Abstract
Background and hypothesis
Pathogenic understanding of the psychotic disorders converges on regulation of dopaminergic signaling in mesostriatocortical pathways. Functional connectivity of the mesostriatal pathways may inform us of the neuronal networks involved.
Study design
This longitudinal study of first episode psychosis (FEP) (49 patients, 43 controls) employed seed-based functional connectivity analyses of fMRI data collected during a naturalistic movie stimulus.
Study results
We identified hypoconnectivity of the dorsal striatum with the midbrain, associated with antipsychotic medication dose in FEP, in comparison with the healthy control group. The midbrain regions that showed hypoconnectivity with the dorsal striatum also showed hypoconnectivity with cerebellar regions suggested to be involved in regulation of the mesostriatocortical dopaminergic pathways. None of the baseline hypoconnectivity detected was seen at follow-up.
Conclusions
These findings extend earlier resting state findings on mesostriatal connectivity in psychotic disorders and highlight the potential for cerebellar regulation of the mesostriatocortical pathways as a target of treatment trials.
Similar content being viewed by others
Introduction
While the pathogenesis of first episode psychosis (FEP) remains poorly understood, converging lines of evidence support the dopamine hypothesis, suggesting mesostriatal dopaminergic pathways to be a final common pathway in psychosis1,2. The functional anatomy and neurochemistry of dopaminergic pathways, based on animal and human research, are becoming established as the substrate of signal within neuroimaging research (reviewed by McCutcheon et al.2). For example, mesostriatal dopamine synthesis increase has repeatedly been observed in positron emission tomography (PET) schizophrenia studies3, while dopaminergic drugs induce psychotic symptoms4, and all known antipsychotic medications block dopaminergic activity in the striatum5. Furthermore, functional striatal abnormalities can already form the basis of predictive markers of schizophrenia6.
However, only a small proportion of known risk factors for psychotic disorders is related directly to dopaminergic pathways. Also, multiple psychosis risk factors and related brain findings point to complex alterations in the brain’s neuronal networks that may contribute to dysregulation of the mesostriatal pathways. One option to assess such up-stream alteration is to study functional connectivity of the striatum and especially the midbrain components of the mesostriatal pathways7. Previous fMRI studies have focused on mesolimbic and mesostriatal functional connectivity2, but much less is known about extra-striatal midbrain functional connectivity in psychotic disorders7,8. In one study using a probabilistic ventral tegmental area (VTA) template seed, Nakamura and colleagues found no group differences in resting state connectivity of the ventral tegmental area between patients with schizophrenia and healthy control subjects7. However, resting state VTA hypoconnectivity with the cerebral cortex is associated with avolition in schizophrenia subjects8. Overall, midbrain functional connectivity and its associations with psychosis symptoms warrant further research.
The midbrain components of the mesostriatocortical pathways are spatially restricted and may be difficult to define based on atlases. Therefore, we used an alternative functional localization approach based on functional connectivity alterations with the striatum. The dorsal striatum is often termed ‘associative’ as it is involved in higher cognitive functions through cortical projections9 and may show the largest dopaminergic dysfunction in schizophrenia1. Conversely, the ventral striatum is associated with aberrant mesolimbic connectivity linked to positive symptoms (hallucinations and delusions) in schizophrenia10 and those at high risk of psychosis11. Recent research has also strongly connected the ventral striatum with reward deficits12.
Our fMRI seed selection approach intends to create seed regions defined by striatal functional activity rather than only its anatomical borders. To do this, seeds were based on voxels linked to ‘associative’ (dorsal) and ‘motivational’ (ventral) striatal functions in research published in the NeuroSynth database (neurosynth.org). Then the midbrain seeds for further connectivity analyses were defined as voxels in the midbrain showing a group difference between FEP patients and control subjects in functional connectivity with either of the striatal seeds. In addition to seed selection, we aimed to complement earlier literature by using a rare longitudinal sample of first-episode psychosis (FEP) patients and a naturalistic movie stimulation, instead of the more common resting state approaches. Functional brain imaging studies within subjects experiencing a naturalistic stimulus, such as viewing a movie, can complement resting state studies as they provide an excellent assay of emotional, motivational, social and cognitive functions in a way that has strong validity for everyday demands of brain function13,14. Compared to resting state studies, naturalistic stimuli may help a subject to stay alert and reduce head movement, which are crucial for connectivity studies. Such stimuli enhance the test-retest reliability of fMRI studies, and have been used successfully in numerous clinical studies15,16,17. Even if the strengths of functional connections depend to some extent on the task used, the overall architecture of the functional connectivity is stable during rest and various tasks, and average connectivity strengths during rest resemble those averaged across multiple different tasks18.
