Abstract
Recent meta-analyses have shown lower zinc and higher copper levels in the serum of people with schizophrenia than in healthy controls. However, the relationship between trace elements (TEs) and the pathophysiology of psychosis, including schizophrenia, remains unclear due to the antipsychotic effects on mineral levels. In this study, we aimed to determine the relationship between zinc and copper levels in hair and psychosis risk among drug-naïve adolescents. This study was conducted as a part of a population-based biomarker subsample study of the Tokyo Teen Cohort Study, including 252 community-dwelling 14-year-old drug-naïve adolescents. Zinc and copper levels in hair were measured using inductively coupled plasma mass spectrometry. The thought problems (TP) scale from the Child Behavior Checklist was used to evaluate psychosis risk. Regression analysis showed that hair zinc levels were negatively correlated with the TP scale (T-score) (β = −0.176, P = 0.005). This result remained significant after adjusting for age and sex (β = −0.175, P = 0.005). In contrast, hair copper levels were not associated with the TP scale (T-score) (β = 0.026, P = 0.687). These findings suggest that lower zinc levels could be involved in the pathophysiology of psychosis, independent of antipsychotics. Further longitudinal studies are required to investigate whether hair zinc level is a useful new biomarker for assessing psychosis risk.
Similar content being viewed by others
Introduction
Schizophrenia is a major mental disorder characterized by psychotic symptoms, flattening of affect, and cognitive impairment, and many people with schizophrenia do not fully recover, resulting in social and occupational dysfunction1. In adolescence, the prevalence of psychotic experiences (PEs) is as high as 8%2, and PEs are considered early indicators of psychosis, including schizophrenia3,4,5. However, the biological mechanism underlying the relationship between adolescent PEs and psychosis is unclear, and there are no established biomarkers for early intervention for psychosis. As the first step to elucidate this issue, it appears reasonable to examine whether trace elements (TEs), one of the potential biomarkers of schizophrenia, also have important roles in psychosis risk among adolescents.
Recent meta-analyses have shown significant differences in the serum levels of several TEs between people with schizophrenia and healthy controls6,7. Lower zinc and higher copper levels were well confirmed in schizophrenia, and this finding was also consistent in studies using hair samples8,9. Furthermore, these levels were significantly associated with symptomatic severity; the positive and negative syndrome scale (PANSS) scores were negatively correlated with serum and hair zinc levels9,10 and positively correlated with hair copper levels8. However, people with schizophrenia may present with different dynamics of TEs compared to drug-naïve people with first-episode psychosis (FEP) or psychosis risk due to the antipsychotic effects on mineral levels, and the mechanism underlying abnormal mineral levels in people with schizophrenia is unknown. Indeed, several studies have shown that TEs are affected by antipsychotics, including aripiprazole, risperidone, and clozapine11,12,13. In drug-naïve people with FEP, some studies showed that zinc levels were lower and copper levels did not significantly differ in serum compared to healthy controls10,11. However, to the best of our knowledge, no studies have been conducted on drug-naïve adolescents with psychosis risk. Therefore, the relationship between TEs and psychosis risk remains unclear.
To understand the role of TEs in the relationship between adolescent PEs and psychosis, we investigated the relationship of zinc and copper levels in hair with the thought problems (TP) scale, which is an indicator of psychosis risk among healthy adolescents14,15.
Results
Table 1 shows the characteristics of the participants who were analyzed. There were no sex differences in terms of age (χ2 = 15.94, P = 0.938), TP scale (T-score) (P = 0.104), or hair zinc levels (P = 0.613). However, hair copper levels were significantly higher in females than in males (P = 0.002) (data not shown).
According to a previous study16, a T-score of the TP scale > 68.5 is considered the cut-off for psychosis. Thus, 80 participants (31.7%) were identified with possible psychosis in the present study. As shown in Fig. 1, the hair zinc levels of the 80 participants were significantly lower than those of the other 172 participants (P < 0.01).
