Abstract
We previously reported that intramuscular injections of ubiquitin ligase CBLB inhibitory pentapeptide (Cblin; Asp-Gly-pTyr-Met-Pro) restored lost muscle mass caused by sciatic denervation. Here, we detected Cblin on the basolateral side of Caco-2 cells after being placed on the apical side, and found that cytochalasin D, a tight junction opener, enhanced Cblin transport. Orally administered Cblin was found in rat plasma, indicating that intact Cblin was absorbed in vitro and in vivo. Furthermore, transgenic Cblin peptide-enriched rice (CbR) prevented the denervation-induced loss of muscle mass and the upregulation of muscle atrophy-related ubiquitin ligases in mice. These findings indicated that CbR could serve as an alternative treatment for muscle atrophy.
Similar content being viewed by others
Introduction
Insulin-like growth factor 1 (IGF-1) signaling is a major pathway of skeletal muscle growth and hypertrophy1. We previously reported that skeletal muscle disuse such as that associated with space flight, tail suspension, and sciatic denervation upregulates expression of the Cblb gene encoding the ubiquitin ligase, Casitas B-lineage lymphoma b (CBLB)2. Overexpressed CBLB protein induces the ubiquitination and degradation of insulin receptor substrate 1 (IRS1), the key adapter protein of IGF-1 signaling, in vitro and in vivo3,4, then induces the expression of genes encoding muscle atrophy-related ubiquitin ligases, such as muscle atrophy F-box protein (MAFbx)5,6 and muscle RING finger 1 (MuRF1)5 by activating forkhead transcription factor 3 (FOXO3)7,8. We also showed that Cblb-deficient mice are resistant to muscle atrophy induced by tail suspension4. These results indicated that CBLB-dependent IRS1 degradation is a critical mediator of muscle atrophy under unloading conditions.
We developed a CBLB inhibitory peptide (Cblin peptide) with the amino acid sequence Asp-Gly-phosphorylated (p) Tyr-Met-Pro to prevent muscle atrophy. This peptide mimics the sequence of tyrosine608-phosphorylated IRS1, interacts with the tyrosine kinase binding (TKB) domain of CBLB, and subsequently inhibits the CBLB-mediated ubiquitination of IRS19. Injecting Cblin peptide into the skeletal muscle of mice with sciatic denervation prevented the loss of muscle mass4. However, repetitive intramuscular injections of the peptide are invasive and stressed the mice.
Many di- and tripeptides derived from food sources exert bioactivities such as antihypertensive effects in vivo10,11,12. Bioactive di- and tripeptides are absorbed into the bloodstream via the intestinal proton-coupled peptide transporter-1, PEPT1, encoded by the Slc15a1 gene13,14. A peptide-binding cavity in PEPT1 allows it to attach to di- and tripeptides, but not to peptides with ≥4 amino acid residues. The orally administered pentapeptide, Arg-Val-Pro-Ser-Leu, which has angiotensin-converting enzyme inhibitory activity in vitro lowers blood pressure in spontaneously hypertensive rats15. Oligopeptides such as Arg-Val-Pro-Ser-Leu, Val-Leu-Pro-Val-Pro16, and Thr-Asn-Gly-Ile-Ile-Arg17 can penetrate Caco-2 cell monolayers, presumably via paracellular tight junctions. We previously discovered that the respective tetra- and pentapeptides Gly-Sar-Sar-Sar and Gly-Sar-Sar-Sar-Sar that have methylated peptide bonds can be absorbed intact in rats, although to a lesser extent than di- and tripeptides18. However, whether Cblin peptide is similarly absorbed requires further investigation.
We previously showed that feeding mice with soy glycinin inhibited muscle atrophy induced by sciatic denervation19. We speculated that this effect might have resulted from proteases in the gastrointestinal tract releasing Cblin-like peptides such as Asp-Ile-Tyr-Asn-Pro from soy glycinin. We considered that consuming foods containing Cblin-like peptides might prevent muscle atrophy. Akama et al. developed a transgenic rice strain that is rich in gamma-aminobutyric acid20, and novel plant systems that stably accumulate Asp-Gly-Tyr-Met-Pro in rice seeds21. The present study aimed to determine whether Asp-Gly-Tyr-Met-Pro is absorbed by the intestine and whether Cblin-rich rice (CbR) can prevent muscle atrophy in rodents.
Results and discussion
Transport of Cblin peptides across Caco-2 cell monolayers
The intestinal transport of Cblin peptides Asp-Gly-pTyr-Met-Pro and Asp-Gly-Tyr-Met-Pro (initial apical concentration: 1 mM) was investigated in Caco-2 cell monolayers with transepithelial electrical resistance (TEER) > 300 Ω · cm2 over a period of 60 min. Basolateral solutions were sampled every 15 min for 60 min and analyzed by LC–TOF/MS. The mass spectrometry (MS) signals ([M + H]+) corresponding to 662.1625 m/z for Asp-Gly-pTyr-Met-Pro and 582.2228 m/z for Asp-Gly-Tyr-Met-Pro were time dependently increased at retention times (RTs) of 19.8 and 19.4 min, respectively (Fig. 1a, b). Therefore, both peptides penetrated Caco-2 cell monolayers and remained intact. Peptide fragments were screened based on their specific m/z values. After 60 min, Asp-Gly-pTyr-Met-Pro was dephosphorylated and Asp-Gly-Tyr-Met-Pro was transported to the basolateral solutions at an estimated concentration that was similar to that of intact Asp-Gly-pTyr-Met-Pro (Fig. 1c). Density-enhanced protein phosphatase-1 or intestinal alkaline phosphatase expressed on the surface of epithelial cells or released into media might be involved in the dephosphorylation process22,23. No other peptide fragments were found, indicating that both peptides are resistant to peptidase degradation in Caco-2 cells (data not shown). The calculated apparent permeability coefficients (Papp) were 3.5 ± 1.2 × 10−7 and 7.0 ± 0.8 × 10−7 cm/s for Asp-Gly-pTyr-Met-Pro and Asp-Gly-Tyr-Met-Pro, respectively. These values were in the same order as the pentapeptide transport model Gly-Sar-Sar-Sar-Sar (Papp: 8.6 ± 0.6 × 10−7 cm/s24; Table 1), indicating that the transport velocity of Cblin peptides across Caco-2 cell monolayers was appropriate.