We hypothesized that striatal functional connectivity with the midbrain differs between FEP patients and healthy controls. We then used the midbrain regions differing in striatal connectivity between groups as a new seed for comparison of connectivity between groups across the brain. Assuming psychosis-related mesostriatal pathways project from these midbrain regions, alteration of connectivity of these regions with other parts of the brain in FEP patients might reflect alteration in regulation of the mesostriatal pathways. Although correlational findings from fMRI connectivity cannot inform directly about causality or the origins of mesostriatal dysfunction, such findings might pinpoint candidate connections for further studies to answer the central question, “What causes the mesostriatal dopaminergic dysfunction in psychotic disorders?”. To further our understanding of the findings, we correlated connectivity strengths with antipsychotic medication dose and symptom severity and compared the findings with those acquired in patients at follow-up imaging approximately 1 year later (1.26 + /−0.35 years).
Methods
Subjects
We included 159 participants (97 patients, aged 18–40 years, 62 age matched controls) from the Helsinki Early Psychosis Study (HEPS), Helsinki University Hospital, Finland. Participants were recruited 2010–2016 and data were collected 2010–2018. In the control group (n = 62) at baseline, one subject was excluded due to a neurological abnormality and one subject was excluded due to fMRI image misalignment that we could not reliably correct. One subject was excluded from symptom correlation analyses due to interviewers not being able to obtain a reliable evaluation of symptom severity at baseline. This provided 60 baseline control subject datasets for our analyses. In the patient group (n = 97) at baseline, twelve had inadequate data acquisition at the time of scanning, three had significant neurological findings, three were excluded due to significant head motion and three subjects had fMRI image misalignment that we could not reliably correct. This yielded 76 baseline FEP patient datasets for our analyses, and 75 baseline FEP patients for symptom correlation analyses. For all follow-up subjects in the patient group (n = 49) and control group (n = 43) there were no fMRI data exclusions required. One subject’s follow-up clinical data was unavailable, thus one dataset was absent from clinical correlation analyses.
The detailed methods about data collection have been reported previously19,20. Briefly, the inclusion criterion for FEP patients was having psychotic symptoms lasting over 24 h, with scores of 4–7 points on the Unusual thought content or Hallucinations in the Brief Psychiatric Rating Scale Extended (BPRS-E)21. We excluded patients with previous psychotic episodes and subjects whose psychotic symptoms were clearly related to substance use or a general medical condition. DSM-IV diagnoses were based on the Research Version of the Structural Clinical Interview for DSM-IV (SCID)22, which was complemented with information from medical records of all lifetime mental health treatment contacts23. BPRS interviews were done by trained psychiatric nurses or psychologists. The structured BPRS assessment by the UCLA Department of Psychiatry and Biobehavioral Sciences was translated into Finnish and adapted as a semistructured interview, and the training was designed and organized as recommended in Ventura et al. (1993)24. The same interviewers were trained to use SCID. Training was organized by a senior psychiatrist (JS) who also reviewed all medical records from the patients and the interviews together with the interviewers. Final diagnostic assessment was made by JS using all available information from the interviews and medical records. The diagnoses reported here are based on the longest follow-up data available from the patient. Diagnostic assessments were done at baseline for control subjects and at 2 months follow-up for patients, then again at the one year follow-up. We complemented BPRS with three items25 during both clinical assessments. At baseline, symptom severity was assessed for the worst lifetime period and for the past week. There was one follow-up assessment at 2 months between the scans to document the initial treatment response. At 12 months, symptom severity was assessed for the past week and for the worst period after the 2-month follow-up measurement (i.e. during the previous 10 months). Chlorpromazine equivalent doses were calculated using the defined daily dose method26.
Image acquisition
Functional magnetic resonance imaging was attempted at baseline for all subjects unless deemed to be at risk due their mental state. We conducted the fMRI recordings at the AMI Centre of Aalto Neuroimaging, Aalto University, Finland. Due to the prescheduled change of the AMI Centre scanner during the study baseline, 32 subjects (19 patients, 13 controls) were scanned using a GE Signa VH/i 3 T scanner (scanner 1) with a 16-channel head coil and 134 subjects (85 patients, 49 controls) using a Siemens Skyra 3 T scanner (scanner 2) with a 32-channel head coil. For both scanners, the parameters for functional blood-oxygenation-level-dependent (BOLD) imaging were 36 slices, slice thickness 4 mm, matrix size 64 × 64, echo time 30 ms, repetition time 1.8 s, flip angle 75°, and field of view 24 cm (voxel size therefore 3.75 × 3.75 × 4 mm). We included participants from both scanners in the same analyses because multisite fMRI studies have shown that a 10% increase in sample size will increase statistical power as opposed to using only single scanner data27. Slices were aligned according to the line connecting the anterior and posterior commissures. T1-weighted structural images with 1 mm3 isotropic voxels and T2-weighted structural images were also acquired, and a clinical neuroradiologist evaluated these scans for brain abnormalities. At follow-up, neuroimaging and clinical assessment were repeated around 12 months using scanner 2 and successfully completed for 52 FEP patients and 43 healthy control subjects.