Regression analysis showed that hair zinc levels were negatively correlated with the TP scale (T-score) (β = −0.176, P = 0.005) (Table 2). Figure 2 also shows the correlation of the TP scale (T-score) with hair zinc levels. This correlation remained significant after adjusting for age and sex (β = −0.175, P = 0.005). In contrast, hair copper levels were not associated with the TP scale (T-score) (β = 0.026, P = 0.687).
Using the interquartile range (IQR), five outliers for the TP scale and four outliers for hair zinc levels were identified. The IQR was defined as the difference of the third quartile (Q3) minus the first quartile (Q1) of distribution, and these outliers were higher than Q3 plus 1.5 times the IQR. There were no outliers that were lower than Q1 minus 1.5 times the IQR. Regression analysis after removing these outliers also showed that hair zinc levels were negatively correlated with the TP scale (T-score) (β = −0.211, P = 0.001). This correlation remained significant after adjusting for age and sex (β = −0.212, P = 0.001) (data not shown).
Discussion
Hair zinc levels were negatively associated with the TP scale among drug-naïve adolescents, suggesting that lower zinc levels could be involved in the pathophysiology of psychosis independent of antipsychotics. In contrast, hair copper levels were not associated with psychosis risk. These findings are consistent with recent meta-analyses that showed lower zinc levels in people with schizophrenia6,7. Interestingly, our findings are also consistent with previous studies of drug-naïve people with FEP, in which zinc levels were lower, and copper levels did not significantly differ in the serum compared to healthy controls10,11. Zinc may play an important role in the development of psychosis. In contrast, higher copper levels in people with schizophrenia may indicate antipsychotic effects.
Preceding studies showed that the increase in hair zinc levels began in later childhood and continued until adolescence, before decreasing over time17 and that the peak zinc levels were generally observed between adolescence and adulthood18,19. All the participants in this study were adolescents, indicating that a deviation from the normal trajectory of hair zinc levels may be involved in the development of psychosis. In addition to previous findings showing an association of lower zinc levels with chronic schizophrenia6,7 or FEP10,11, drug-naïve adolescents with psychotic symptoms also showed lower hair zinc levels. These findings suggest that hair zinc levels may be a new biomarker of psychosis risk in a population with wide-ranging ages. However, further longitudinal studies are required to examine the utility of hair zinc levels as a biomarker of psychosis.
Several explanations could be considered for the association between lower zinc levels and the pathophysiology of psychosis risk. First, zinc is an essential TE in numerous biological processes20 and has the highest concentration in the brain compared to other organs in the human body21. Zinc also serves as an endogenous neuromodulator of several important glutamate receptors, including N-methyl-d-aspartate (NMDA) receptors, γ-amino butyric acid receptors, and the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid/kainate receptors22,23. In zinc-deficient young rats, increased extracellular glutamate was observed in the hippocampus23,24. In the cortical synaptic membranes of guinea pigs, zinc deficiency decreased the concentration of NMDA receptors25. Notably, these findings are consistent with the glutamate hypothesis for schizophrenia26. Second, zinc is a component of more than 300 enzymes, and it is involved in systemic physiology, including antioxidant and anti-inflammatory effects21. In in vivo and in vitro cell culture experiments, zinc deficiency was associated with increased oxidative (thiobarbituric acid-reactive substances and protein carbonyl content) and inflammatory (IL-1β, IL-6, and TNF-α) factors27,28. Recently, the relationship between these factors and the pathophysiology of schizophrenia has been confirmed in human studies29,30. Thus, lower zinc levels may affect psychosis development by inducing glutamate dysfunction, oxidative stress, and inflammation.
