Abstract
TJP2/ZO-2-inactivating mutations in humans cause progressive cholestatic liver disease. Liver-specific deletion of Tjp2 in the mouse (Tjp2 cKO mice) leads to mild progressive cholestasis without an overt degradation of the bile-blood barrier (BBB). These mice are more susceptible to cholic acid (CA) induced liver injury. Interestingly, while initially also more susceptible, Tjp2 cKO mice develop tolerance to a DDC-supplemented diet. The DDC diet induces an exacerbated ductular reaction in Tjp2 cKO mice, which arises from the transdifferentiation of hepatocytes to cholangiocytes. Consequently, this transdifferentiation is only observed if Tjp2 is inactivated in hepatocytes, but not if deleted in cholangiocytes. The DDC-diet-induced hepatocyte transdifferentiation in Tjp2 cKO mice requires Yap and Wwtr1/Taz, whose protein expression is upregulated in hepatocytes lacking Tjp2, but is independent of Notch2. Although inactivating Tjp2 is sufficient for the upregulation of Yap and Wwtr1/Taz protein, efficient transdifferentiation requires the DDC-diet insult. Thus, Tjp2 negatively regulates Yap/Taz-mediated transdifferentiation of hepatocytes to cholangiocytes in response to DDC-diet-induced liver injury. Furthermore, transdifferentiation is regulated at multiple levels and the type of injury inflicted on the Tjp2 deficient liver plays an important role in the resulting pathophysiology.
Similar content being viewed by others
Introduction
The liver plays key roles in the metabolism of amino acids, lipids, and carbohydrates, as well as in the production and excretion of bile. Hepatocytes carry out metabolic and detoxification reactions and produce bile. Bile acids are excreted into the canaliculi, from where the bile drains into intrahepatic bile ducts lined by bile duct epithelial cells or cholangiocytes, from where it is delivered to the gall bladder via the extrahepatic bile duct. Tight junctions (TJs) between adjacent hepatocytes seal the canaliculus to form the bile-blood barrier, which restricts the diffusion of bile from the canaliculus to the serosal tissue compartment.
As a major metabolic detoxification hub, the liver is continuously exposed to bile acids and other cytotoxic chemicals. These insults commonly result in increased plasma bile acid levels, reduced liver function, and expansion of bile duct epithelial cells (e.g. a ductular reaction) and, if chronic, ultimately lead to liver fibrosis and cirrhosis. The organ has developed a remarkable array of regenerative strategies to cope with injury. Hepatocytes can proliferate and reconstitute a significant mass of the damaged tissue. If hepatocyte proliferation is insufficient, still poorly characterized liver progenitor cells (known as oval cells in the mouse) are activated to generate cholangiocytes and hepatocytes. DDC-diet-induced liver injury activates the transdifferentiation of hepatocytes into cholangiocytes, a process that is regulated by the Notch1 and Hippo2 signaling pathways.
TJs of the BBB are composed of different integral transmembrane proteins, including members of the claudin (for example, Cldn1) and MARVEL (for example, Ocln) protein families. Cytosolic adaptor proteins such as ZO-1/Tjp1 and ZO-2/Tjp2 tether these transmembrane proteins to the underlying actomyosin cytoskeleton. In addition to their structural roles, Tjp1and Tjp2, act as hubs to regulate major signaling pathways. Through their multiple protein-protein interaction domains they associate with and modulate, often in response to cell–cell contact cues, different signaling factors, and transcriptional regulators. Tjp1 or Tjp2, for example, have been implicated in regulating the cytosolic/nuclear localization of the Hippo pathway transcriptional effectors Yap and Wwtr1/Taz3,4,5,6,7,8.
