Abstract
In this study, experiments on amyloid β peptide25-35-induced mice were performed to provide in vivo evidence on the potential of the blood–brain barrier transportable soy dipeptide, Tyr-Pro, in combating memory impairment. We demonstrated for the first time that oral administration of Tyr-Pro (100 mg/kg, twice a day) in mice for 16 days significantly improved impaired memory by spontaneous alternation and shortened step-through latency in amyloid β-induced mice.
Similar content being viewed by others
Introduction
In a recent study, we provided strong evidence that dipeptides possessing Pro, such as Gly-Pro and Tyr-Pro, can be transported across the blood–brain barrier (BBB) in an intact form into the parenchyma of peptide-perfused mouse brain1. Thus far, several animal reports have hinted at the memory-improving effect of peptides, such as Leu-His, which attenuates microglial activation and emotional disturbances2, Met-Lys-Pro3, and Trp-Tyr4, which prevent cognitive decline. In the report of administered Trp-[carboxyl-14C]Tyr4, radioactive substances were detected in mouse brain, while no evidence on accumulation of the intact dipeptide in the brain parenchyma was provided. In contrast, using our proposed phytic acid-aided MALDI-MS/MS imaging analysis1,5, we pointed out the first finding that the BBB-transportable Tyr-Pro from soybean hydrolysate1 subsequently accumulated in the hippocampus, cerebral cortex, hypothalamic area, striatum, and cerebellum of mouse brain. The accumulated regions mainly regulate memory6; therefore, in vivo experiments using memory-impaired mice will provide insight into the benefits of BBB-transportable Tyr-Pro against cognitive impairment. Amyloid β peptide (Aβ)25-35-induced mice were used for this study, since the Aβ25-35-induction was confirmed to cause the impairment of cognitive brain function7,8.
Results and discussion
Acute Alzheimer’s disease (AD) has been typically simulated in mice by inducing cognitive impairment through Aβ25-35 injection (6 nmol per mouse i.c.v.)7,8, and soy dipeptide Tyr-Pro1 was orally administered at the dose of 100 mg/kg twice a day, as indicated in Fig. 1a. Regarding the Y-maze test, Tyr-Pro significantly improved reduced spontaneous alternation induced by Aβ (Fig. 1c) (p < 0.05), while the administration for 16 days did not affect locomotive activity of mice (no significant differences were noted in the number of total entries in the long arm between groups, Fig. 1b). This indicated that BBB transportable soy dipeptide Tyr-Pro can be potentially active in preventing reduction of working memory in mice.
Tyr-Pro (100 mg/kg) was orally administered twice a day for 16 days, except for days of i.c.v. injection of Aβ25–35 peptide and the behavioural tests (Tyr-Pro administration once a day) (a). The mice received Aβ25–35 peptide injection (6 nmol/mouse, i.c.v.) on the 7th day and Tyr-Pro administration was performed after recovery from anaesthesia. The Y-maze test was started at 60 min after Tyr-Pro administration on the 14th day. The number of total arm entries (b) and percentage of spontaneous alternations (c) were evaluated. Acquisition trial on the 15th day (d) and the retention trial on the 16th day (e) were performed in the passive avoidance test. Both trials were started at 60 min after Tyr-Pro administration. Data for corresponding mice in sham (n = 10), vehicle (n = 8) and Tyr-Pro (n = 10) are depicted as closed circle, square and triangle, respectively. Two data points corresponding to mice out of normal distribution in the control group [shown as open square in (d)] were eliminated as per the interquartile outlier test. Details of the test are described in supplemental information. Data are shown as median (solid bar), and first and third quartiles (dotted lines). Statistical significance was determined by Fisher’s PLSD test and Mann–Whitney U test for Y-maze test and passive avoidance test, respectively. ***p < 0.001 vs. sham control and #p < 0.05 vs. Aβ25-35 alone. N.S. indicates no significance.
Next, the step-through type passive avoidance test9 was performed to evaluate the benefits of Tyr-Pro in long-term memory in Aβ25-35-injected AD model mice. The interquartile outlier test allowed the elimination of two mice that showed unusually higher acquisition trial latency (Fig. 1d), and the three groups (sham, vehicle and Tyr-Pro groups) displayed no significant differences in the spectrum of step-through latency in the acquisition trial on day 15. On the 16th day, a significant impairment in the long-term memory or shortened latency time (p < 0.001) compared to the sham group was observed in Aβ25-35-injected mice (Fig. 1e). In contrast, the step-through latency time in Aβ25-35-injected mice did not significantly reduce upon Tyr-Pro administration (p < 0.05) (Fig. 1e). This indicated that Tyr-Pro could serve as a player in preventing long-term memory impairment or Aβ25-35-induced AD cognitive deficiency in mice.