It remains unclear how hair zinc levels were lowered among adolescents at psychosis risk. One possible explanation is that genetic variations in zinc homeostasis may alter their early neurodevelopment. Zinc homeostasis is maintained by metallothioneins (MTs)31 and two families of zinc transporters: ZnTs (Zn transporters) and ZIPs (Zrt- and Irt-like proteins)32. Recent genome-wide association studies have revealed that a single nucleotide polymorphism in zinc homeostasis genes (ZnT3, ZIP8, and ZIP12) is related to the risk of schizophrenia33,34,35. Furthermore, schizophrenia-like behavioral disturbances, including reduced pre-pulse inhibition and deficits in learning, memory, and social interactions, were observed in the animal models with a targeted disruption of zinc homeostasis genes (ZnT3 and MT3)36,37. Thus, further studies are required to clarify the effect of genetic heterogeneity in zinc homeostasis on psychosis risk.
Our findings suggest important clinical implications for the early identification and intervention of psychosis. In clinical and community settings, it could be recommended that adolescents with lower hair zinc levels receive zinc supplementation, dietary guidance, and psychosocial support. Indeed, a double-blind, randomized, placebo-controlled trial in people with schizophrenia showed that zinc sulfate add-on therapy to risperidone significantly decreased the PANSS scores compared to risperidone monotherapy38.
We have discussed the association between zinc and psychosis, particularly in schizophrenia. However, zinc may be widely involved in the pathophysiology of other mental disorders due to its role in numerous biological processes20. Some meta-analyses have shown that lower zinc levels were also observed in people with depression39 and neurodevelopmental disorders, including autism spectrum disorder40 and attention-deficit hyperactivity disorder41. Thus, further studies in adolescents will be required to investigate the relationship between lower zinc levels and the risk of these disorders.
Strengths and limitations
This study had several strengths. First, adolescents without antipsychotic medications participated in the study, which allowed us to exclude the antipsychotic effects on mineral levels. Therefore, our findings suggest the essential role of TEs in the pathophysiology of psychosis. Second, using a few strands of hair to measure stable mineral levels was less invasive than the collection of blood samples.
This study had some limitations. First, we did not conduct medical interviews, including the structured interview for prodromal syndromes (SIPS) and the comprehensive assessment of ARMS (CAARMS), but used the TP scale, which has only seven items and was evaluated by the primary caregivers. Although previous studies showed an association between the TP scale and psychosis risk14,15, they may have underestimated or overestimated the children’s abnormal behaviors, and this observer bias was not considered in the present study. Second, regression analysis showed that hair zinc levels were significantly correlated with the TP scale (T-score); nonetheless, the adjusted R2 in the analysis was low, indicating that further validation is required regarding the use of hair zinc levels as a clinical biomarker. Additionally, the study design was cross-sectional and the sample size was not large although participants were recruited from a large-scale population-based birth cohort study. Therefore, further longitudinal studies with larger samples size are required to confirm the causal relationship between hair zinc levels and psychosis development. Third, we could compare the TP scale between adolescents at risk of psychosis and people with psychosis using internationally standardized T-scores. However, regarding the hair mineral levels, we could not compare psychosis risk with psychosis because we could not obtain the hair samples from individuals with psychosis, and measurement methods and standard values differ between studies. In the future, it will be necessary to investigate the difference in hair mineral levels between adolescents with psychosis risk and psychosis.
Conclusion
In a population-based birth cohort study of adolescents, lower hair zinc levels could be associated with psychosis risk among drug-naïve adolescents, suggesting its involvement in the pathophysiology of psychosis, independent of antipsychotics. Further longitudinal studies are required to investigate hair zinc level as a new biomarker for assessing psychosis risk.
Methods
Participants
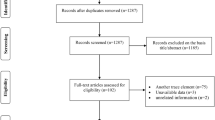
This study was conducted as part of the population-based biomarker subsample study of the Tokyo Teen Cohort Study (pb-TTC), which was a large-scale population-based birth cohort study conducted in the Tokyo Metropolitan area with >3000 adolescent-caregiver dyads (TTC, http://ttcp.umin.jp/). The pb-TTC included 345 adolescents (mean [SD], 13.5 [0.6] years), of which 282 adolescents participated in a second survey at age 14, and both hair mineral levels and the TP scale were examined in 254 adolescents. Two adolescents received antipsychotic medication during the study, and the other 252 adolescents were finally selected for statistical analysis to exclude the effect of antipsychotics on mineral levels (Fig. 3).