Stimuli
During fMRI, the participants were shown five scenes in sequence from the fantasy film Alice in Wonderland, directed by Tim Burton (Walt Disney Pictures, Burbank, CA, 2010; dubbed into Finnish). The total duration was 7 min and 20 s, equaling 245 echo-planar imaging volumes. This film is partly animated with human actors present in every scene. The scenes show Alice’s wedding followed by her trip through a rabbit hole to Wonderland and subsequent fantasy events (see28 for full details). Scenes were projected without breaks using Presentation software (Neurobehavioral Systems Inc., USA) to a semi-transparent screen, visible to the subject via a mirror placed on the head coil. The soundtrack was delivered to the subject via plastic tubes connected to earplugs. Foam pads were added in and around the head coil for noise cancellation, and the sound level was adjusted to be comfortable for each subject.
Data pre-processing
The fMRI data analysis steps are shown in Fig. 1. The fMRI data were pre-processed using DPARSF 5.0 software within DPABI29 run in MATLAB (Mathworks, Natick, USA). This included slice timing correction, realignment, co-registration to T1 images, and spatial normalization by DARTEL to the Montreal Neurological Institute (MNI) space. To reduce the effect of individual functional differences and to better fit the assumption of normal distribution, images were smoothed with an 8 mm full-width-at-half-maximum Gaussian kernel. We used framewise displacement (FD)30 to calculate a score for movement for each time point (1 time point = 1 repetition time) and a mean score across all time points, excluding subjects with mean FD Jenkinson > 0.2 mm or percent of FD Power > 0.5 mm > 0.2 (i.e., 20%) from further analyses.
A First fMRI connectivity analysis with striatal seeds. In the second connectivity analysis (not shown), the midbrain regions differing between groups were used as a new seed region. B The functional connectivity results generated by our seed-based approach were analysed by group level comparisons between FEP patients and healthy controls. C Functional connectivity measurements of FEP patients (FC eigenvalues) were correlated with antipsychotic drug dose equivalents and the severity of positive or negative symptoms composite scores at baseline and follow-up.
Functional connectivity methodology
After pre-processing, DPARSF 5.0 was used to compute functional connectivity with a seed-based approach. Firstly, two striatal seed masks were created using the NeuroSynth database (neurosynth.org) by search terms ‘associative’ and ‘motivational’ with respective.nii file outputs generated. This approach intends to create seed regions defined by striatal functional activity rather than its precise anatomical borders. To generate a representative dorsal associative striatal seed (Seed 1, Fig. 2A), the motivational output was subtracted from the associative output in SPM12. To generate a representative ventral striatal seed mask (Seed 2, Fig. 2B), the associative output was subtracted from the motivational output.
A Seed 1. A dorsal striatal seed mask based on NeuroSynth ‘motivational’ output subtracted from the NeuroSynth ‘associative’ output. B Seed 2. A ventral striatal seed mask created by subtracting the ‘associative’ output from the ‘motivational’ output. C Seed 3. Our second analysis utilised the midbrain regions carrying significant hypoconnectivity with the dorsal striatum (Seed 1) as a new seed, Seed 3.
The functional connectivity images generated by our seed-based approach were compared between FEP patients and healthy control subjects in a longitudinal analysis using MRM toolbox31 and in two-sample t-tests in SPM12, both with and without adjustment for scanner type. We used region of interest analysis within one midbrain anatomical mask (WFU PickAtlas, RRID:SCR_007378). All analyses were corrected for multiple comparisons at the cluster level with primary voxel-level threshold p = 0.001. The resulting significant midbrain clusters were used for subsequent mask-based eigenvariate extraction of connectivity values for each FEP subject, to quantify striato-midbrain connectivity. These values were extracted for both baseline data and follow-up data, with the same masks used upon baseline data and follow-up data, to allow us to make regional FC comparisons over time as a post hoc analysis. Our second analysis utilized the midbrain clusters that differed in connectivity with the dorsal striatum (Seed 1) between groups as a new midbrain seed (Seed 3, Fig. 2C). This approach aimed to utilize localization of the functionally defined midbrain alterations to further assess midbrain-related circuitries of altered connectivity. To estimate validity of the midbrain connectivity findings, we compared them with average midbrain connectivity of 1000 subjects in the Neurosynth database.