Thus far, few peptides have been reported that exhibit a protective effect against cognitive decline. Mizushige et al.10 reported anxiolytic-like activity of Tyr-Leu in ddY mice via activation of serotonin 5-HT1A, dopamine D1 and γ-aminobutanoic acid (GABA) receptors. Effects of Leu-His2 on microglial activation and emotional disturbance and preventive effects of Met-Lys-Pro3 and Trp-Tyr4 on cognitive decline allowed us to speculate the potential effect of small peptides on brain health; however, no substantial evidence exists for their intact BBB transport and presence (or distribution) in brain parenchyma of orally administered peptides. Our previous study was the first evidential report that dipeptides possessing Pro (Gly-Pro and Tyr-Pro) from 18 dipeptide candidates can cross the BBB system in intact form, followed by the distribution in the hippocampus and cerebral cortex in peptide-perfused mice brain1. Moreover, in our preliminary data, Tyr-Pro was absorbed into blood circulation in its intact form in ICR mice after its single oral administration (shown in Supplemental Fig. 1), which suggesting a possible access of orally administered Tyr-Pro to brain tissue. Hence, the present study clearly demonstrated, for the first time, that BBB-transportable and orally absorbed Tyr-Pro can improve cognitive impairment in Aβ25-35-induced mice.
Considering that AD therapeutic drugs, such as donepezil11 targeted acetylcholinesterase (AChE) to increase acetylcholine, neurotransmitter metabolism, such as acetylcholine metabolism in the central nerve system (CNS) at memory controlling regions in brain6 might be a possible target of Tyr-Pro. However, it was impossible to directly monitor acetylcholine in the present study, because acetylcholine is instable and quickly metabolized to choline and acetate by AChE, and a tactical sampling techniques, such as microdialysis in living mice12, is essential. Thus, the effect of Tyr-Pro on protein expression of choline acetyltransferase (ChAT) and AChE in the hippocampus and cerebral cortex of mouse brain was evaluated using a micro-capillary protein electrophoresis system in this study. As shown in Fig. 2d–f, the expression of ChAT in the cerebral cortex in Tyr-Pro group was significantly higher than that in vehicle group (p < 0.05), with an increasing tendency of ChAT protein accumulation in the hippocampus in the Tyr-Pro group (p = 0.116) (Fig. 2a–c). In contrast, no significant changes in AChE expression were observed in both regions of the brain (Supplemental Fig. 2). Collectively, we speculate that long-term (16 days) administration of BBB-transportable Tyr-Pro in the acute AD model mice may ameliorate Aβ-impaired acetylcholine metabolism by promoting acetylcholine production, particularly in the cerebral cortex, thereby improving both short- and long-term memory impairment in the CNS.
Protein levels of ChAT in the hippocampus (a–c) and the cerebral cortex (d–f) were measured using a Wes instrument based on capillary electrophoresis immunoassay as described in supplemental information. The chemiluminescent signal is displayed as a virtual blot-like image (a, d) and eletropherogram (b, e) based on the molecular weight. Protein expression of ChAT was normalised by the electropherogram peak area of applied total protein in each lane, and the data are expressed as the ratio against the sham group. All data are presented as plot with the mean (solid bar) ± standard error (dotted lines). Statistical significance determined by Fisher’s PLSD test is represented as #p < 0.05 vs. Aβ25-35 alone. N.S. indicates no significance.
In conclusion, the present study demonstrates for the first time that soy dipeptide, Tyr-Pro, can potentially improve impaired cognitive deficits in both working and long-term memories in Aβ-injected AD model mice. Although the present study provides evidence of in vivo brain benefits of Tyr-Pro, possibly through cholinergic neurotransmission pathway via enhanced ChAT protein expression, acetylcholine amount in brain tissue, the involvement of other possible pathways such as glucose13 and N-methyl-d-aspartate metabolisms14, and influence of composed amino acids should be evaluated both in vivo and in vitro when probing for Tyr-Pro-induced brain health as its intact peptidic form. In addition, further investigations into the bioavailability (absorption into blood and accumulation in brain) of Tyr-Pro after long-term administration and the mechanism of Tyr-Pro on the acetylcholine system in vitro experiments are needed. Taken together, possible memory-benefits of Tyr-Pro should be considered when examining the physiological functions of dietary small peptides.
