). Sixty-eight HBV-naïve adults with NAFLD were enrolled through the Canadian HBV network and completed three-dose HBV or HBV/HAV vaccine (Engerix-B®, or Twinrix®, GlaxoSmithKline). Anti-HBs titers were measured at 1–3 months post third dose. In 31/68 subjects enrolled at the coordinating-site, T-cell proliferation and follicular T-helper cells (pTFH) were assessed using PBMC. Immune response was also studied in NAFLD mice. NAFLD patients were stratified as low-risk-obesity, BMI < 35 (N = 40) vs. medium-high-risk obesity, BMI > 35 (N = 28). Anti-HBs titers were lower in medium/high-risk obesity, 385 IU/L ± 79 vs. low-risk obesity class, 642 IU/L ± 68.2, p = 0.02. High-risk obesity cases, N = 14 showed lower vaccine-specific-CD3+ CD4+ T-cell response compared to low-risk obesity patients, N = 17, p = 0.02. Low vaccine responders showed dysfunctional pTFH. NAFLD mice showed lower anti-HBs levels and T-cell response vs. controls. In conclusion, we report here that obese individuals with NAFLD exhibit decreased HBV vaccine-specific immune responses.
Similar content being viewed by others
Introduction
The implementation of universal childhood hepatitis B virus (HBV) vaccination in >200 countries has led to a significant decline in the global incidence of chronic hepatitis B and hepatocellular carcinoma (HCC), and has been hailed as the first vaccine known to prevent cancer1. All available HBV vaccines contain the major HBV surface antigen (HBsAg) and induce antibody and T cell responses in >85% of immunocompetent adults2,3,4. The Centres for Disease Control and Prevention recommends the HBV vaccine to all individuals with chronic liver disease (i.e., persons with hepatitis C virus (HCV) infection, diabetes, autoimmune hepatitis, alcohol, and nonalcoholic fatty liver disease (NAFLD))5.
Over the last 2 decades, the obesity epidemic has contributed to the development of the metabolic syndrome and NAFLD6,7,8. Obesity leads to a chronic inflammatory state and negatively affects immunity as evidenced by higher rates of vaccine failure, immunity against foreign pathogens, and infection complications9. In general, there is limited knowledge regarding the impact of obesity and NAFLD on immune functions per se. Studies in animal models suggest altered lymphocyte responsiveness to mitogens, dysregulated cytokine expression, decreased macrophage, natural killer cell, and dendritic cell function to pathogens10,11. In general, obesity can disrupt overall immune system integrity and lead to alterations in leukocyte development, migration, and diversity. Although HBV vaccination is strongly recommended in all individuals with chronic liver disease, there is limited data on HBV vaccine responses in adults with obesity and NAFLD. Moreover, most studies only focussed on B-cell immunity (i.e., antibody to HBV surface (anti-HBs) responses) to the vaccine. Some studies have found that obesity was associated with poor response to HBV vaccine in preadolescents and in adults with body mass index (BMI) >30 kg/m2 compared to non-obese individuals12,13. Reduced anti-HBs seroconversion rates were also found in adults with diabetes and renal disease compared to healthy adults14,15. Although one study found a high prevalence of HBV vaccine unresponsiveness in ~70% of pediatric patients with NAFLD16, another reported no difference in vaccine response rates in children with NAFLD compared to healthy controls17.
We conducted a prospective interventional study to assess the efficacy of HBV vaccination in HBV-naïve adults with NAFLD in Canada, a low HBV endemic country that did not implement universal childhood immunization until the mid 1990s18. In this study, we assessed anti-HBs titers, ex vivo B- and T-cell responses, and the role of a special subset of CD4+ T cells (i.e, peripheral T follicular helper cells, pTFHs) involved in generation and maintenance of memory B and plasma cells following completion of three-dose HBV vaccine series (i.e., EngerixB® or Twinrix®). Moreover, we provide supportive parallel HBV immunization data from a unique high-fat diet (HFD)-induced mouse model of NAFLD.
Results
Summary of baseline demographic and clinical data
In total, 386 patients were screened of which 356 were excluded, usually due to prior HBV exposure or vaccination (N = 193) or age (N = 127). Three withdrew from the study or were lost to follow-up. Overall, 68 HBV naive NAFLD adults completed the standard HBV primary vaccination and stratified as BMI < 35 (low-risk obesity) or BMI > 35 (medium high-risk obesity) based on Health Canada nomogram19. Figure 1 describes the experimental design of this multisite, phase IV, open-label interventional study. The clinical characteristics of enrolled patients are included in Table 1. The overall median age was 50 years, 35 females, 78% (53/68) Caucasian,16% (11/68) diabetic, 40 had BMI < 35 vs. 28 with BMI > 35. In 31 enrolled at the coordinating site, ex vivo B- and T-cell responses (17 with BMI < 35 and 14 with BMI > 35) (Table 2) were also assessed.