Post hoc analysis on extracted FC values
Extracted FC values were normally distributed (Shapiro-Wilk test) and tested for Pearson’s partial correlations with positive and negative symptom composite scores. These correlations were controlled for scanner type and antipsychotic dose as antipsychotics may affect striatal connectivity32. FC values between time points and scanner groups were compared using paired and unpaired samples Student’s t-tests, respectively. These methods were completed in SPSS Statistics (Version 27, IBM, New York). For FEP patients, evaluation of FC changes over time in correlation with negative or positive symptoms was achieved using repeated measures correlation analysis (rmcorr)33 in R Studio (rstudio.com, Boston, MA). This method relies on repeated measures of FC within each subject and specifically tests for a common association with other variables within each subject. It then provides a correlation plot over time for the FEP group. Unlike ANOVA, potentially confounding variables between subjects such as sex, age and BMI have no influence on the rmcorr correlation analysis.
To test the hypothesis that functional connectivity was associated with the severity of positive and negative symptoms, we calculated two positive symptom and two negative symptom composite sum scores with BPRS-E21 and SANS25 items as follows: Negative 1; BPRS 16 (blunted affect) + SANS global rating for Anhedonia/Asociality + SANS global rating for Alogia + SANS global rating for Avolition/Apathy. Negative 2; BPRS (16 (blunted affect) + 17 (emotional withdrawal) + 18 (motor retardation)) x 0.33. Positive 1 and 2; past week (Positive 1) and worst ever episode (Positive 2) sum of BPRS 10 (hallucinations) + 11 (unusual thought content, i.e. delusions) + 12 (bizarre behaviour) + 15 (conceptual disorganization, i.e. positive formal thought disorder).
Ethical standards
All procedures contributing to this work comply with the ethical standards of the Finnish national and institutional committees on human experimentation, and with the Helsinki Declaration of 1975, revised in 2008. The study protocol was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa (diary numbers 257/12/03/03/2009 and 226/13/03/03/2013), and written informed consent was obtained from each subject before participation.
Results
Sample characteristics are shown in Table 1. First episode psychosis (FEP) patients and control subjects did not differ regarding age or sex either at baseline or follow up. Our movie-based fMRI identified dorsal striatal functional hypoconnectivity with the midbrain at baseline presentation of FEP, as compared with controls (Table 2 and Fig. 3). Our repeated measures longitudinal analysis identified no significant FC changes over time, either corrected or uncorrected for scanner type (p > 0.05). Therefore, masks of the voxels of significantly reduced FC found at baseline were used to extract equivalently located FC values from follow-up data for FC comparisons only as a post hoc analysis.
Images of the baseline clusters of significant functional hypoconnectivity of the midbrain with Seed 1 (dorsal striatum, Fig. 2A). There were no clusters of midbrain hypoconnectivity with Seed 2 (ventral striatum, Fig. 2B). All findings were made by groupwise comparisons between FEP patients (n = 76) and healthy matched controls (n = 60) at baseline. No significant connectivity changes between these regions were seen at follow-up 12 months later. Primary voxel p-value of 0.001 was used in image and in cluster level statistics.
Clusters of hypoconnectivity of the striatum with the midbrain baseline are shown in Fig. 3. The midbrain cluster coordinates did not change when comparisons were corrected for scanner type at baseline. Specifically, reduced baseline FC was only seen with our dorsal (associative) striatal seed (Seed 1, Fig. 2A), and not with our ventral (motivational) striatal seed (Seed 2, Fig. 2B). Controlling for scanner type, dorsal striato-midbrain FC was negatively correlated with antipsychotic drug dose equivalents at baseline only (r = −0.289, p = 0.012, Fig. 4). There were no partial correlations of baseline FC with positive or negative symptoms (p > 0.05). In FEP patients, inter-regional functional connectivity was not significantly different between baseline and follow-up (longitudinal analyses and paired samples Student’s t-tests, p > 0.05). When evaluating correlations between functional connectivity and negative symptom, positive symptom, or antipsychotic dose changes over time, the rmcorr approach yielded no correlations over the duration between baseline and follow-up (p > 0.05).