Methods
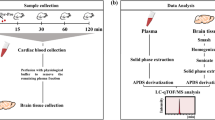
All the animal procedures were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. The experimental protocol was reviewed and approved by the Animal Studies Committee of Nihon Bioresearch Inc. (Study No. 390066, Gifu, Japan). Five-week-old male ddY mice with 23–28 g body weight (Japan SLC Inc., Shizuoka, Japan) were used in this study. The experimental schedules are shown as Fig. 1a. Tyr-Pro (100 mg/kg) was orally administered twice a day for 16 days, except for days of i.c.v. injection of Aβ25–35 peptide and behavioural tests (Tyr-Pro administration once a day). The mice received i.c.v. injection of Aβ25–35 peptide at 6 nmol/mouse on the 7th day and Tyr-Pro administration was performed after recovery from anaesthesia, according to a previous report with several modifications15. Spontaneous alternation performance (Y-maze test) was started at 60 min after Tyr-Pro administration on the 14th day. Passive avoidance test (acquisition trial on the 15th day and retention trial on the 16th day) was started at 60 min after Tyr-Pro administration on each day. After the passive avoidance test, hippocampus and cerebral cortex of mouse brain were taken and stored at −80 °C until analysis. Other detail methods are available in supplemental information.
Data availability
The data supporting the findings reported herein are available on reasonable request from the corresponding author.
References
Tanaka, M. et al. Brain-transportable dipeptides across the blood-brain barrier in mice. Sci. Rep. 9, 5769 (2019).
Ano, Y., Kita, M., Kitaoka, S. & Furuyashiki, T. Leucine–Histidine dipeptide attenuates microglial activation and emotional disturbances induced by brain inflammation and repeated social defeat stress. Nutrients 11, 2161 (2019).
Min, L.-J. et al. Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLoS ONE 12, e0171515 (2017).
Ano, Y. et al. Tryptophan-related dipeptides in fermented dairy products suppress microglial activation and prevent cognitive decline. Aging 11, 2949–2967 (2019).
Hong, S.-M., Tanaka, M., Yoshii, S., Mine, Y. & Matsui, T. Enhanced visualization of small peptides absorbed in rat small intestine by phytic-acid-aided matrix-assisted laser desorption/ionization-imaging mass spectrometry. Anal. Chem. 85, 10033–10039 (2013).
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I. & Moser, M.-B. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 7, 663–678 (2006).
Clark, R. E. & Martin, S. J. Interrogating rodents regarding their object and spatial memory. Curr. Opin. Neurobiol. 15, 593–598 (2005).
Takeda, S. et al. Validation of Aβ1–40 administration into mouse cerebroventricles as an animal model for Alzheimer disease. Brain Res. 1280, 137–147 (2009).
Lorenzini, C. A., Bucherelli, C. & Giachetti, A. Passive and active avoidance behavior in the light-dark box test. Physiol. Behav. 32, 687–689 (1984).
Mizushige, T. et al. Aromatic amino acid-leucine dipeptides exhibit anxiolytic-like activity in young mice. Neurosci. Lett. 543, 126–129 (2013).
Godyń, J., Jończyk, J., Panek, D. & Malawska, B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. 68, 127–138 (2016).
Nirogi, R., Mudigonda, K., Kandikere, V. & Ponnamaneni, R. Quantification of acetylcholine, an essential neurotransmitter, in brain microdialysis samples by liquid chromatography mass spectrometry. Biomed. Chromatogr. 24, 39–48 (2010).
Patching, S. G. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol. Neurobiol. 54, 1046–1077 (2017).
Kandimalla, R. & Reddy, P. H. Therapeutics of neurotransmitters in Alzheimer’s disease. J. Alzheimers Dis. 57, 1049–1069 (2017).
Tajima, H. et al. A humanin derivative, S14G-HN, prevents amyloid-β-induced memory impairment in mice. J. Neurosci. Res. 79, 714–723 (2005).
Acknowledgements
The authors thank Ms Kaori Miyazaki at Kyushu University for technical assistance.
Author information
Authors and Affiliations
Contributions
M.T. and T.M. designed this study. H.K., A.Y., A.N., F.T. and S.D. performed all the analytical experiments and analysed the results. M.T., H.K., A.Y., A.N. and T.M. wrote the paper. All authors considered and discussed the results and designed whole experimental strategy. Y.K. commented on the paper. T.M. edited the paper and supervised the whole project. All the authors have read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, M., Kiyohara, H., Yoshino, A. et al. Brain-transportable soy dipeptide, Tyr-Pro, attenuates amyloid β peptide25-35-induced memory impairment in mice. npj Sci Food 4, 7 (2020). https://doi.org/10.1038/s41538-020-0067-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-020-0067-3
This article is cited by
-
Dietary amino acid intake and sleep duration are additively involved in future cognitive decline in Japanese adults aged 60 years or over: a community-based longitudinal study
BMC Geriatrics (2023)
-
A memory-improving dipeptide, Tyr-Pro, can reach the mouse brain after oral administration
Scientific Reports (2023)
-
Stable Isotope Dilution LC/ESI-SRM/MS Analysis for Highly Polar Bioactive Dipeptides Found in Fermented Brown Rice Product using a Porous Graphitic Carbon Column
Food Analytical Methods (2023)
-
A trip of peptides to the brain
Food Production, Processing and Nutrition (2020)