Chromatograms show apical to basolateral transepithelial transport of 1 mM Asp-Gly-pTyr-Met-Pro (a) and Asp-Gly-Tyr-Met-Pro (b) in Caco-2 cell monolayers over 60 min. Samples from the basolateral side at 15, 30, 45, and 60 min were analyzed using LC–TOF/MS with [M + H]+ at m/z 662.1625 for Asp-Gly-pTyr-Met-Pro and 582.2228 for Asp-Gly-Tyr-Met-Pro. (c) Dephosphorylation of Asp-Gly-pTyr-Met-Pro monitored in basolateral solution after 60 min of transport by extracting ions with m/z 582.2228 corresponding to Asp-Gly-Tyr-Met-Pro ions.
Transport pathway studies showed that the Papp values of Asp-Gly-pTyr-Met-Pro and Asp-Gly-Tyr-Met-Pro (Fig. 2a, b) were not affected by 10 mM Gly-Sar, a PEPT1 substrate, suggesting that PEPT1 is not involved in transporting the two pentapeptides. These results concurred with those of a study showing that PEPT1 only recognizes and transports peptides with ≤3 amino acid residues25. Meanwhile, the Papp values of both peptides were significantly higher in monolayers incubated with cytochalasin D, a paracellular tight junction opener, than in untreated membranes (Fig. 2c, d). These results indicated that Cblin passively diffuses across paracellular tight junctions like several other oligopeptides26. We could not rule out the involvement of other pathways in the intestinal absorption of Cblin pentapeptides. The transcytosis mechanism is involved in transporting the decapeptide Tyr-Trp-Asp-His-Asn-Asn-Pro-Gln-Ile-Arg that is derived from rapeseed protein27 and β-casein, a 17-residue peptide (193–209)28. Chothe et al. reported that some transport systems in intestinal epithelial cell lines might be specific to oligopeptides with >3 residues29.
Effects of 10 mM Gly-Sar on transport of 1 mM Asp-Gly-pTyr-Met-Pro (a) and Asp-Gly-Tyr-Met-Pro (b) across Caco-2 cell monolayers for 60 min. Basolateral peptide concentrations were determined after 60 min by LC–TOF/MS. Effects of cytochalasin D on transport of 1 mM Asp-Gly-pTyr-Met-Pro (c) and Asp-Gly-Tyr-Met-Pro (d) across Caco-2 cell monolayers for 60 min. Caco-2 cell monolayers were incubated with or without cytochalasin D (0.5 μg/mL) for 30 min. Basolateral peptide concentrations were determined by LC–TOF/MS. Values are expressed as means ± SEM, n = 4–6. *P < 0.05, **P < 0.01. NS, not significant.
Absorption of Asp-Gly-Tyr-Met-Pro into rat circulation
Sprague-Dawley (SD) rats were fasted for 16 h, then administered p.o. with 100 mg/kg of body weight (BW) of Asp-Gly-Tyr-Met-Pro, and blood was sampled at 0, 15, 30, 45, and 60 min thereafter. Absolute plasma Asp-Gly-Tyr-Met-Pro concentrations were determined using an internal standard comprising plasma spiked with a final concentration of 50 pmol/mL Asp-Gly-Tyr-Met-Pro labeled with stable isotopes (Asp-Gly[13C2; 15N]-Tyr-Met-Pro) (Fig. 3a, box). A peak corresponding to m/z of the Asp-Gly-Tyr-Met-Pro peak on MS was undetectable in plasma at 0 min (Fig. 3a). Absorbed Asp-Gly-Tyr-Met-Pro peaked at 2.78 ± 0.17 pmol/mL in plasma samples at 15 min (Fig. 3b and Table 2), then decreased over the next 45 min (Fig. 3a, b). The pentapeptide at 50 and 200 mg/kg BW was also absorbed (Supplementary Table 1). We previously showed that the orally administered pentapeptide, Gly-Sar-Sar-Sar-Sar, is absorbed into rat circulation18. Although less Asp-Gly-Tyr-Met-Pro than Gly-Sar-Sar-Sar-Sar was absorbed, the model pentapeptide was more resistant to peptidase degradation due to having methylated peptide bonds18. Therefore, we screened plasma samples for peptide fragments generated by enzymatic degradation based on m/z values and found tetra- and dipeptide metabolites corresponding to the m/z values of Gly-Tyr-Met-Pro and Met-Pro, respectively (Fig. 3c, d). Exopeptidases such as aminopeptidase and peptidyl dipeptidase expressed on the brush border membrane might have been responsible for generating these peptide fragments30. Other metabolites such as amino acids and hydrophilic peptides might not have been retained on reversed phase columns under our experimental conditions. The bioavailability of Asp-Gly-Tyr-Met-Pro and its metabolism require further clarification.
a Absorption of Asp-Gly-Tyr-Met-Pro in 8-week-old Sprague-Dawley rats. Stacked intensity-time chromatograms of Asp-Gly-Tyr-Met-Pro with [M + H]+ of m/z 582.2228 and 50 pmol/mL of labeled internal standard (IS) Asp-Gly(13C2; 15N)-Tyr-Met-Pro ([M + H]+: m/z 585.2228) (insert) created by sampling plasma at 0, 15, 30, 45, and 60 min from tail veins of rats dosed with 100 mg/kg BW Asp-Gly-Tyr-Met-Pro. b Time course of appearance of Asp-Gly-Tyr-Met-Pro in plasma of rats orally administered with 100 mg/kg BW determined by LC–TOF/MS. Data are expressed as means ± SEM (n = 5). c, d Extracted ions corresponding to those of possible metabolites, Gly-Tyr-Met-Pro ([M + H]+: m/z 467.1959) and Met-Pro ([M + H]+: m/z 247.1111) were detected in rat plasma at 30 min after administration.