Groupwise, functional connectivity between the midbrain seed (Seed 3) and areas of the cerebellum was reduced at baseline in patients when compared with healthy controls (Fig. 5). Connectivity of 1000 subjects in Neurosynth database suggested these cerebellar regions to be connected with the midbrain seeds in healthy subjects (r = 0.04–0.07) supporting validity of these connections. The cerebellum showed no significant groupwise FC changes with the midbrain at follow-up 12 months later (p > 0.05). In FEP patients, midbrain-cerebellar functional connectivity was not significantly different between baseline and follow-up (longitudinal analyses and paired samples Student’s t-test, p > 0.05). Controlling for scanner type, midbrain-cerebellar FC did not correlate with antipsychotic drug dose equivalents at baseline or follow-up (p > 0.05). The midbrain-cerebellar FC did not correlate with negative symptoms or antipsychotic dose at baseline or follow-up (p > 0.05). As with striatal FC, the rmcorr approach showed no significant correlations between midbrain-cerebellum FC and either positive symptom, negative symptom, or antipsychotic dose changes over the 12 months duration from baseline to follow-up (p > 0.05).
Images of the clusters of significant cerebellar functional hypoconnectivity with the midbrain (Seed 3, Fig. 2C). The greyscale images show two representative slice locations, which are both compiled in the line diagram row. Primary voxel p value of 0.001 was used in image and in cluster level statistics.
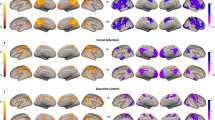
Taken together, our results show groupwise functional hypoconnectivity between the dorsal striatum (Seed 1, Fig. 2A) and midbrain regions (Fig. 3A), and between those same midbrain regions and areas of the cerebellum (Fig. 5). This three-point network of hypoconnectivity found in our FEP study at baseline is illustrated in Fig. 6. The main findings and their correlations are summarized in Table 2.
The three linked regions include clusters within the dorsal striatum (Seed 1, orange), the midbrain (Seed 3, green) and the cerebellum (purple). The whole brain surface representation is created by a previously published method in BrainNet Viewer27. Primary voxel p-value of 0.001 was used in image and in cluster level statistics.
Discussion
Our research identified dorsal striatal hypoconnectivity with the midbrain during naturalistic stimulus processing at the time of FEP presentation (baseline). Furthermore, the midbrain regions that showed reduced connectivity with the dorsal striatum showed hypoconnectivity with the cerebellum, which is suggested to be involved in regulation of the mesostriatocortical dopaminergic pathways34. Mesostriatal and meso-cerebellar hypoconnectivity were not seen at follow-up.
We focused on the connectivity of the mesostriatal interactions that have now become highly implicated in psychosis35. Aberrant striatal connectivity has been well documented and reviewed2. In line with our findings, dopaminergic dysfunction has most frequently been seen in the dorsal (associative) striatum36,37,38. Supporting this, fMRI studies have shown dorsal striatal hypoconnectivity with cortical networks in schizophrenia39,40,41,42,43 and in patients with clinical high risk of psychosis44. Midbrain connectivity is of special interest as dopaminergic activity characterized by increased synthesis and release of dopamine in psychotic disorders originate in the midbrain7,8.
Mesostriatal connectivity has previously been positively associated with positive symptoms40 and negatively with negative symptoms45. Furthermore, functional striatal resting state abnormalities that can predict schizophrenia severity do include hypoconnectivity between the striatum and the midbrain6. Our findings extend these lines of evidence by suggesting mesostriatal hypoconnectivity prevails during naturalistic stimulus processing in FEP, in association with medication dose. No significant individual or repeated measures functional connectivity correlations with positive symptoms were found. We also observed lower baseline dorsal striato-midbrain connectivity in association with higher baseline antipsychotic drug dose equivalents in FEP. In line with this, fMRI before antipsychotic treatment46,47 as well as during treatment48 of early schizophrenia has shown decreased midbrain connectivity with the dorsal striatum during the resting state. The present study did not find associations between antipsychotic use and longitudinal functional connectivity changes, but interestingly, antipsychotic dose correlated negatively with meso-striatal connectivity at the baseline. This finding may reflect blocking of mesostriatal interactions during antipsychotic treatment and supports the assumption that the present fMRI findings may reflect functioning of the mesostriatal dopaminergic activity.
Little is known about midbrain connectivity beyond the striatum7. Therefore, it is interesting that the midbrain regions showing group difference in their striatal connectivity also differed between groups in their connectivity with the cerebellum. The cerebellum is known to be important in cognitive and emotional-motivational circuitries beyond its motor functions. It was involved in the influential hypothesis of cognitive dysmetria of Andreasen and coworkers49, and further evidence has supported the role of the cerebellum in schizophrenia34. Structural connections are known to exist at least between the fastigial cerebellar nuclei and the midbrain, and stimulation of the fastigial and dentate nuclei alters dopamine functioning in the medial prefrontal cortex34. In line with this, the present midbrain and cerebellar regions were connected in a large sample of healthy subjects in Neurosyth fMRI database. The present findings support further studies to assess the potential role of the cerebellum in regulation of mesostriatocortical dopaminergic activity in psychosis. Such studies could have important therapeutic implications regarding, for example, targeting transcranial magnetic stimulation of the cerebellum34.