Anti-HBs titers in adults with NAFLD after completion of three-dose HBV vaccine series negatively correlated with BMI
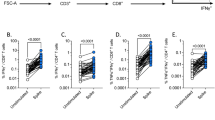
In total, 85% (58/68) of study participants developed protective anti-HBV surface levels (anti-HBs > 10 IU/L) (Table 3). HBV vaccine nonresponse (anti-HBs < 10 IU/L) was found in 10/68 NAFLD patients. The rates of vaccine non-response were similar in low- vs. high-risk obesity class patients with NAFLD (i.e., 15% (6/40) of patients with BMI < 35 and 14.2% (4/28) of patients with BMI > 35 were nonresponders) (Table 4). Poor response to the vaccine was not related to age, gender, smoking status, metabolic syndrome (diabetes, dyslipidemia, and hypertension), and fibrosis stage (including patients who underwent liver biopsy, Supplementary Table 1). All the vaccine nonresponders had increased waist circumference indicating abdominal obesity and risk of obesity related health problems based on Health Canada recommendations (i.e., ≥102 cm for males and ≥82 cm for females)20 (Table 4). Interestingly, a negative correlation (r = −0.3, p = 0.03) was observed between anti-HBs and BMI (Fig. 2A). In addition, humoral vaccine response was not associated with age and sex (Fig. 2B, C).
A Spearman’s rank correlation between anti-HBs levels with BMI, in N = 68 vaccinated NAFLD patients. The data show a moderate negative correlation between BMI and anti-HBs titers (Spearman’s Rank correlation co-efficient r = −0.3, p = 0.03). B Anti-HBs titers were comparable between males and females (p = 0.7, Mann–Whitney U test). C No association was noted between age and anti-HBs levels. D Anti-HBs levels in low and high-risk obesity patients with NAFLD. Anti-HBs levels were significantly lower (p = 0.02) in the high risk obesity group vs. low-risk obesity group NAFLD patients (Mann–Whitney U test). Data is presented as mean ± standard deviation.
NAFLD patients with BMI > 35 show weaker humoral immune responses to hepatitis B vaccine compared to those with BMI < 35
Overall, NAFLD patients with BMI > 35 showed significantly lower mean anti-HBs levels compared to low-risk obesity class patients with BMI < 35. The mean anti-HBs levels in patients with BMI > 35 were 385 IU/L ± 79 compared to 642 IU/L ± 68.2 in patients with BMI < 35 (p = 0.02; Fig. 2D).
Assessment of total memory B cells
Previous studies have shown that individuals who are nonresponders to the HBV vaccine (anti-HBs < 10 IU/L) show a strong anamnestic response to a booster dose suggesting an effect on memory B cells. Analyses of PBMC were performed in subjects (N = 31/68) enrolled at the coordinating site. The proportion of total memory B cells CD45+ CD19+ CD27+ in PBMC from NAFLD patients was comparable between patients with BMI > 35 vs. BMI < 35, p > 0.05 (Supplementary Fig. 1).
Assessment of memory T cells and HBsAg-specific-proliferation of CD3 + CD4+ TH cells
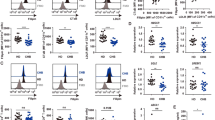
Long term T cell-mediated protection depends on the generation of a pool of memory cells to protect against future pathogen challenge. The two types of memory T cells central to a vaccine response are central memory T cells (TCM), having a reactive function (similar to memory B cells), and effector memory cells (TEM) that are protective (similar to antibodies)21. The gating strategy for memory T cells is shown in Fig. 3A. The proportion of circulating TEM and TCM cells between NAFLD adults with BMI < 35 and BMI > 35 at baseline and after vaccination showed no significant difference (Fig. 3B, C). CD4+ T cells showed HBsAg-specific-proliferation in 12/17 (70.5%) cases in patients with BMI < 35 group compared to 5/14 (35.7%) with BMI > 35 (Fig. 3D, E). In addition, high-risk obesity NAFLD patients (mean SI 3.1 ± 0.62) showed a reduced proliferation compared to low-risk cases (mean stimulation index (SI) 5.1 ± 0.68), p = 0.02. Further, gating of CD3+ CD4+ CFSE low cells revealed comparable frequencies of TEM cells between both groups (Fig. 3F). In 2/17 cases with BMI < 35, and 1/13 BMI > 35 we found antigen specific proliferation of CD8+ cells (Supplementary Fig. 2). This was unexpected given that CD8+ T cell responses are difficult to induce by proteins and these responses are likely due to cross presentation of antigen to CD8+ T cells.