Characterization of Cblin peptide-enriched rice (CbR)
Akama et al. recently constructed transgenic CbR to create a foodstuff with antimuscle atrophy properties21. Transgenic rice plants were enriched with Cblin peptide by inserting DNA fragments encoding 15 Gln-Asp-Gly-Tyr-Met-Pro-Trp (Cblin-like peptide) repeats processed by chymotrypsin digestion into the rice glutelin gene21. Previous studies have shown that four repeats of novokinin hexapeptide and six of lactostatin pentapeptide both with Gln-Arg spacers increased the expression efficacy of functional peptides in rice grain31,32. We used Trp as the spacer and avoided basic Arg, which carries a positive charge that reportedly interferes with binding to CBLB33. We preserved the backbone structure and inserted Gln and Trp for chymotrypsin recognition and digestion in the stomach to increase the efficiency of expression and release of the inserted peptide sequence, instead of changing the terminal amino acids Asp and Pro, which might result in a loss of function. Akama et al. also confirmed the accumulation of Cblin-like peptides by immunoblotting with an antibody specific to repeats of Gln-Asp-Gly-Tyr-Met-Pro-Trp. We evaluated the digestibility of 15 Cblin peptide repeats in transgenic rice seeds. Glutelin was extracted from CbR or nontransgenic control rice (non-Tg), then digested in vitro with chymotrypsin to identify released Cblin-like peptide using high-performance liquid chromatography (HPLC) and LC–TOF/MS. As digestion progressed, the HPLC peak at RT 26–27 min became intensified in samples derived from CbR (Fig. 4b), but not from non-Tg (Fig. 4a). The RT (27.0 min) of the synthetic peptide standard (Gln-Asp-Gly-Tyr-Met-Pro-Trp) on HPLC (Fig. 4c) matched that of CbR-derived samples (Fig. 4b). To confirm the generation of Cblin-like peptide in the chymotrypsin hydrolysate of CbR, the HPLC fraction at 27.0 min was analyzed by LC–TOF/MS. The RT for standard Cblin-like peptide was 21.4 min and its apparent m/z was [M + 2H]2+ m/z 448.6840 (Fig. 4f). The MS signal of the 27.0 min HPLC fraction of CbR samples was significant, with a signal-to-noise ratio of 18.0 at 24.1 min in the LC–TOF/MS analysis (Fig. 4e), whereas an MS peak at that RT was undetectable in non-Tg-derived samples (Fig. 4d). This indicated that a peptide with the same m/z and RT values as Cblin-like peptide occurs in CbR and can be generated in the gut by digestion.
Chromatograms of chymotrypsin-digested glutelin-derived peptides from nontransgenic (non-Tg; a) or Cblin-enriched (CbR; b) rice, and synthetic standard peptide Gln-Asp-Gly-Tyr-Met-Pro-Trp (c). Fraction at 27.0 min was collected and further analyzed by LC–TOF/MS while monitoring [M + 2H]2+ of m/z 448.6840 for Gln-Asp-Gly-Tyr-Met-Pro-Trp (d–f). Arrows indicate MS signals corresponding to RT (21.4 min, upper panel) and m/z ([M + 2H]2+: m/z 448.6840, lower panel) of standard Gln-Asp-Gly-Tyr-Met-Pro-Trp. AU, arbitrary units; ND, not detected.
Akama et al. have described the composition of CbR in detail21. They found no significant differences in the contents of protein, amino acids, amylose, and starch between non-Tg and CbR. They and others also constructed transgenic rice strains that are rich in other functional peptides using the same technique as that for CbR34,35. They showed that the contents of moisture, protein, fat, carbohydrate, fiber, amino acids, fatty acids, minerals (sodium, potassium, iron, calcium, magnesium, and zinc), and vitamins (B1, B2, B6, and niacin) in transgenic rice plants and nontransgenic rice seeds were equivalent. These results suggested that nutritional contents other than overexpressed Gln-Asp-Gly-Tyr-Met-Pro-Trp are essentially identical between non-Tg and CbR and that nonsignificant differences in the nutritional contents are within natural ranges.
Ability of CbR to prevent muscle atrophy in vivo
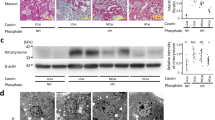
We evaluated the anti-muscle atrophy effects of oral intake of CbR in mice with denervated sciatic nerves. We measured the BW and food consumption of mice fed with rice containing CbR or non-Tg. The BW of mice in both groups increased by 13% during the experimental period with no significant difference (Fig. 5a). The amounts of food consumed by both groups were also similar (Fig. 5b). The wet weight of the gastrocnemius (Ga) and soleus (Sol) muscles decreased by 24% and 45%, respectively, in mice with sciatic denervation that were orally administered with non-Tg (Table 3). We found that CbR significantly suppressed the 13% rate of weight loss caused by denervation in the Ga (Table 3). The intake of CbR suppressed weight loss in the Sol, but this significantly differed between denervated and sham-operated muscle (Table 3). We investigated whether the anti-muscle atrophy effect of CbR correlates with the inhibited degradation of IRS1 protein in Ga muscles of mice fed with CbR. Denervation decreased the total amount of IRS1 protein in mice supplemented with non-Tg. Feeding with CbR restored IRS1 protein even after denervation (Fig. 5c). Along with IRS1 protein preservation, oral CbR intake also suppressed the expression of genes encoding muscle atrophy-related ubiquitin ligases that are regulated by IGF-1 signaling. Expression of the gene encoding the muscle atrophy-related ubiquitin ligase, Mafbx, was 2.7-fold higher in denervated Ga muscles of mice fed with non-Tg (Fig. 5d). Meanwhile, Mafbx mRNA expression in muscle did not significantly differ between denervated and sham-operated mice fed with CbR (Fig. 5d). Expression of the ubiquitin ligase gene, Murf1, was also 2.7-fold higher in mice fed with non-Tg, but significantly suppressed in mice fed with CbR (Fig. 5e). These results indicated that the oral intake of CbR prevented muscle atrophy in mice. Therefore, a standard diet supplemented with CbR might offer an alternative approach to prevent, and perhaps cure muscle atrophy in humans.