Written informed consent was obtained from each participant and their primary caregiver before participation. The research ethics committee of the Tokyo Metropolitan Institute of Medical Science approved this study.
Measurement of hair mineral levels
Serum mineral levels fluctuate with diurnal variation and dietary intake42. However, hair mineral levels stably reflect the accumulation of TEs in the human body over months to years43 and are confirmed as the best indicators of mineral levels in the body44. Furthermore, hair sampling is a less invasive measurement than blood collection.
Hair samples (~3 cm in length and weighing 0.1 g) were obtained from each participant, with the hair cut close to the scalp. The samples were sent to the La Belle Vie research laboratory, and mineral levels (zinc and copper) were measured using the methods as described below45.
Hair samples (75 mg) were weighed into 50 mL plastic tubes and washed twice using acetone and then with 0.01% Triton solution, in accordance with the procedures recommended by the Hair Analysis Standardization Board46. The washed hair samples were mixed with 10 mL 6.25% tetramethylammonium hydroxide (TMAH, Tama Chemical) and 50 µL of 0.1% gold solution (SPEX Certi Prep.) and then dissolved at 75 °C with shaking for 2 h. After cooling the solution to room temperature, an internal standard (Sc, Ga, and In) solution was added. The volume was adjusted gravimetrically, and the obtained solution was used for mineral analysis. The mineral concentrations were measured with inductively coupled plasma mass spectrometry (Agilent-7500ce) using the internal standard method47,48,49 and are expressed as ng/g hair (ppb). For quality control of the mineral analysis, a certified human hair reference material supplied by the National Institute for Environmental Studies of Japan (certified reference material no. 13) was used50.
Assessment of psychosis risk
The Child Behavior Checklist (CBCL) is a simple questionnaire tool developed by Achenbach that comprehensively evaluates children’s emotional and behavioral problems51. CBCL is widely used to predict mental disorders meeting Diagnostic and Statistical Manual of Mental Disorders criteria52,53,54, and the TP scale, a subscale of CBCL, has been shown to be an indicator of psychosis risk among adolescents14,15.
The TP scale was completed by caregivers (mainly mothers) and included the following seven items: (i) ‘Can’t get his/her mind off certain thoughts; obsessions’, (ii) ‘Hears sounds or voices that aren’t there’, (iii) ‘Repeats certain acts over and over; compulsions’, (iv) ‘Sees things that aren’t there’, (v) ‘Stares blankly’, (vi) ‘Strange behavior’ and (vii) ‘Strange ideas’51,55. All the responses were rated on a 3-point scale: not true = 0, somewhat/sometimes true = 1, and very true/often true = 2. The TP scale was defined as the total score of the seven items (possible range: 0–14). The standard values of CBCL vary according to country56,57. As such, TP scores were converted into T-score based on Japanese standard values55 for international comparisons.
Statistical analysis
Data are presented as the mean [SD]. Non-parametric analyses using the Mann–Whitney U test or χ2 test were performed to examine sex differences and compare hair zinc levels between participants with TP scale (T-score) > 68.5 and TP scale (T-score) ≤ 68.5. The threshold for significance was defined as P < 0.05. Regression analysis was used to examine the correlation between hair mineral levels (zinc and copper) and the TP scores among adolescents. Age and sex were covariates due to their possible correlation with the TP scale51,55. The significance level (α) was set to 0.05 for two-tailed tests. All statistical analyses were performed using IBM SPSS for Mac OS, version 26.0 (IBM Corp., New York, USA).
Data availability
The data described in the manuscript, code book and analytic code will be made available upon request pending.
References
Jauhar, S., Johnstone, M. & McKenna, P. J. Schizophrenia. Lancet 399, 473–486 (2022).
Kelleher, I. et al. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol. Med. 42, 1857–1863 (2012).