Study considerations and future research
The reduction in dorsal striato-midbrain-cerebellar connectivity were characteristic for FEP patients during the baseline study when symptoms were, on average, more severe. Larger longitudinal studies are needed to assess whether such changes could represent a state-like dysfunction in connectivity that underpin such symptoms.
The present study used naturalistic stimuli in lieu of resting state data, and thus we cannot directly compare these two methods of obtaining fMRI data. It is possible that the present movie stimulus elicits different connectivity of the midbrain with the striatum and cerebellum than the resting state. Further research would ideally contain data obtained both at rest and during naturalistic stimuli, to assess the relationships between changes occurring in each state. Furthermore, analyses of dynamic connectivity during a task can be used to link task characteristics to changes in connections of interest. Importantly, naturalistic stimuli may help to reduces the likelihood of possible sources of error related to sleepiness and head movement, uncontrollability of psychological state during imaging and the validity of the stimulus for everyday life (fixation cross versus a meaningful movie).
We defined midbrain seeds based on their functional connectivity in the present sample, which may enhance validity of seed regions for the study questions. The resulting midbrain seeds were small (altogether 621 mm2), comparing to only about 11 original voxels in image acquisition. Considering the accuracy of DARTEL normalization50 and smoothing the data to compensate for interindividual differences, the seed size appears sufficient.
In summary, the present study supports continued clinical and research attention upon functional hypoconnectivity within dorsal striatal dopaminergic interactions and associated cerebellar activity in early psychosis. Robust evaluation of striatal functional connectivity abnormalities within the mesostriatal system especially6 with the added context of patient risk factors may enhance our predictive models for psychosis severity when employed as it first presents. Our results provide novel insight on functional connectivity changes that could eventually help to access causal explanation for positive and negative symptoms in psychotic disorders.
Data availability
To protect vulnerable study subjects, data of the present study is not publicly available. Inquiries about the data can be addressed to the corresponding author.
References
McCutcheon, R., Beck, K., Jauhar, S. & Howes, O. D. Defining the locus of dopaminergic dysfunction in schizophrenia: A meta-analysis and test of the mesolimbic hypothesis. Schizophr. Bull. 44, 1301–1311, https://doi.org/10.1093/schbul/sbx180 (2018).
McCutcheon, R. A., Abi-Dargham, A. & Howes, O. D. Schizophrenia, dopamine and the Striatum: From biology to symptoms. Trends Neurosci. 42, 205–220, https://doi.org/10.1016/j.tins.2018.12.004 (2019).
Hazlett, E. A., Vaccaro, D. H., Haznedar, M. M. & Goldstein, K. E. Reprint of: F-18Fluorodeoxyglucose positron emission tomography studies of the schizophrenia spectrum: The legacy of Monte S. Buchsbaum, M.D. Psychiatry Res. 277, 39–44, https://doi.org/10.1016/j.psychres.2019.06.014 (2019).
Borovac, J. A. Side effects of a dopamine agonist therapy for Parkinson’s disease: A mini-review of clinical pharmacology. Yale J. Biol. Med. 89, 37–47 (2016).
Awad A. G. Revisiting the concept of subjective tolerability to antipsychotic medications in schizophrenia and its clinical and research implications: 30 Years Later. CNS Drugs. 33. https://doi.org/10.1007/s40263-018-0588-3 (2019).
Li, A. et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 26, 558–565, https://doi.org/10.1038/s41591-020-0793-8 (2020).
Nakamura, Y. et al. Differences in functional connectivity networks related to the midbrain dopaminergic system-related area in various psychiatric disorders. Schizophr. Bull. 46, 1239–1248, https://doi.org/10.1093/schbul/sbz121 (2020).
Giordano, G. M. et al. Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: a resting state functional MRI study. Eur. Neuropsychopharmacol. 28, 589–602, https://doi.org/10.1016/j.euroneuro.2018.03.013 (2018).
Howes, O. D. et al. Elevated striatal dopamine function linked to prodromal signs of schizophenia. Arch. Gen. Psychiatry 66, 13–20, https://doi.org/10.1001/archgenpsychiatry.2008.514 (2009).