A Gating strategy for T cells. B Proportion of circulating central memory cells (TCM, CCR7+ CD45RA−). C Proportion of circulating effector memory cells (TEM, CCR7− CD45RA−) gated on live CD3+ CD56− CD4+ cells at baseline and after vaccination in NAFLD patients. The data show no differences in the percentage of the cells at baseline and post vaccination between low and high-risk obesity NAFLD cases (Krushkal–Wallis test). D Representative CFSE proliferation assays from a low (showed proliferation) and high risk patient (who did not show proliferation) using PBMC. Every sample was cultured in triplicates with DMSO controls, HBsAg stimulated cells (5 µg) for ~8 days and positive control anti-CD3 (1 µg/mL) + anti-CD28 (5 µg/mL) beads for 4 days. E Stimulation index (SI) in low risk vs. high-risk cases: high risk obesity class NAFLD patients showed weaker T cell proliferation compared to low-risk patients, p = 0.02 (Mann–Whitney U test). F Proportion of effector memory cells among the proliferated CD4+ T cells: TEM frequencies between 12 low-risk and 5 high-risk obesity patients that showed HBsAg specific T-cell proliferation were comparable (Mann–Whitney U test). Data are presented as mean ± standard deviation. G Correlation between anti-HBs levels, IU/L and CD4+ T cell stimulation index: Spearman’s Rank correlation co-efficient = 0.6, p = 0.001. CFSE carboxyfluorescein diacetate succinimidyl ester, PBMC peripheral blood mononuclear cells.
Correlation between HBsAg-specific-T-cell response and humoral response
A direct correlation has been reported previously between serological and T-cell responses22. We found a positive correlation between serum anti-HBs levels and CD4+ T cell SI in 31 patients (Fig. 3G).
Serum CXCL13 and IL-21 and peripheral T-follicular helper cells (pTFHs) in NAFLD patients at baseline and post vaccination
Initially, we compared the pTFH cell response in NAFLD patients with BMI > 35 and BMI <35 at baseline and post vaccination and found no difference among the groups (Supplementary Fig. 3). We then grouped patients on the basis of antibody levels given that pTFH cells, a subset of CD4+ T cells are involved in generation and maintenance of memory B and plasma cells23. We evaluated two important effectors, serum CXCL13 and IL-21 levels and proportion of pTFH between non/low responders, anti-HBs < 100 IU/L and normal-high responders (anti-HBs > 100 IU/L). No differences were observed in the baseline and post vaccination cytokine levels in both the groups (Fig. 4A, B). However, we found a negative correlation between baseline serum CXCL13 levels and anti-HBs levels (Fig. 4C). Interestingly, post vaccination increase in pTFH was noted in only in the normal-high responders but not in the non/low responders (Fig. 4D).
A Serum CXCL13 B serum IL-21 levels in pg/mL at baseline and post vaccination in 31 NAFLD patients. C Spearman’s rank correlation between baseline CXCL13 and anti-HBs levels in NAFLD, r = −0.5, p = 0.009. D Frequency of pTFH in PBMC among NAFLD patients. E HBsAg specific CXCL13 production by pTFH cocultured with B cells. Sorting experiments were carried out in N = 19/21 responders and N = 9 non/low responders. Data are presented as mean ± standard deviation. R normal and high responders, anti-HBs > 100 IU/L, NR/LR nonresponders and low responders, anti-HBs < 100 IU/L, unstim unstimulated, stim stimulated.
Antigen specific release of CXCL13 and IL-21 by sorted pTFHs
Coculture of pTFHs with B cells in the presence of HBsAg showed a significant antigen specific increase in both CXCL13 and IL-21 in the normal-high responders but not in the non-low responders suggesting dysfunctional pTFHs in this group (Fig. 4E, F).
Anti-HBs antibody levels and T-cell responses in a mice model of NAFLD
Mice models who were fed a HFD to induce NAFLD and given the HBV vaccine are depicted in Fig. 5. The liver histology of mice on HFD showed hallmarks of fatty liver disease (<66% steatosis in zone 2 and 3) confirming HFD-induced NAFLD (Fig. 6A). HFD mice both in group V→N (vaccinated before NAFLD was induced) and in N→V group (vaccine administered after NAFLD was induced by HFD) exhibited lower anti-HBs antibody levels than controls (p < 0.05) (Fig. 6B). The N→V group mice also showed a lower CD4+ T-cell response than normal mice and a similar trend was observed in V→N study mice compared to controls (Fig. 6C).