Body weight (a) and food consumption (b) during experimental period in mice fed with diets containing non-transgenic (non-Tg; unfilled circles) or Cblin-supplemented (CbR; filled circles) rice. Results are shown as means ± SEM (n = 5–6 per group). Expression of IRS1 protein (c) and Mafbx (d) and Murf1 (e) genes associated with muscle atrophy in sciatic denervated or sham-operated gastrocnemius muscles of mice fed with diets containing non-Tg or CbR. Results are shown as means ± SEM (n = 5−6 per group). *P < 0.05 (S vs. D). †P < 0.01 (non-Tg vs. CbR). D, denervated; S, sham-operated.
We previously found that Cblb is expressed at very low levels in normal skeletal muscle. It is upregulated by oxidative stress in skeletal muscle under unloading conditions and is insensitive to insulin/IGF-136. These findings indicated that CbR exerts beneficial effects only on skeletal muscles that are insensitive to insulin/IGF-1 and are accompanied by Cblb upregulation. Accordingly, we estimated that CbR cannot increase the sensitivity of normal skeletal muscle to insulin/IGF-1. Due to low Cblb expression in skeletal muscle under normal (loaded) circumstances, CbR imposes minimal side effects, because symptoms such as decreased food intake and diarrhea were not found in mice fed with CbR.
We used LC–TOF/MS to analyze CbR-derived peptides in serum and skeletal muscles of mice fed with the transgenic rice. Uptake of the intact Cblin-like heptapeptide, Gln-Asp-Gly-Tyr-Met-Pro-Trp, was undetectable in serum from mice fed with CbR (Supplementary Fig. 2a, b). Although the signals seemed to correspond to the m/z of the intact Cblin-like heptapeptide in serum from mice fed with CbR, we could not consider that they were from the heptapeptide-derived peak because the RT of 14.5 min did not match that of the synthetic heptapeptide. Akama et al. constructed a CbR containing 15 repeats of Gln-Asp-Gly-Tyr-Met-Pro-Trp21 that is released by chymotrypsin digestion (Fig. 4). Chymotrypsin cleaves C-terminal tryptophan, tyrosine, and phenylalanine on peptide chains; thus, Gln-Asp-Gly-Tyr-Met-Pro-Trp, Gln-Asp-Gly-Tyr, or Met-Pro-Trp peptides might be released after oral CbR intake and chymotrypsin digestion. We detected an MS signal at 482.1882 that corresponded to Gln-Asp-Gly-Tyr (Supplementary Fig. 2e). In addition to this peak, several characteristic peaks with diverse m/z values in serum from mice fed with CbR corresponded to predicted metabolites with ≥4 amino acid residues. The MS signals at 710.2814, 613.2286, 582.2228, and 485.1701 corresponded to Gln-Asp-Gly-Tyr-Met-Pro, Gln-Asp-Gly-Tyr-Met, Asp-Gly-Tyr-Met-Pro, and Asp-Gly-Tyr-Met, respectively (Supplementary Fig. 2c, d, f, g and Supplementary Table 4). The Cblin-like heptapeptide, Gln-Asp-Gly-Tyr-Met-Pro-Trp, released in the stomach might undergo unpredicted modifications, which would generate more peptide fragments during the absorption process. In fact, we detected Gly-Tyr-Met-Pro and Met-Pro in the circulation of rats orally administered with Asp-Gly-Tyr-Met-Pro. However, a CbR-specific signal was not detected in skeletal muscle from mice fed with CbR (Supplementary Fig. 3). Although a signal corresponding to m/z 596.2537 that is the estimated value of Tyr-Met-Pro-Trp was detected in skeletal muscle of mice fed with CbR, it was not found in all four analyzed mice (Supplementary Fig. 3h) and it was undetectable in serum (Supplementary Fig. 2h). Based on these findings, we speculated that orally administered CbR is digested in the gut, then characteristic shorter peptides released into the circulation elicit bioactivities that restore muscle mass. The lack and/or the difficulty of reproducibility in detection might be due to low content of the heptapeptide and its metabolites in skeletal muscle. Intra- and interindividual differences such as age18, the time of day37, and the duration of feeding can alter the absorption and distribution of peptides after oral intake. Muscle peptide accumulation after oral intake has not been reported. Therefore, amounts of muscle peptide uptake and the involved mechanism require further evaluation. We plan to detect the peptides in skeletal muscle considering these factors and elucidate the absorption and muscle accumulation of this peptide, its metabolites, and the individual bioactivities of identified metabolite fragments.
As described above, CbR-derived Gln-Asp-Gly-Tyr-Met-Pro-Trp and its metabolic fragments containing the backbone structure seems to be responsible for the functionality of CbR. The amino acid sequence of Cblin pentapeptide was designed to inhibit interaction between CBLB and IRS1 proteins based on the ability of CBLB to recognize and bind to phosphorylated tyrosine38. Metabolites containing tyrosine might also inhibit CBLB binding to IRS1 via their tyrosine residues even when they are not phosphorylated. We previously elucidated the crystal structure of the TKB domain of CBLB complexed with Cblin and found that Tyr in Cblin is essential to conserve hydrophobic interaction between CBLB and Cblin9. In accordance with this, we confirmed the suppressive effect of the shortened Cblin pentapeptide, Gly-pTyr-Met, on CBLB-mediated ubiquitination of IRS1 in vitro9. Furthermore, the modified Cblin peptide Asp-Ile-Tyr-Asn-Pro containing intact (non-phosphorylated) tyrosine also suppresses CBLB-mediated IRS1 ubiquitination19. We did not detect all metabolites in mice given CbR, but those of Cblin-like peptide containing Tyr might have synergistically functioned with intact peptides to inhibit CBLB activity and restore muscle mass. We have scant understanding of which fragments are generated, and how they contribute to the functionality of CbR, but we plan to investigate the absorption behavior and bioactivity of Gln-Asp-Gly-Tyr-Met-Pro-Trp, and screen possible peptide fragments generated by dietary CbR and the relative contribution of each to CbR functionality. These complex topics will be time-consuming, but we will elucidate them.