Epstein, J., Stern, E. & Silbersweig, D. Mesolimbic activity associated with psychosis in schizophrenia: Symptom-specific PET studies. Ann. NY. Acad. Sci. 877, 562–574, https://doi.org/10.1111/j.1749-6632.1999.tb09289.x (1999).
Winton-Brown, T. et al. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl. Psychiatry 7, e1245–e1245, https://doi.org/10.1038/tp.2017.174 (2017).
Gomes, F. V. Altered Ventral Striatum–Hippocampus connectivity during reward processing as an Endophenotype for Psychosis. Biol Psychiatry 91, e7–e9, https://doi.org/10.1016/j.biopsych.2021.10.019 (2022).
Finn, E. S. et al. Idiosynchrony: From shared responses to individual differences during naturalistic neuroimaging. Neuroimage 215, 116828, https://doi.org/10.1016/j.neuroimage.2020.116828 (2020).
Hasson, U. & Honey, C. J. Future trends in Neuroimaging: Neural processes as expressed within real-life contexts. Neuroimage 62, 1272–1278, https://doi.org/10.1016/j.neuroimage.2012.02.004 (2012).
Vanderwal, T., Kelly, C., Eilbott, J., Mayes, L. C. & Castellanos, F. X. Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage 122, 222–232, https://doi.org/10.1016/j.neuroimage.2015.07.069 (2015).
Wang, J. et al. Test–retest reliability of functional connectivity networks during naturalistic fMRI paradigms. Hum. Brain Mapp. 38, 2226, https://doi.org/10.1002/HBM.23517 (2017).
Yang, Z. et al. Individualized psychiatric imaging based on inter-subject neural synchronization in movie watching. Neuroimage 216, 116227, https://doi.org/10.1016/J.NEUROIMAGE.2019.116227 (2020).
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S. & Petersen, S. E. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251, https://doi.org/10.1016/j.neuron.2014.05.014 (2014).
Rikandi, E. et al. Connectivity of the precuneus-posterior cingulate cortex with the anterior cingulate cortex-medial prefrontal cortex differs consistently between control subjects and first-episode psychosis patients during a movie stimulus. Schizophr. Res. 199, 235–242, https://doi.org/10.1016/j.schres.2018.03.018 (2018).
Mäntylä, T. et al. Altered activation of innate immunity associates with white matter volume and diffusion in first-episode psychosis. PLoS One 10, e0125112, https://doi.org/10.1371/journal.pone.0125112 (2015).
Ventura, J. et al. Brief Psychiatric Rating Scale Expanded version 4.0: Scales anchor points and administration manual. Int. J. Meth. Psychiatr. Res. 13, 221–244 (1993).
First, M., Spitzer, R. L., Gibbon, M. L., Williams, J. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. In: (SCID-I/P).; 2002.
Gorgens, K. A. Structured Clinical Interview For DSM-IV (SCID-I/SCID-II). In: Encyclopedia of Clinical Neuropsychology. Springer New York; 2011:2410–2417. https://doi.org/10.1007/978-0-387-79948-3_2011
Ventura, J., Green, M. F., Shaner, A. & Liberman, R. P. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters. Int. J. Methods Psychiatr. Res. 3, 221–244 (1993).
Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br. J. Psychiatry 155, 49–52, https://doi.org/10.1192/s0007125000291496 (1989).
Leucht, S., Samara, M., Heres, S. & Davis, J. M. Dose equivalents for antipsychotic drugs: The DDD method. Schizophr. Bull. 42, S90–S94, https://doi.org/10.1093/schbul/sbv167 (2016).
Suckling, J. et al. Components of variance in a multicentre functional MRI study and implications for calculation of statistical power. Hum. Brain Mapp. 29, 1111–1122, https://doi.org/10.1002/hbm.20451 (2008).
Rikandi, E. et al. Precuneus functioning differentiates first-episode psychosis patients during the fantasy movie Alice in Wonderland. Psychol. Med. 47, 495–506, https://doi.org/10.1017/S0033291716002609 (2017).
Yan, C. G., Wang, X. D. I., Zuo, X. N. & Zang, Y. F. DPABI: Data processing & analysis for (Resting-State) brain imaging. Neuroinformatics 14, 339–351, https://doi.org/10.1007/s12021-016-9299-4 (2016).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841, https://doi.org/10.1006/nimg.2002.1132 (2002).
McFarquhar, M. et al. Multivariate and repeated measures (MRM): A new toolbox for dependent and multimodal group-level neuroimaging data. Neuroimage 132, 373, https://doi.org/10.1016/J.NEUROIMAGE.2016.02.053 (2016).
Sarpal, D. K. et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72, 5–13, https://doi.org/10.1001/jamapsychiatry.2014.1734 (2015).