Nuevo, R., Van, Os,J., Arango, C., Chatterji, S. & Ayuso-Mateos, J. L. Evidence for the early clinical relevance of hallucinatory-delusional states in the general population. Acta Psychiatr. Scand. 127, 482–493 (2013).
Welham, J. et al. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol. Med. 39, 625–634 (2009).
Poulton, R. et al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch. Gen. Psychiatry 57, 1053–1058 (2000).
Saghazadeh, A. et al. Trace elements in schizophrenia: a systematic review and meta-analysis of 39 studies (N = 5151 participants). Nutr. Rev. 78, 278–303 (2019).
Baj, J. et al. Beyond the mind-serum trace element levels in schizophrenic patients: a systematic review. Int. J. Mol. Sci. 21, 9566 (2020).
Rahman, A. et al. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol. Trace Elem. Res. 127, 102–108 (2009).
Ghanem, A. A. et al. Copper and zinc levels in hair of both schizophrenic and depressed patients. Mansoura J. Forens. Med. Clin. Toxicol. 17, 89–102 (2009).
Ma, J. et al. Association between serum essential metal elements and the risk of schizophrenia in China. Sci. Rep. 10, 10875 (2020).
Chen, X. et al. Association of serum trace elements with schizophrenia and effects of antipsychotic treatment. Biol. Trace Elem. Res. 181, 22–30 (2018).
Sussulini, A. et al. Elemental fingerprinting of schizophrenia patient blood plasma before and after treatment with antipsychotics. Eur. Arch. Psychiatry Clin. Neurosci. 268, 565–570 (2018).
Santa Cruz, E. C., Madrid, K. C., Arruda, M. A. Z. & Sussulini, A. Association between trace elements in serum from bipolar disorder and schizophrenia patients considering treatment effects. J. Trace Elem. Med. Biol. 59, 126467 (2020).
Simeonova, D. I., Nguyen, T. & Walker, E. F. Psychosis risk screening in clinical high-risk adolescents: a longitudinal investigation using the Child Behavior Checklist. Schizophr. Res. 159, 7–13 (2014).
Hamasaki, Y., Nakayama, T., Hikida, T. & Murai, T. Combined pattern of childhood psycho-behavioral characteristics in patients with schizophrenia: a retrospective study in Japan. BMC Psychiatry 21, 57 (2021).
Salcedo, S. et al. Diagnostic efficiency of the CBCL thought problems and DSM- oriented psychotic symptoms scales for pediatric psychotic symptoms. Eur. Child Adolesc. Psychiatry 27, 1491–1498 (2018).
Celik, B. et al. Assessment of hair zinc in the school children in Kayseri, Turkey. Biol. Trace Elem. Res. 196, 343–348 (2020).
Meng, Z. Q. Age- and sex-related differences in zinc and lead levels in human hair. Biol. Trace Elem. Res. 61, 79–87 (1998).
Bales, C. W. et al. Zinc, magnesium, copper, and protein concentrations in human saliva: age- and sex-related differences. Am. J. Clin. Nutr. 51, 462–469 (1990).
Kambe, T., Tsuji, T., Hashimoto, A. & Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 95, 749–784 (2015).
Mocchegiani, E., Bertoni-Freddari, C., Marcellini, F. & Malavolta, M. Brain, aging and neurodegeneration: role of zinc ion availability. Prog. Neurobiol. 75, 367–390 (2005).
Smart, T. G., Xie, X. & Krishek, B. J. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog. Neurobiol. 42, 393–441 (1994).
Frederickson, C. J., Suh, S. W., Silva, D., Frederickson, C. J. & Thompson, R. B. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 130, 1471S–1483S (2000).
Takeda, A., Hirate, M., Tamano, H. & Oku, N. Release of glutamate and GABA in the hippocampus under zinc deficiency. J. Neurosci. Res. 72, 537–542 (2003).
Browning, J. D. & O’Dell, B. L. Zinc deficiency decreases the concentration of N-methyl-d-aspartate receptors in guinea pig cortical synaptic membranes. J. Nutr. 125, 2083–2089 (1995).