We estimate that trypsin, which prefers peptide linkages where the carboxylic acid group is provided by a basic amino acid (arginine, lysine, and histidine), does not digest Cblin-like peptide. On the other hand, pepsin has specific preferences, as it selectively targets bonds containing the amine group of aromatic amino acids (tryptophan, tyrosine, and phenylalanine). CbR-derived Cblin-like peptides might be digested as: Gln-Asp-Gly ↓ Tyr-Met-Pro ↓ Trp. However, pepsin is responsible for digesting only ~15% of dietary protein39. Upon CbR feeding, both pepsin and chymotrypsin would function to release Cblin-like peptide and its metabolites, but more peptides might be generated by chymotrypsin than pepsin.
The sequence encoding repeated-Cblin-like peptides derived from CbR is inserted into that encoding glutelin, the major storage protein in rice. Glutelin is stored within protein body (PB)-II in the rice endosperm, while another storage protein, prolamine, is stored in PB-I40. Whereas PB-II is easily digested in humans by proteolytic enzymes, PB-I is resistant to digestion by gastrointestinal enzymes such as pepsin and is excreted41. Although we could not exclude the possibility that some nutrients in CbR affect glutelin digestibility, the deposition sites of inserted peptides are considered important to determine the efficiency of glutelin digestion and to acquire the beneficial effect of the inserted peptides. Takaiwa et al. developed transgenic rice seeds overexpressing cedar pollen allergen and found that the tolerogenic dose required to achieve allergen-specific immune tolerance by oral feeding differs between transgenic seeds that use PB-I or PB-II as deposition sites42. We estimate that PB-II sites containing glutelin with Cblin-like peptides are easily digested in the mouse GI tract and release these peptides, but future studies are needed to determine the digestion and absorption mechanism in vivo.
Coexisting components in food matrices might affect di-/tripeptide absorption by inhibiting or facilitating PEPT1 function43. For example, egg-white protein hydrolysate contains oligopeptides that inhibit peptidase-mediated epithelial hydrolysis, thus contributing to the absorption of longer peptides44. The metabolic behavior and functionality of Gln-Asp-Gly-Tyr-Met-Pro-Trp and its fragment peptides upon CbR intake remained to be determined. Here, CbR digestion in vitro seemed to generate several peptide fragments (Fig. 4). How these fragments and other protein/non-protein components of rice affect the digestion and absorption of Cblin peptide remains unknown. Exosomes or exosome-like vesicles might be involved in the intestinal uptake and systemic delivery of Cblin peptides. Further study is needed to uncover the absorption mechanism of Cblin peptides in the CbR food matrix.
The present study showed that intact Cblin peptide can circulate in the blood of rodents by passing from epithelial cells via tight junctions. Cblin peptide might synergistically function with its metabolites to suppress muscle atrophy in rodents. A standard diet supplemented with CbR could become an alternative approach to preventing, and perhaps even curing, muscle atrophy in humans.
Methods
Transport of Cblin pentapeptides across Caco-2 cell monolayers
Cell culture and transport experiment were performed as described24,45. Caco-2 cells were cultured using BD BioCoat Intestinal Epithelium Differentiation Environment kits (BD Bioscience, Franklin Lakes, NJ, USA). Caco-2 cells grown in 0.9 cm2 Falcon cell culture inserts (polyethylene terephthalate membranes; pore size, 1.0 μm; Becton Dickinson and Co.) with a type I collagen-coated membrane (Cellmatrix type IA Collagen Gel Culturing Kit; Nitta Gelatin Inc., Osaka, Japan) were gently rinsed twice with Hanks balanced salt solution (HBSS; 137 mM NaCl, 5 mM KCl, 5.5 mM D-glucose, 4 mM NaHCO3, 0.75 mM NaHPO4·12H2O, 0.4 mM KH2PO4, 0.8 mM MgSO4·7H2O, and 1.26 mM CaCl2·2H2O, pH 6.0 adjusted with 10 mM 2-(N-morpholino)-ethanesulfonic acid). A portion (1.5 mL) of HBSS buffer (pH 6.0) was added to the apical side, and HBSS buffer (pH 7.4 adjusted with 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) was added to the basolateral side. After equilibration for 15 min at 37 °C, the apical buffer was replaced with fresh HBSS (pH 6.0) containing 1 mM peptide and 0.5% DMSO. Portions (60 μL) of solutions were drawn from the apical or basolateral sides at 15, 30, 45, and 60 min to determine amounts of transported peptides. Monolayer integrity was evaluated by measuring TEER using a Millicell® ERS-2 Voltohmmeter (Merck KGaA, Darmstadt, Germany), and monolayers with a TEER of >300 Ω · cm2 were included in the experiments. The apparent permeability coefficient (Papp, cm/s) was calculated as
where dC/dt is the change in concentration on the basolateral side over time (mM/s), V is the volume of solution in the basolateral compartment (1.5 mL), A is the surface area of the membrane (0.9 cm2), and C0 is the initial concentration on the apical side (mM).
We investigated the involvement of PEPT1 in peptide transport by competition studies using 10 mM Gly-Sar, a PEPT1 substrate46. We also investigated peptide transport over paracellular tight junctions by incubating monolayers with the tight junction opener, cytochalasin D (0.5 μg/mL) for 30 min47 before evaluating transport.