A Liver histology of control and HFD mice showing hallmarks of fatty liver disease. Greater than 66% steatosis was noted in zone 2 and 3. B Qualitative ELISA showing anti-HBs in NAFLD mice vs. controls. NAFLD mice showed significantly lower levels of anti-HBs than controls (p < 0.05, ANOVA test). C In vitro proliferation of CD3+ CD4+ splenocytes at ~day 5 in response to 5 µg HBsAg. Mice in the NAFLD→vaccination (N→V) group showed significantly lower proliferation than controls (p < 0.05, ANOVA). The same trend was observed in the Vaccination→NAFLD (V→N) group. Data are represented as mean ± standard deviation.
Discussion
This novel interventional study was designed to evaluate the efficacy of hepatitis B vaccine using a standard dosing schedule in HBV naïve/ previously unvaccinated adults with a clinical diagnosis of NAFLD. Overall, this study determined that upon completion of the full vaccination regimen, using the approved recombinant hepatitis B vaccine, seroprotection was induced in ~85% of subjects. A similar rate of nonresponse (15–20% of patients with anti-HBs < 10 IU/L) to the HBV vaccine have been shown in persons with diabetes and inflammatory bowel disease24,25. Importantly, this reduced antibody response was more pronounced in high-risk obesity class NAFLD patients with BMI > 35 compared to individuals with a lower BMI < 35 at only ~1 month post primary vaccination. Similar to others, we noted a negative correlation between response to vaccination and BMI26. However, it is also noteworthy that 3 of 10 nonresponders in the study were cases with a BMI 25–30 (i.e., overweight but nonobese individuals). We found comparable rates of non-immunity (~15%) in both low- vs. high-risk obesity groups, and hence addresses the potential issue of sufficient needle length for immunization of obese individuals27. Older age at the time of vaccination (>60 years) has been associated with inadequate antibody response28. The median age in the group in our study was ~50 years and no correlation between age and humoral response was observed. Further, known factors for vaccine non-response such as liver fibrosis stage (i.e., cirrhosis), metabolic syndrome (i.e., diabetes, hypertension, and dyslipidemia), gender or smoking status were not associated with antibody response in the current study29.
Antibody response to hepatitis B vaccine involves both B and T cells. We analyzed T cell responses in 31 NAFLD patients and found reduced HBsAg-specific-CD4+ T cell response in patients with BMI > 35 compared to patients with BMI < 35. We noted a comparable frequency of TEM among the proliferated cells in low- and high-risk obesity groups, however, we did not investigate the cytokine production by these cells. Prior studies found intact T cell responses in healthy individuals immunized with the recombinant HBV vaccine and the traditional plasma derived vaccine in the absence of antibodies30. In obesity, cell death and enlarged adipocytes occurs due to a constant pro-inflammatory state. Additionally, there is a reduced proportion of CD4+ T cells, which are activated by nonconventional means involving leptin and result in sub-optimal CD4+ T cell differentiation and proliferation in response to antigens31,32. Moreover, studies in NAFLD mice models suggest decreased antigen processing and presentation in splenic dendritic cells compared to controls in association with an impaired T cell response33.
Dysfunctional pTFH cells have been reported in nonresponders with anti-HBs < 10 IU/L to the HBV vaccine in healthy subjects and also in influenza vaccine nonresponders34,35. It is noteworthy that in NAFLD, low responders in addition to nonresponders show dysfunctional pTFHs. Studies have shown that prevaccination levels of CXCL11 and CXCL12 in healthy individuals and CXCL13 in HIV+ subjects may predict hepatitis B-vaccine response34,36. Thus, the results of our current study suggest that baseline CXCL13 could potentially be a biomarker for vaccine response in NAFLD, and an area for future investigation.
There is evidence regarding the role of human leukocyte antigen (HLA) alleles in response to HBV vaccination with HLA-DRB1 and HLA-DQB1 being associated with strong antibody responses37. We did not perform HLA typing in our study; however, we conducted parallel preclinical studies in a NAFLD mice model to account for vaccine responses within a model of identical genetic backgrounds. We noted blunted anti-HBs and T cell response in mice fed a HFD compared to normal weight mice on normal diet. In the first experiment, mice were fed HFD for 9 weeks and then immunized. This group recapitulates the cohort of NAFLD patients receiving the vaccine. In the second group, both animal groups were immunized at baseline and then fed either a normal vs. HFD, and hence potentially represents the human scenario of HBV vaccination in non-obese children who may subsequently develop adult onset obesity and NAFLD. In agreement with our results, Miyake et al.33 found impaired HBsAg-specific-humoral and T-cell responses in NAFLD mice who received a similar diet. It would be interesting to develop a mouse model that recapitulates nonalcoholic steatohepatitis (NASH) and study mechanism(s) of HBV vaccination response in NAFLD vs. NASH.