Kim, J. S., Kornhuber, H. H., Schmid-Burgk, W. & Holzmuller, B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 20, 379–382 (1980).
Doboszewska, U. et al. Alterations of bio-elements, oxidative, and inflammatory status in the zinc deficiency model in rats. Neurotox. Res. 29, 143–154 (2016).
Gammoh, N. Z. & Rink, L. Zinc in infection and inflammation. Nutrients 9, 624 (2017).
Muller, N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 44, 973–982 (2018).
Murray, A. J., Rogers, J. C., Katshu, M., Liddle, P. F. & Upthegrove, R. Oxidative stress and the pathophysiology and symptom profile of schizophrenia spectrum disorders. Front. Psychiatry 12, 703452 (2021).
Mocchegiani, E. et al. Zinc-bound metallothioneins as potential biological markers of ageing. Brain Res. Bull. 55, 147–153 (2001).
Liuzzi, J. P. & Cousins, R. J. Mammalian zinc transporters. Annu. Rev. Nutr. 24, 151–172 (2004).
Perez-Becerril, C., Morris, A. G., Mortimer, A., McKenna, P. J. & de Belleroche, J. Common variants in the chromosome 2p23 region containing the SLC30A3 (ZnT3) gene are associated with schizophrenia in female but not male individuals in a large collection of European samples. Psychiatry Res. 246, 335–340 (2016).
Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Lam, M. et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 51, 1670–1678 (2019).
Koumura, A. et al. Metallothionein-3 deficient mice exhibit abnormalities of psychological behaviors. Neurosci. Lett. 467, 11–14 (2009).
Thackray, S. E., McAllister, B. B. & Dyck, R. H. Behavioral characterization of female zinc transporter 3 (ZnT3) knockout mice. Behav. Brain Res. 321, 36–49 (2017).
Mortazavi, M. et al. Efficacy of zinc sulfate as an add-on therapy to risperidone versus risperidone alone in patients with schizophrenia: a double-blind randomized placebo-controlled trial. Iran. J. Psychiatry Behav. Sci. 9, e853 (2015).
Wang, J., Um, P., Dickerman, B. A. & Liu, J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients 10, 584 (2018).
Zhang, J. et al. Trace elements in children with autism spectrum disorder: a meta-analysis based on case-control studies. J. Trace Elem. Med. Biol. 67, 126782 (2021).
Ghoreishy, S. M., Ebrahimi Mousavi, S., Asoudeh, F. & Mohammadi, H. Zinc status in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis of observational studies. Sci. Rep. 11, 14612 (2021).
Ayodele, J. T. & Bayero, A. S. Lead and zinc concentrations in hair and nail of some Kano inhabitants. Afr. J. Environ. Sci. Technol. 3, 164–170 (2009).
Lakshmi Priya, M. D. & Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 142, 148–158 (2011).
Lech, T. Lead, copper, zinc, and magnesium levels in hair of children and young people with some disorders of the osteomuscular articular system. Biol. Trace Elem. Res. 89, 111–125 (2002).
Takeuchi, H. et al. Association of hair iron levels with creativity and psychological variables related to creativity. Front. Hum. Neurosci. 7, 875 (2013).
Cranton, E. M., Bland, J. S., Chatt, A., Krakovitz, R. & Wright, J. V. Standardization and interpretation of human hair for elemental concentrations. J. Holist. Med. 4, 10–20 (1982).
Yasuda, H. et al. High toxic metal levels in scalp hair of infants and children. Biomed. Res. Trace Elem. 16, 39–45 (2005).
Yasuda, H., Yonashiro, T., Yoshida, K., Ishii, T. & Tsutsui, T. Mineral imbalance in children with autistic disorders. Biomed. Res. Trace Elem. 16, 285–292 (2005).
Yasuda, H. et al. Association between aging and minerals in male Japanese adults. Anti Aging Med 4, 38–42 (2007).
Yoshinaga, J., Morita, M. & Okamoto, K. New human hair certified reference material for methylmercury and trace elements. Z. Anal. Chem. 357, 279–283 (1997).