Quantitation of transported oligopeptides
Oligopeptides were separated at 40 °C using a linear gradient of 0.1% formic acid (FA) in MS grade water (solvent A) and 0.1% FA in acetonitrile (ACN) (solvent B) for 20 min at a flow rate of 0.2 mL/min, and an Agilent 1200 series HPLC system (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a Cosmosil 5C18-MS-II column (Φ 2.0 mm × 150 mm, Nacalai Tesque Inc., Kyoto, Japan). The MS conditions comprised: drying gas, N2; flow rate, 8.0 L/min; drying gas temperature, 200 °C; drying gas pressure, 1.6 bar; HV capillary voltage, −4500 V; capillary exit, 70.0 V; skimmer 1, 50.0 V; hexapole 1, 23.0 V; hexapole RF, 100.0 Vpp; skimmer 2, 23.0 V; lens 1 transfer, 52.0 μs, and mass range m/z 100–1000. A calibration solution of 10 mM sodium formate in 50% ACN was injected at the beginning of each run. Data were analyzed using Bruker Data Analysis version 3.2 software (Bruker, Billerica, MA, USA). Calibration curves were prepared using 0.1–10 μM standards.
Detection of orally administered oligopeptides in rats
Seven-week-old male SD (Crj:SD) rats (Charles River Japan, Kanagawa, Japan) were housed for 1 week at 21 ± 1 °C with lights on from 8 a.m. to 8 p.m. and given a standard MF chow diet (Oriental Yeast Co., Tokyo, Japan) with access to water ad libitum. The rats were fasted for 16 h before being orally administered once with Asp-Gly-Tyr-Met-Pro (100 mg/kg BW) dissolved in milli-Q water. Blood samples from the tail veins of the rat at 0, 15, 30, 45, and 60 min thereafter were placed in chilled microtubes containing ethylenediaminetetraacetic acid disodium salt (EDTA-2Na, 0.2 mg) and protease inhibitors (aprotinin, 0.1 mg; chymostatin, 0.1 mg), then plasma was obtained by immediate centrifugation at 3500 × g for 15 min. The plasma samples (100 μL) were spiked with Asp-Gly-Tyr-Met-Pro labeled with stable isotopes (Asp-Gly[13C2; 15N]-Tyr-Met-Pro) at a final concentration of 50 pmol/mL. Plasma was precipitated with ice cold ACN containing 0.1% FA, separated by centrifugation at 14,000 × g for 15 min, then the supernatant was collected and dried using a centrifugal evaporator. Pellets were resuspended in 25 μL of 0.1% FA and analyzed under the LC–TOF/MS conditions described above. Plasma concentrations of Asp-Gly-Tyr-Met-Pro were calculated using the internal standard method (Eq. (2)) where y is the ratio between the observed peak area of Asp-Gly-Tyr-Met-Pro and the internal standard Asp-Gly(13C2; 15N)-Tyr-Met-Pro, and x is the peptide concentration (pmol/mL)
Detection of Cblin-like peptides released from Cblin-enriched transgenic rice seeds after chymotrypsin digestion in vitro
Akama et al. have constructed transgenic Cblin peptide-enriched rice (CbR)21. Rice flour (1 g) prepared from CbR or non-transgenic rice (non-Tg) was delipidized with acetone (10 mL) and dried at ambient temperature for 24 h. Glutelin was then extracted from the rice flour twice with 4 mL of 0.02 M NaOH (pH 12.0) at 20 °C for 30 min and separated by centrifugation at 3000 × g for 30 min. Glutelin precipitated from combined supernatants by adjusting the isoelectric point to pH 5.0 was washed twice with distilled water and dissolved in 100 mM Tris-HCl (pH 7.8) containing 10 mM CaCl2. Glutelin protein content was measured using Pierce BCA Protein Assay Kits (Thermo Fisher Scientific Inc., Waltham, MA, USA). Glutelin (1 mg) was digested in vitro with chymotrypsin (Sigma Aldrich Corp., St. Louis, MO, USA) at a ratio (w/w) of 1:60 at 30 °C for 120 min. Thereafter, protease inhibitor tablets (Sigma Aldrich Co.) were added, then samples were concentrated by vacuum evaporation and dissolved in milli-Q water containing 0.1% trifluoroacetic acid (TFA) for HPLC analysis.
Samples were separated using a Hitachi 5110 HPLC system connected to a Chromaster Organizer, Interface Box, 5430 Diode Array Detector, and 5110 Pump (Hitachi Ltd., Tokyo, Japan) with a TSKgel ODS-80Ts column (2.0 mm × 25 cm, 5 μm; Tosoh Co., Kanagawa, Japan), with a gradient elution of 0.1% TFA (solvent A) in ultrapure water to 0.1% TFA in ACN (solvent B) over 60 min at a flow rate of 0.2 mL/min: 0–5 min, 5% B isocratic; 5–30 min, 5–60% B; 30–40 min, 60–70% B; 41–45 min, 70–100% B; 45–50 min, 100% B isocratic; 50–55 min, 100–5% B; 55–60 min, 5% B isocratic. Chromatograms were obtained at 220 nm. Data signals were processed using a Chromaster System Manager (Hitachi Ltd.). The fraction at RT 27.0 min (corresponding to synthetic Gln-Asp-Gly-Tyr-Met-Pro-Trp) was further analyzed by LC–TOF/MS as described above except for the capillary exit and hexapole RF being 60 V and 100 Vpp, respectively.
Determination of effects of transgenic Cblin peptide-enriched rice on mice with sciatic denervation
Six-week-old male Jcl:ICR mice (Japan SLC Inc., Shizuoka, Japan) were housed with access to a standard diet and water ad libitum under lights on from 8 a.m. to 8.0 p.m. The experimental diet comprised powdered Cblin rice grains (CbR) and non-transgenic rice (non-Tg) mixed with the standard diet (50% w/w). Supplementary Table 2 shows the composition of the diets. The mice were then fed with the diet containing either CbR or non-Tg for 7 days, then their sciatic nerves were unilaterally transected under anesthesia. The contralateral innervated (sham-operated) muscles of the same animals served as controls. Seven days later, the mice were sacrificed and the Sol and Ga muscles were dissected, weighed, and frozen in liquid nitrogen.