Our study did not include an age-matched healthy arm, however, a pooled analysis of previous studies in ~2000 healthy immunocompetent adults predicted >80% seroconversion rates in adults up to 60 years of age28. Additionally, we did not stratify patients with lean NAFLD (BMI < 25), low-risk (BMI: 25–29.9), medium-risk (BMI 30–34.9), and high-risk obesity cases (BMI > 35) or perform comparisons based on waist circumference. However, the current study of 68 adults is one of the largest reported to date regarding HBV vaccine responses in obese adult patients with confirmed diagnosis of NAFLD.
As per clinical guidelines and vaccine monographs, approaches for inducing vaccine response in non and low responders include repeating the vaccination series, a single booster dose or immunization with higher doses. The use of a two-dose novel vaccine, Heplisav-B®-induced high seroprotection rates in diabetics >70 years38. Although this vaccine is approved by the U.S. Food and Drug Administration (FDA), it is not yet approved by Health Canada. Whether NAFLD patients benefit from this vaccine is an interesting area of future investigation. It is known that 5–10% of healthy adults are nonresponders to the HBV vaccine, 15–50% of vaccine recipients are anti-HBs negative within 5–10 years after vaccination39. The results of the current study are remarkable in that 15% (10/68) of NAFLD patients were non-responders to the primary vaccination as early as 1-month post vaccination. Gara et al.40 determined that anti-HBs titers decline more slowly among at-risk adults compared to childhood vaccine recipients. Although a recent study showed vaccine-specific-T-cell responses lasting at ~20–30 years after adult immunization41, to date no studies have explored long-term humoral and adaptive immune response to the HBV vaccine in NAFLD adults. We plan to conduct a follow-up study to assess long-term responses (i.e., antibody waning, the persistence of T-cell immunity and anamnestic response) to the vaccine in individuals enrolled in this study.
In summary, this study showed significantly reduced HBV vaccine-specific-antibody and T cell responses in NAFLD patients with a BMI > 35 compared to low-risk obesity class NAFLD patients with BMI < 35. The hepatitis B vaccine is one of the most important human vaccines recommended worldwide. Obesity and NAFLD impairs normal immune functioning and may further perpetuate chronic disease development, and complications of the metabolic syndrome. This clinical study, together with complementary animal model data, can inform vaccine strategies, development of improved immunization protocols and highlight the impaired host immune response in high-risk obesity class individuals with NAFLD.
Patients and methods
Study participants and vaccines
Sixty-eight HBV naive NAFLD patients (18–60 years) in this study were recruited from specialized NAFLD clinics at five Canadian centers from August 2016–2019. Exclusion criteria included subjects <18 and >60 years of age, pregnancy, human immunodeficiency virus+, HCV+, decompensated cirrhosis, and those with serological evidence of HBV exposure or immunization (HBsAg, anti-HBs) and total HBV core antibody (anti-HBc). All patients provided signed informed consent under an approved ethics protocol (Conjoint Health Research Ethics Board REB16-0274) according to the principles of Good Clinical Practice and the Declaration of Helsinki. The study was registered on ClinicalTrials.gov (NCT02985450). The in-clinic review included identifying risk factors for NAFLD (obesity/hyperlipidemia/metabolic syndrome/type 2 diabetes) in the absence of significant alcohol consumption and/or an ultrasound showing confirmed steatosis. The diagnosis of NASH vs. NAFLD is based on histopathological criteria (i.e., steatosis, lobular inflammation, and hepatocellular ballooning)8 which requires a liver biopsy42,43. However, few study patients underwent liver biopsy due to invasive nature in the current study and was often not clinically indicated if patients had normal transient elastography. All adults received the standard three-dose regimen (0–6 months) of Engerix B® (20 µg of HBsAg and 500 µg aluminum hydroxide, Glaxosmithkline, GSK) or a combined HBV/HAV vaccine (Twinrix®,GSK) as per Canadian immunization guidelines44. The vaccines were purchased by patients or health practitioners and administered as an intramuscular injection into the deltoid muscle of the nondominant arm by clinic nurse.
Assessment of anti-HBs antibodies after vaccination
Anti-HBs titers were assessed in patients at 1–3 months after complete vaccination (i.e., three doses of Engerix B® or Twinrix B®) by a chemiluminescent microparticle immunoassay (detection range, 0–1000 IU/L, Abbott Architect, Mississauga, ON, Canada). HBV seroprotection was defined as anti-HBs ≥ 10 IU/L after complete vaccination45.