Achenbach, T. M. Manual for the Child Behavior Checklist/4-18 and 1991 Profile (Department of Psychiatry, University of Vermont, Burlington, 1994).
Bolhuis, K. et al. Psychotic-like experiences in pre-adolescence: what precedes the antecedent symptoms of severe mental illness? Acta Psychiatr. Scand. 138, 15–25 (2018).
Hofstra, M. B., van der Ende, J. & Verhulst, F. C. Child and adolescent problems predict DSM-IV disorders in adulthood: a 14-year follow-up of a Dutch epidemiological sample. J. Am. Acad. Child Adolesc. Psychiatry 41, 182–189 (2002).
Roza, S. J., Hofstra, M. B., van der Ende, J. & Verhulst, F. C. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. Am. J. Psychiatry 160, 2116–2121 (2003).
Itani, T. et al. Standardization of the Japanese version of the Child Behavior Checklist/4-18. Psychiatr. Neurol. Paediatr. Jpn. 41, 243–252 (2001).
De Groot, A., Koot, H. M. & Verhulst, F. C. Cross-cultural generalizability of the child behavior checklist cross-informant syndromes. Psychol. Assess. 6, 225–230 (1994).
Verhulst, F. C. & Achenbach, T. M. Empirically based assessment and taxonomy of psychopathology: cross-cultural applications. A review. Eur. Child Adolesc. Psychiatry 4, 61–76 (1995).
Acknowledgements
We thank Nanako Obata, Izumi Nohara, Mai Hatakenaka, and Ikuyo Kito for their technical assistance. We thank Dr. Yasue Horiuchi and Dr. Hiroaki Ishida (Tokyo Metropolitan Institute of Medical Science) for their helpful advice. We would also like to sincerely thank all the adolescents and their families who participated in this study and the entire TTC team. This work was supported by JSPS KAKENHI (grant numbers JP16H06395, JP16H06398, JP16H06399, JP16K15566, JP16K21720, JP17H05930, JP17H05931, JP19H00972, JP19H04877, JP19K17055, JP20H01777, JP20H03608, JP20H03951, JP20H03596, JP21H05171, JP21H05173, JP21H05174, JP22K07609, and JP21K10487), JST-Mirai Program (grant number JPMJMI21J3), AMED (grant numbers JP19dm0207069, JP18dm0307001, and JP18dm0307004), Moonshot R&D (grant number JPMJMS2021), UTokyo Center for Integrative Science of Human Behavior (CiSHuB) and the International Research Center for Neurointelligence (WPI-IRCN) at The University of Tokyo Institutes for Advanced Study (UTIAS).
Author information
Authors and Affiliations
Contributions
K.T., M.M., S.Y., A.N., and M.A. designed the research; K.T., M.M., S.Y., K.Toriumi, S.A., K.S., K.E., Y.M., Y.T., S.Y., S.U., M.I., M.H.-H., H.T., K.K., A.N., and M.A. conducted the research; K.T., M.M., S.Y., Y.T., S.U., M.I., A.N., and M.A. analyzed the data; and K.T., M.M., and S.Y. wrote the paper. A.N. and M.A. had primary responsibility for the final content. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.M., S.Y., K.Toriumi, S.A., K.K., A.N., and M.A. received grant support from the Japan Society for the Promotion of Science during the conduct of the study. K.K. received grant support from MSD, Astellas, Sumitomo Dainippon Pharma, Eisai, Lily, Takeda, and Mitsubishi Tanabe Pharma. K.K. reports personal fees from Daiichi Sankyo, Otsuka, Meiji Seika Pharma, MSD, Yoshitomi, Astellas, Mochida, Sumitomo Dainippon, Eisai, and Fuji-Film RI Pharma outside the submitted work. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tabata, K., Miyashita, M., Yamasaki, S. et al. Hair zinc levels and psychosis risk among adolescents. Schizophr 8, 107 (2022). https://doi.org/10.1038/s41537-022-00307-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00307-y