All animal experiments proceeded according to the guidelines for animal experiments at Tokushima University and Kyushu University. The Ethics Review Committee for Animal Experimentation at these institutions approved all the experimental protocols described herein (Permission Nos. T27–113 and A30-015-4).
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using ISOGEN (Nippon Gene, Osaka, Japan). Single-stranded cDNA was synthesized using M-MLV Reverse Transcriptase (Promega). Real-time RT-PCR proceeded using SYBR-Green Master Mix (Thermo Fisher Scientific Inc., Waltham, MA, USA) and a Real-time PCR system (Thermo Fisher Scientific Inc.). Supplementary Table 3 shows the primer sequences. Amounts of target mRNA were normalized relative to that of Actb.
Immunoblotting
Proteins were extracted from mouse muscles into Tris-HCl buffer, pH 7.5, containing 150 mM NaCl, 5 mM EDTA, 1% TritonX-100, 10 mM NaF, 2 mM Na3VO4, 10 μM MG132, and protease inhibitor tablets (Roche Diagnostics, Basel, Switzerland). Total protein extracts were resolved by SDS-PAGE and transferred to PVDF membranes. PBS-Tween containing 4% Block Ace Powder (DS Pharma Biomedical Co. Ltd., Osaka, Japan) was used to block non-specific binding. Then, the membranes were incubated with primary antibodies at 4 °C overnight. Chemiluminescent blot was detected using ImmunoStar LD (Wako Pure Chemical Industries, Osaka, Japan) and signals were quantified by densitometry. All blots or gels were derived from the same experiment and were processed in parallel. The primary antibodies were anti-IRS1 (Merck Millipore Burlington, MA, USA) and anti-ACTIN (ABclonal Technology, Woburn, MA, USA).
Statistical analysis
Values were statistically evaluated by Student’s t tests using Excel-Toukei 2015 software (Social Survey Research Information Co. Ltd., Osaka, Japan), and are expressed as means ± SEM. Statistical significance among Papp of Asp-Gly-pTyr-Met-Pro, Asp-Gly-Tyr-Met-Pro, and Gly-Sar-Sar-Sar-Sar was assessed by one-way analyses of variance (ANOVA) followed by Tukey post hoc tests. Data from mice fed with non-Tg or CbR were statistically evaluated by two-way ANOVA followed by Tukey–Kramer multiple comparisons for post hoc analysis using Excel-Toukei 2015 software. Values with P < 0.05 were considered significantly different.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and supplementary information.
References
Glass, D. J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 37, 1974–1984 (2005).
Nikawa, T. et al. Skeletal muscle gene expression in space-flown rats. FASEB J. 18, 522–524 (2004).
Suzue, N. et al. Ubiquitin ligase Cbl-b downregulates bone formation through suppression of IGF-I signaling in osteoblasts during denervation. J. Bone Miner. Res. 21, 722–734 (2006).
Nakao, R. et al. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol. Cell. Biol. 29, 4798–4811 (2009).
Bodine, S. C. et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 (2001).
Gomes, M. D., Lecker, S. H., Jagoe, R. T., Navon, A. & Goldberg, A. L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl Acad. Sci. USA 98, 14440–14445 (2001).
Sandri, M. et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004).
Stitt, T. N. et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 (2004).
Ohno, A. et al. Structural analysis of the TKB domain of ubiquitin ligase Cbl-b complexed with its small inhibitory peptide, Cblin. Arch. Biochem. Biophys. 594, 1–7 (2016).
Kawasaki, T. et al. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hum. Hypertens. 14, 519–523 (2000).
Sipola, M. et al. Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. J. Physiol. Pharmacol. 52, 745–754 (2001).
Majumder, K. et al. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 8, e82829 (2013).
Adibi, S. A. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology 113, 332–340 (1997).
Palacín, M., Estévez, R., Bertran, J. & Zorzano, A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev. 78, 969–1054 (1998).
Ding, L., Wang, L., Zhang, Y. & Liu, J. Transport of antihypertensive peptide RVPSL, ovotransferrin 328-332, in human intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 63, 8143–8150 (2015).
Lei, L., Sun, H., Liu, D., Liu, L. & Li, S. Transport of Val-Leu-Pro-Val-Pro in human intestinal epithelial (Caco-2) cell monolayers. J. Agric. Food Chem. 56, 3582–3586 (2008).
Ding, L., Wang, L., Yu, Z., Zhang, T. & Liu, J. Digestion and absorption of an egg white ACE-inhibitory peptide in human intestinal Caco-2 cell monolayers. Int. J. Food Sci. Nutr. 67, 111–116 (2016).
Hanh, V. T. et al. Effect of aging on the absorption of small peptides in spontaneously hypertensive rats. J. Agric. Food Chem. 65, 5935–5943 (2017).
Abe, T. et al. Soy glycinin contains a functional inhibitory sequence against muscle-atrophy-associated ubiquitin ligase Cbl-b. Int. J. Endocrinol. 2013, 907565–907565 (2013).
Akama, K. et al. Seed-specific expression of truncated OsGAD2 produces GABA-enriched rice grains that influence a decrease in blood pressure in spontaneously hypertensive rats. Transgenic Res. 18, 865–876 (2009).
Akama, K. et al. Functional rice with tandemly repeated Cbl-b ubiquitin ligase inhibitory pentapeptide prevents denervation-induced muscle atrophy in vivo. Biosci. Biotechnol. Biochem. 85, 1415–1421 (2021).
Dörfel, M. J. & Huber, O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 807356–807356 (2012).
Estaki, M., DeCoffe, D. & Gibson, D. L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 20, 15650–15656 (2014).
Hong, S. M., Tanaka, M., Koyanagi, R., Shen, W. & Matsui, T. Structural design of oligopeptides for intestinal transport model. J. Agric. Food Chem. 64, 2072–2079 (2016).