TJP2-inactivating familial mutations in humans are associated with progressive cholestasis9,10,11,12. The role of Tjp2 in liver biology has been explored using mouse models13,14 as well as liver organoids derived from Tjp2 knock-out or patient-specific induced pluripotent stem cells15. While mice lacking Tjp2 are embryonic lethal16, the liver-specific inactivation results in mild progressive cholestasis14 without an overt defect in the BBB13,14. Tjp2 deficiency presents with a redistribution of radixin and less canalicular microvilli14,15, previously linked to cholestasis17, as well as changes in bile acid transporter and detoxification enzyme expression and distribution13,14,15.
Here, we characterized the effect of a DDC diet on Tjp2 cKO mice. Interestingly, Tjp2 cKO mice develop tolerance to prolonged DDC-diet feeding, likely due to the increased formation of duct-like structures that facilitate the clearance of bile. This DDC-diet-induced ductular reaction arises from an enhanced transdifferentiation of hepatocytes to cholangiocytes, which requires Yap/Wwtr1 and is negatively modulated by Tjp2.
Results
Liver-specific inactivation of Tjp2 leads to an exacerbated ductular reaction in response to DDC-diet
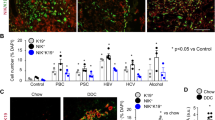
The body weight of control or Tjp2 cKO mice fed normal chow supplemented with 0.1% DDC (DDC-diet) gradually declined (Fig. 1a). In contrast to controls, however, the body weight of Tjp2 cKO animals fed DDC diet stabilized after week 2 and then partially recovered, suggesting that these mice had adapted to cope with the effect of the DDC-diet. Liver-to-body weight ratios were not only significantly higher in Tjp2 cKO mice compared to controls, but the increase between 7 and 28 days of DDC diet was also more pronounced in the absence of hepatic Tjp2 (Fig. 1b). While the histology of control and Tjp2 cKO livers were comparable after 7 days of DDC diet (Fig. 1c), cell proliferation, as shown by more Ki67-positive cells detected by immunofluorescence microscopy, was enhanced in livers of Tjp2 cKO mice fed DDC diet for 28 days (Fig. 1d, e). Interestingly, however, the number of Ki67-positive hepatocytes, identified by Hnf4α staining, was indistinguishable between controls and Tjp2 cKO livers. Similar results were obtained by using Edu incorporation to monitor cell proliferation (Supplementary Fig. 1a, b), pointing to the expansion of a non-hepatocyte cell pool in the liver of Tjp2 cKO mice fed DDC-diet. The number of Hnf4α-positive hepatocytes per liver area remained similar (Supplementary Fig. 1c, d) and no difference in cell or hepatocyte proliferation was observed between livers of control and Tjp2 cKO mice fed a regular chow (Supplementary Fig. 1e, f).
a Change of relative body weight for control and Tjp2 cKO mice fed DDC diet for 28 days. b Liver to body weight ratio of indicated mouse lines after 7- and 28-day DDC-diet. c H&E staining for control and Tjp2 cKO mouse liver after 7- and 28-day DDC-diet. Scale bar: 100 μm. d, e Immunofluorescence microscopy and quantification of Ki67-positive cells and Ki67-positive HNF4α-positive hepatocytes in the liver of mice fed DDC diet for 28 days. Scale bar: 50 μm. f, g Immunohistochemistry staining for Ck19 showing the periportal area and quantification of the Ck19-positive liver area from random liver sections after a 28-day DDC-diet. Scale bar: 100 μm. h, i Western blot analysis and quantification of Ck19 expression in control and Tjp2 cKO mouse liver samples after a 28-day DDC diet. j, k Immunohistochemistry staining and quantification of Sox9-positive cells after a 28-day DDC-diet. Scale bar: 100 μm. l, m Immunohistochemistry staining and quantification of A6-positive liver area after a 28-day DDC diet. Scale bar: 100 μm. n, o Sirius red staining and quantification of Sirus red-positive liver area after a 28-day DDC diet. Scale bar: 50 μm. Data in (a, b, e, g, i, k, m, o) shown as mean ± SD, unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns—not significant (p > 0.05), with p < 0.05 considered a significant difference. (a, b, e, o): n = 10, (g): n = 7, (k, m): n = 8, (i) n = 3 mice per cohort, data from blot (h).