Bakdash, J. Z. & Marusich, L. R. Repeated measures correlation. Front. Psychol. 8, 456, https://doi.org/10.3389/fpsyg.2017.00456 (2017).
Parker, K. L., Narayanan, N. S. & Andreasen, N. C. The therapeutic potential of the cerebellum in schizophrenia. Front. Syst. Neurosci. 8, 163, https://doi.org/10.3389/fnsys.2014.00163 (2014).
Howes O. D., Hird E. J., Adams R. A., Corlett P. R., McGuire P. Aberrant salience, information processing and dopaminergic signalling in people at clinical high risk for psychosis. Biol. Psychiatry. Published online March 31, 2020. https://doi.org/10.1016/j.biopsych.2020.03.012
Kegeles, L. S. et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry 67, 231–239, https://doi.org/10.1001/archgenpsychiatry.2010.10 (2010).
Mizrahi, R. et al. Increased stress-induced dopamine release in psychosis. Biol. Psychiatry 71, 561–567, https://doi.org/10.1016/j.biopsych.2011.10.009 (2012).
Howes, O. D. et al. Midbrain dopamine function in schizophrenia and depression: A post-mortem and positron emission tomographic imaging study. Brain 136, 3242–3251, https://doi.org/10.1093/brain/awt264 (2013).
Fornito, A. et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70, 1143–1151, https://doi.org/10.1001/jamapsychiatry.2013.1976 (2013).
Yoon, J. H., Minzenberg, M. J., Raouf, S., D’Esposito, M. & Carter, C. S. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol. Psychiatry 74, 122–129, https://doi.org/10.1016/j.biopsych.2012.11.018 (2013).
Orliac, F. et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr. Res. 148, 74–80, https://doi.org/10.1016/j.schres.2013.05.007 (2013).
Karcher, N. R., Rogers, B. P. & Woodward, N. D. Functional Connectivity of the Striatum in Schizophrenia and Psychotic Bipolar Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 956–965, https://doi.org/10.1016/j.bpsc.2019.05.017 (2019).
Peters, H. et al. Changes in extra-striatal functional connectivity in patients with schizophrenia in a psychotic episode. Br. J. Psychiatry 210, 75–82, https://doi.org/10.1192/bjp.bp.114.151928 (2017).
Dandash, O. et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr. Bull. 40, 904–913, https://doi.org/10.1093/schbul/sbt093 (2014).
Xu, P. et al. Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophr. Res. 208, 353–359, https://doi.org/10.1016/j.schres.2019.01.023 (2019).
Hadley, J. A. et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 39, 1020–1030, https://doi.org/10.1038/npp.2013.305 (2014).
Martino, M. et al. Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naïve patients with schizophrenia. Schizophr. Bull. 44, 419–431, https://doi.org/10.1093/schbul/sbx067 (2018).
Gangadin, S. S., Cahn, W., Scheewe, T. W., Hulshoff Pol, H. E. & Bossong, M. G. Reduced resting state functional connectivity in the hippocampus-midbrain-striatum network of schizophrenia patients. J. Psychiatr. Res. 138, 83–88, https://doi.org/10.1016/j.jpsychires.2021.03.041 (2021).
Andreasen, N. C., Paradiso, S. & O’Leary, D. S. “Cognitive Dysmetria” as an Integrative Theory of Schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry. Schizophr. Bull. 24, 203–218, https://doi.org/10.1093/OXFORDJOURNALS.SCHBUL.A033321 (1998).
Klein, A. et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802, https://doi.org/10.1016/j.neuroimage.2008.12.037 (2009).
Acknowledgements
We thank all participants, Laura Hietapakka Marita Kattelus, Sanna Leppänen, Teemu Mäntylä, Tuula Mononen, and Eva Rikandi for their contribution to data collection. This work was financially supported by the Sigrid Jusélius Foundation (J.S. and T.T.R.), the Finnish Cultural Foundation (J.S.), the Academy of Finland (grants #278171 and #323035 to J.S. and #315861 to T.T.R.), the Finnish Medical Foundation (N.H., T.T.R.), State funding for university-level health research (Hospital District of Helsinki and Uusimaa #TYH2013332, #TYH2014228, #TYH2017128 to T.K.), and the European Union’s Seventh Framework Programme for project METSY (# 602478 to J.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hayward, N.M.E.A., Triana, A.M., Panula, J.M. et al. Connectivity alterations of mesostriatal pathways in first episode psychosis. Schizophr 9, 15 (2023). https://doi.org/10.1038/s41537-023-00339-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-023-00339-y