Isolation of peripheral blood mononuclear cells (PBMC)
PBMC were separated by density gradient centrifugation from ~20 mL of heparinized blood, and ~107 cells/vial were cryopreserved. PBMC were only isolated at the coordinating center at baseline (i.e., before the vaccination) and post vaccination for assessment of cellular response.
Assessment of circulating memory B and T cells
The proportion of memory B and T cells in PBMC at baseline and post vaccination was assessed using flow cytometry. Approximately, 5 × 105 PBMC were stained for memory B-cell markers using anti-CD19, anti-CD45, and anti-CD27. Memory T cells were identified using anti-CD3, anti-CD4, anti-CD8, anti-CD56, anti-CD45RA, and anti-CCR7 using multi-parameter flow cytometry (BD FACS Canto II, Toronto, ON, Canada) Catalog and lot numbers of the antibodies used can be found in Supplementary Table 2. Prior to staining, cells were stained with fixable viability dye FVS 510. Antibodies were used at dilutions recommended by the vendor. Appropriate isotype and compensation controls were used and a minimum of 100,000 events was acquired. Data were analyzed using FACS DIVA and Flowjo v11 (Treestar Inc., San Carlos, CA).
HBsAg-specific proliferation assays
Fresh (or cryopreserved in 4 patients) PBMC (~106) were labeled with 1 µM carboxyfluorescein-diacetate-succinimidyl-ester (CFSE; BD Horizon, San Diego, CA) according to vendor’s protocol. Labeled PBMC were stimulated with 5 µg HBsAg (adw) (ARP, Waltham, MA) in RPMI 1640 with 10% FBS and 2 mmol/L glutamine. Anti-CD3 (1 µg/mL) and anti-CD28 (5 µg/mL) (BD Biosciences, San Jose, CA) stimulated cells served as a positive control. Unstimulated DMSO-treated cells were used as negative controls. Cells were cultured in triplicates and plates incubated at 37 °C with 5% CO2 for ~8 days. Cell proliferation was assessed on day 8. SI was calculated as % CFSE low cells in stimulated cells / % CFSE low cells in the unstimulated control46. SI > 3 was considered positive for HBsAg-specific proliferation. Cells were stained using the memory T-cell panel and analyzed by flow-cytometry.
Peripheral follicular helper T cell responses (pTFH)
Baseline and post-vaccination PBMC were labeled with anti-CD4 and anti-CXCR5 antibodies. In addition, CD19+ and CD4+ CXCR5+ cells were sorted (>85% purity) using BD FACS ARIA II (Supplementary Fig. 4) and 30,000 cells were cocultured (1:1) for 5 days in the presence of 3.5 µg of HBsAg + 2 µg/mL anti-CD28. Staphylococcal enterotoxin B (Sigma, Oakville, ON, Canada) was used as a positive control. Cell culture supernatant and serum levels of CXCL13 and IL-21 were measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., Oakville, ON, Canada).
HBV vaccination in a mice model of NAFLD
Mice were purchased from Jackson Inc. (Bar Harbour, ME) and housed at the University of Calgary in a pathogen-free facility. All experiments were approved by the University of Calgary animal care committee in accordance with the Canadian Council for Animal care (AC16-0040). C57BL/6 male mice (6–8 weeks old, N = 40;10/group), were fed either a normal chow vs. HFD (40% fat, 40% sucrose, Dyets Inc., Bethelem, PA)47 and administered 2 doses of Engerix-B® (0.05 µg/g body weight) 2 weeks apart, intramuscularly, either after inducing NAFLD phenotype (N→V) or at baseline prior to HFD (V→N). The induction of NAFLD phenotype in mice was confirmed on liver histology. Briefly, liver lobes were harvested from NAFLD and control mice, fixed in formalin and embedded in paraffin. Sections were cut at 4 µm diameter and stained with hematoxylin and eosin. Slides were blinded and evaluated by a clinical pathologist to assess for liver steatosis, inflammation, and hepatocyte ballooning.
Assessment of anti-HBs and T cell response in a mice model of NAFLD
Blood was collected before vaccination and every 2 weeks post booster via tail vein bleeding and by cardiac puncture at the final time point. Whole blood was collected in heparinized tubes and spun at 1000g for 10 min to obtain plasma. Anti-HBs levels in control and HFD mice were determined using a qualitative ELISA (Aviva Biosystems Biology, San Diego, CA). Spleens were harvested at the final time point for lymphocyte proliferation assessment. A single-cell suspension of splenocytes was prepared and CFSE labeled as described above and ~106 cells were stimulated with 5 µg of HBsAg. Concanavalin A (Con-A) (4 µg/mL) stimulated and DMSO treated cells served as positive and negative controls, respectively. On day 4–5, splenocytes were assessed for the proliferation of CD3+ CD4+ T cells using flow-cytometry (Supplementary Table 2).