Newstead, S. et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. EMBO J. 30, 417–426 (2011).
Shen, W. & Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 8, 4306–4314 (2017).
Xu, F. et al. Transepithelial transport of YWDHNNPQIR and its metabolic fate with cytoprotection against oxidative stress in human intestinal Caco-2 cells. J. Agric. Food Chem. 65, 2056–2065 (2017).
Regazzo, D. et al. The (193-209) 17-residues peptide of bovine β-casein is transported through Caco-2 monolayer. Mol. Nutr. Food Res. 54, 1428–1435 (2010).
Chothe, P., Singh, N. & Ganapathy, V. Evidence for two different broad-specificity oligopeptide transporters in intestinal cell line Caco-2 and colonic cell line CCD841. Am. J. Physiol. Cell Physiol. 300, C1260–C1269 (2011).
Picariello, G., Ferranti, P. & Addeo, F. Use of brush border membrane vesicles to simulate the human intestinal digestion. Food Res. Int. 88, 327–335 (2016).
Yamada, Y. et al. Anti-hypertensive activity of genetically modified soybean seeds accumulating novokinin. Peptides 29, 331–337 (2008).
Wakasa, Y. et al. The hypocholesterolemic activity of transgenic rice seed accumulating lactostatin, a bioactive peptide derived from bovine milk β-lactoglobulin. J. Agric. Food Chem. 59, 3845–3850 (2011).
Cherlyn, N. et al. Structural basis for a novel intrapeptidyl H-bond and reverse binding of c-Cbl-TKB domain substrates. EMBO J. 27, 804–816 (2008).
Takagi, H. et al. Biochemical safety evaluation of transgenic rice seeds expressing T cell epitopes of Japanese cedar pollen allergens. J. Agric. Food Chem. 54, 9901–9905 (2006).
Lechasseur, B. et al. Incipient malunion of an isolated humeral trochlea fracture treated with an elbow hemiarthroplasty: case report. J. Hand Surg. Am. 40, 271–275 (2015).
Uchida, T. et al. Reactive oxygen species upregulate expression of muscle atrophy-associated ubiquitin ligase Cbl-b in rat L6 skeletal muscle cells. Am. J. Physiol. Cell Physiol. 314, C721–C731 (2018).
Okamura, A. et al. Bile acid-regulated peroxisome proliferator-activated receptor-α (PPARα) activity underlies circadian expression of intestinal peptide absorption transporter PepT1/Slc15a1. J. Biol. Chem. 289, 25296–25305 (2014).
Finkelstein, L. D. & Shimizu, Y. Role of phosphoinositide 3-kinase and the Cbl adaptor protein in coupling the alpha4beta1 integrin to mitogen-activated protein kinase signalling. Biochem. J. 345, 385–392 (2000).
Norman, A. W. & Henry, H. L. Hormones 3rd edn, Ch. 7 (Academic Press, 2015).
Ogawa, M. et al. Mutants for rice storage proteins: 2. Isolation and characterization of protein bodies from rice mutants. Theor. Appl. Genet. 78, 305–310 (1989).
Ogawa, M. et al. Purification of protein body-I of rice seed and its polypeptide composition. Plant Cell Physiol. 28, 1517–1527 (1987).
Takagi, H. et al. Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnol. J. 3, 521–533 (2005).
Shen, W. & Matsui, T. Intestinal absorption of small peptides: a review. Int. J. Food Sci. Tech. 54, 1942–1948 (2019).
Wang, L., Ding, L., Du, Z., Yu, Z. & Liu, J. Hydrolysis and transport of egg white-derived peptides in Caco-2 cell monolayers and everted rat sacs. J. Agric. Food Chem. 67, 4839–4848 (2019).
Takeda, J., Park, H.-Y., Kunitake, Y., Yoshiura, K. & Matsui, T. Theaflavins, dimeric catechins, inhibit peptide transport across Caco-2 cell monolayers via down-regulation of AMP-activated protein kinase-mediated peptide transporter PEPT1. Food Chem. 138, 2140–2145 (2013).
Ma, K., Hu, Y. & Smith, D. E. Peptide transporter 1 is responsible for intestinal uptake of the dipeptide glycylsarcosine: studies in everted jejunal rings from wild-type and Pept1 null mice. J. Pharm. Sci. 100, 767–774 (2011).
Ding, L. et al. Transport of egg white ace-inhibitory peptide, Gln-Ile-Gly-Leu-Phe, in human intestinal Caco-2 cell monolayers with cytoprotective effect. J. Agric. Food Chem. 62, 3177–3182 (2014).
Acknowledgements
This study was supported by JSPS Research Fellowship for Young Scientists to W.S. (No. 19J20948), JSPS KAKENHI to T.N. (JP18H04981 and JP19H04054) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, and AMED-CREST to T.N. (JP19gm0810009h0104). The authors thank Arisa Ochi, Hina Ishikawa, and Yuki Nomura (Tokushima University) for technical assistance.
Author information
Authors and Affiliations
Contributions
R.N., W.S., Y.Sh., K.K., K.O., A.O., T.U., M.T., K.A., T.M., and T.N. designed the study protocol. R.N., W.S., Y.Sh., K.K., Y.Sa., A.U., K.O., M.N., M.T., K.A., and T.M. conducted experiments. R.N., W.S., Y.Sh., K.K., Y.Sa., K.O., A.O., M.T., K.A, T.M., and T.N. analyzed data. R.N., W.S., Y.Sa., M.T., K.A, T.M., and T.N. contributed to writing the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakao, R., Shen, W., Shimajiri, Y. et al. Oral intake of rice overexpressing ubiquitin ligase inhibitory pentapeptide prevents atrophy in denervated skeletal muscle. npj Sci Food 5, 25 (2021). https://doi.org/10.1038/s41538-021-00108-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-021-00108-0
This article is cited by
-
In vitro and in silico characterization of adiponectin-receptor agonist dipeptides
npj Science of Food (2021)