As expected, after 28 days of DDC-diet, the control livers presented with a ductular reaction, characterized by tubular structures in the portal region (Fig. 1f). In the Tjp2 cKO liver, these Ck19 ductular structures did not remain localized to the portal area but were found further in the liver parenchyma, as shown by a larger Ck19-positive periportal area (Fig. 1g). Immunohistochemistry staining and quantification for Sox9 (Fig. 1j, k) and the oval cell marker A6 (Fig. 1l, m) confirmed the ductular nature of these cells and their higher abundance and broader distribution in the liver of DDC-diet-fed Tjp2 cKO mice. Consistent with the ductular reaction and the larger Ck19-positive area, Ck19 expression, assessed by Western blot (Fig. 1h, i) or qRT-PCR (Supplementary Fig. 1g), was highly increased in livers of mice fed DDC-diet for 28 days. mRNA expression of Epcam and Osteopontin, two genes involved growth of cholangiocytes, was strongly increased in DDC-diet-fed mice, and more so in Tjp2 cKO animals as compared to controls. While the fraction of fibrotic tissue in control and Tjp2 deficient livers was comparable (Fig. 1n, o), Sirius red staining intensity, restricted to the portal area in controls, was reduced in Tjp2 cKO livers but distributed throughout the whole liver tissue (Fig. 1n). Despite the different pattern of Sirius red staining, qRT-PCR and Western blot analysis and quantification of different fibrosis markers confirmed the comparable fibrosis in control and Tjp2 cKO mouse livers (Supplementary Fig. 1h–k). Abundant DDC-mediated porphyrin deposition was seen in control liver sections, but significantly less so in livers lacking Tjp2 (Fig. 1c), likely due to reduced protein levels of Alas-1, the rate limiting enzyme in porphyrin synthesis (Supplementary Fig. 1l–o).
Mice with hepatic deletion of Tjp2 develop tolerance to the DDC-diet
Tjp2 cKO mice show progressive cholestasis (Fig. 2a–e and14) and are more susceptible to a CA-diet14. In animals fed the DDC diet for 7 days, blood and liver biochemistry parameters (e.g., BA, AP, ALT, AST, and bilirubin levels) were elevated as compared to control chow-fed cohorts, but, except for AP and bilirubin, comparable for DDC-diet fed Tjp2 cKO and control mice (Fig. 2). However, by 28 days, while BA levels remained elevated in controls, they were significantly lower in mice lacking hepatic Tjp2 (Fig. 2a, b). Cholestasis severity, assessed by AP levels, was lower in DDC-diet-fed Tjp2 cKO mice and did not worsen with time as compared to corresponding control animals (Fig. 2c). Compared to corresponding controls, Tjp2 cKO mice also showed milder DDC-diet-induced liver injury, with ALT levels significantly lower (Fig. 2d) and AST (Fig. 2e) levels trending lower at 28 days of DDC-diet feeding. Liver function, monitored by bilirubin levels, was initially more severely affected by the DDC diet in Tjp2 cKO mice (Fig. 2f). However, while liver function continued to deteriorate in controls, it improved in Tjp2 cKO animals by 28 days of DDC-diet feeding, consistent with the lower BA levels and liver injury. Thus, while more susceptible to a CA-diet14, Tjp2 cKO mice develop tolerance to chronic DDC-diet feeding, suggesting that the type of liver injury influences the pathophysiology in these mice.
Biochemical analysis of liver and plasma markers for control and Tjp2 cKO mice fed DDC diet for 7 and 28 days. a Plasma BA, (b) liver BA, (c) AP, (d) ALT, (e) AST, (f) Bilirubin. Data shown as mean ± SD, unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns—not significant (p > 0.05), with p < 0.05 considered a significant difference. n = 10 mice per cohort.