Statistical analysis
Data were analyzed using Graphpad Prism 7 (La Jolla, CA). Demographic, clinical, and laboratory parameters were compared using measures of central tendency; Mann–Whitney or ANOVA were used to compare anti-HBs titers; Mann–Whitney U test and Kruskal–Wallis test with post hoc Dunn’s test for multiple comparisons was used to compare proliferation and immunophenotyping data. Nonparametric Spearman’s rank correlation test was used for correlation analysis; p values < 0.05 were considered
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All the relevant information is in the paper in the form of tables, figures, and supplementary material.
References
WHO | Global health sector strategy on viral hepatitis 2016–2021. (WHO, 2017).
Bauer, T. & Jilg, W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 24, 572–577 (2006).
Simons, B. C. et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J. Infect. Dis. 214, 273–280 (2016).
Rosado, M. M. et al. Switched memory B cells maintain specific memory independently of serum antibodies: the hepatitis B example. Eur. J. Immunol. 41, 1800–1808 (2011).
Schillie, S. et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 67, 1–31 (2018).
Obesity in Canada—Obesity Canada. https://obesitycanada.ca/obesity-in-canada/ (2019).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Liver Health Month-Check Your Engine—Canadian Liver Foundation. https://www.liver.ca/liver-health-month-2018-checkyourengine/ (2019).
Andersen, C. J., Murphy, K. E. & Fernandez, M. L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 7, 66–75 (2016).
Ma, C. et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257 (2016).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019).
Bandaru, P., Rajkumar, H. & Nappanveettil, G. The impact of obesity on immune response to infection and vaccine: An insight into plausible mechanisms. Endocrinol Metab. Synd. 2, 113 (2013).
Liu, F., Guo, Z. & Dong, C. Influences of obesity on the immunogenicity of hepatitis B vaccine. Hum. Vaccin. Immunother. 13, 1014–1017 (2017).
Schillie, S. F., Spradling, P. R. & Murphy, T. V. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes Care 35, 2690–2697 (2012).
Roni, D. A. et al. Safety and efficacy of hepatitis B vaccination in cirrhosis of liver. Adv. Virol. 2013, 1–5 (2013).
Mehta, M., Slaughter, C., Xanthakos, S. A. & Kohli, R. High prevalence of hepatitis B non-immunity in paediatric non-alcoholic fatty liver disease patients. Dig. Liver Dis. 46, 760–761 (2014).
Seniuta, J., Miskiewicz, K. & Szenborn, L. Good response to the primary course and booster dose of hepatitis B vaccine in children with non-alcoholic fatty liver disease. Dig. Liver Dis. 49, 105 (2017).
Polaris Observatory Collaborators, D. et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 3, 383–403 (2018).
Body Mass Index (BMI) Nomogram—Canada.ca. https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/healthy-weights/canadian-guidelines-body-weight-classification-adults/body-mass-index-nomogram.html (2019).
Canadian Guidelines for Body Weight Classification in Adults—Canada.ca. https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/healthy-weights/canadian-guidelines-body-weight-classification-adults/questions-answers-public.html#a4 (2019).
Gray, J. I., Westerhof, L. M. & MacLeod, M. K. L. The roles of resident, central and effector memory CD4 T-cells in protective immunity following infection or vaccination. Immunology 154, 574–581 (2018).
Lu, C. et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J. Infect. Dis. 197, 1419–1426 (2008).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Van Der Meeren, O. et al. Prospective clinical trial of hepatitis B vaccination in adults with and without type-2 diabetes mellitus. Hum. Vaccin. Immunother. 12, 2197–2203 (2016).
Jiang, H. et al. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis. Vaccine 35, 2633–2641 (2017).
Liu, J. et al. Robust antibody and cytokine response to hepatitis b vaccine among not-in-treatment patients with chronic hepatitis C: an open-label control study in China. J. Infect. Dis. 216, 327–335 (2017).
Middleman, A. B., Anding, R. & Tung, C. Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics 125, e508–e512 (2010).
Van Der Meeren, O., Crasta, P., Cheuvart, B. & De Ridder, M. Characterization of an age-response relationship to GSK’s recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum. Vaccin. Immunother. 11, 1726–1729 (2015).
Roukens, A. H. & Visser, L. G. Human Vaccines Hepatitis B Vaccination Strategy in Vaccine Low and Non-responders: A Matter of Quantity of Quality? https://doi.org/10.4161/hv.7.6.14986 (2011).
Carollo, M. et al. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine 31, 506–513 (2013).
Green, W. D. & Beck, M. A. Obesity altered T cell metabolism and the response to infection. Curr. Opin. Immunol. 46, 1–7 (2017).
Mauro, C. et al. Obesity-induced metabolic stress leads to biased effector memory CD4+ T cell differentiation via PI3K p110δ-Akt-mediated signals. Cell Metab. 25, 593–609 (2017).
Miyake, T. et al. Impaired dendritic cell functions disrupt antigen-specific adaptive immune responses in mice with nonalcoholic fatty liver disease. J. Gastroenterol. 45, 859–867 (2010).
Doi, H. et al. Immune determinants in the acquisition and maintenance of antibody to hepatitis B surface antigen in adults after first-time hepatitis B vaccination. Hepatol. Commun. 3, 812–824 (2019).
Pallikkuth, S. et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood 120, 985–993 (2012).
Paris, R. M. et al. Lower baseline germinal center activity and preserved Th1 immunity are associated with hepatitis B vaccine response in treated HIV infection. Pathog. Immun. 2, 66–88 (2017).
Li, Y. et al. Clear and independent associations of several HLA-DRB1 alleles with differential antibody responses to hepatitis B vaccination in youth. Hum. Genet. 126, 685–696 (2009).
Hyer, R. N. & Janssen, R. S. Immunogenicity and safety of a 2-dose hepatitis B vaccine, HBsAg/CpG 1018, in persons with diabetes mellitus aged 60–70 years. Vaccine 37, 5854–5861 (2019).
Klinger, G., Chodick, G. & Levy, I. Long-term immunity to hepatitis B following vaccination in infancy: real-world data analysis. Vaccine 36, 2288–2292 (2018).
Gara, N. et al. Durability of antibody response against hepatitis B virus in healthcare workers vaccinated as adults. Clin. Infect. Dis. 60, 505–513 (2015).
Van Damme, P. et al. Persistence of HB sAg‐specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J. Viral Hepat. https://doi.org/10.1111/jvh.13125 (2019).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, P1388–1402 (2016).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver. Diseases https://doi.org/10.1002/hep.29367/suppinfo (2017).
Hepatitis B. Vaccine: Canadian Immunization Guide—Canada.ca. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-7-hepatitis-b-vaccine.html (2019).
Jack, A. D., Hall, A. J., Maine, N., Mendy, M. & Whittle, H. C. What level of hepatitis B antibody is protective? J. Infect. Dis. 179, 489–492 (1999).
Al-Ayoubi, J. et al. Hepatitis E virus ORF 1 induces proliferative and functional T-cell responses in patients with ongoing and resolved hepatitis E. Liver Int. 38, 266–277 (2018).
Davis, R. P. et al. Optimization of in vivo imaging provides a first look at mouse model of non-alcoholic fatty liver disease (NAFLD) using intravital microscopy. Front. Immunol. 10, 2988 (2019).
Seo, M. H. et al. Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 28, 40–45 (2019).
Acknowledgements
We acknowledge Dr. Karen Poon from the Nicole Perkins Microbial Communities Core of the Snyder Institute, the University of Calgary for the cell sorting experiments. We would also like to thank Dr. Stefan Urbanski for assistance with pathology slide review, Keith Lau and Aaron Lucko for processing blood samples and Dr. Sarah Haylock-Jacobs, and all other research coordinators in the Canadian HBV Network. Dr. Carla S. Coffin is supported by funding from the Canadian Institutes of Health Research (CIHR) [Funding Reference Number (FRN) 144160] and GlaxoSmithKline Biologicals (GSK) SA (study: 204732). GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy but the authors are solely responsible for final content and interpretation. GSK funded the human component of the study.
Author information
Authors and Affiliations
Contributions
S.S.J. and C.S.C. conceived, designed the study, and wrote the paper. S.S.J. performed the experiments and analyzed the data. M.M.M., E.T., C.L.C., A.R., E.M.K., S.J., and M.G.S. recruited patients to the study and provided feedback on the paper. R.P.D. and C.N.J. designed and executed mice experiments in the study and provided feedback on the paper. All authors read and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
C.S.C.: Investigator-Initiated Research Grant/Research Materials—G.S.K., Gilead Sciences. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joshi, S.S., Davis, R.P., Ma, M.M. et al. Reduced immune responses to hepatitis B primary vaccination in obese individuals with nonalcoholic fatty liver disease (NAFLD). npj Vaccines 6, 9 (2021). https://doi.org/10.1038/s41541-020-00266-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-020-00266-4