Deletion of Tjp2 in hepatocytes, not cholangiocytes, mediates the enhanced DDC-diet-induced ductular reaction in the Tjp2 cKO liver
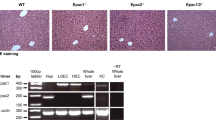
Tjp2 is inactivated in both hepatocytes and cholangiocytes of Tjp2 cKO mice14. To determine whether the enhanced DDC-induced ductular reaction in these animals was due to the absence of Tjp2 from a particular cell type, we took advantage of mice where Tjp2 can be inducibly-inactivated in cholangiocytes (Tjp2 icKOCC) or hepatocytes (Tjp2 icKOHC)14.
As assessed by H&E staining, Tjp2 icKOCC mice fed the DDC diet for 28 weeks showed similar liver histology as controls (Fig. 3a). Immunohistochemical staining for Ck19 and quantification (Fig. 3a, b) showed no difference in the extent of ductular reaction in Tjp2 icKOCC livers compared to controls. Staining for the LacZ reporter confirmed efficient activation of Cre recombinase in cholangiocytes, with most of the ductular reaction originating from these LacZ-positive cholangiocytes.
a H&E, Ck19, and LacZ staining of liver sections from control and Tjp2 icKOCC mice after a 28-day DDC-diet. Scale bar: 100 μm. b Quantification of the Ck19-positive liver area. c Liver to body weight ratio for control and Tjp2 icKOHC mice after a 28-day DDC-diet. d H&E staining for control and Tjp2 icKOHC mice after a 28-day DDC-diet. Scale bar: 100 μm. e, f Ck19 staining showing periportal areas from control and Tjp2 icKOHC mice after a 28-day DDC diet and quantification from random liver sections. Scale bar: 100 μm. g, h Sox9 staining of liver sections from control and Tjp2 icKOHC mice after a 28-day DDC diet and quantification. Scale bar: 100 μm. i, j A6 staining of liver sections from control and Tjp2 icKOHC mice after a 28-day DDC diet and quantification. Scale bar: 100 μm. Data in (b, c, f, h, j) shown as mean ± SD, unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns = not significant (p > 0.05), with p < 0.05 considered a significant difference. (b): n = 6, (c, f): n = 7, (h, j): n-8 mice per cohort. icKOHH or icKOCC, tamoxifen-induced hepatocyte or cholangiocyte deletion, respectively.
In contrast, Tjp2 icKOHC mice fed the DDC diet presented with a higher liver-to-body weight ratio (Fig. 3c). H&E staining revealed duct-like structures further into the liver parenchyma (Fig. 3d), like what was observed in Tjp2 cKO livers. Immunohistochemistry staining for Ck19 (Fig. 3e, f), Sox9 (Fig. 3g, h), and A6 (Fig. 3i, j) confirmed that the cells of these duct-like structures expressed cholangiocyte markers.
Conditional inactivation of Tjp2 facilitates DDC-diet-induced transdifferentiation of hepatocytes to cholangiocytes
Several recent studies have established that hepatocytes can transdifferentiate into cholangiocytes18,19. Since hepatocyte deletion of Tjp2 led to the DDC-induced expansion of cholangiocyte-like cells throughout the liver tissue, we tested if these cholangiocytes were derived from transdifferentiated hepatocytes, using tamoxifen-treated Tjp2F/F AlbCreERT2 Rosa26:Lox-STOP-Lox-LacZ mice14 for lineage tracing. One week after the tamoxifen regime, the resulting control Rosa26:LacZ or Tjp2 icKOHC Rosa26:LacZ mice were fed the DDC diet for 28 days before the collection and analysis of livers. Cre was selectively expressed in hepatocytes as shown by LacZ expression in Ck19-negative cells. In livers of Tjp2 icKOHC Rosa26:LacZ mice, the duct-like structures showed intense blue LacZ staining, with many of the strong LacZ-positive cells also labeling for Ck19 (Fig. 4a). Hepatocytes with a fainter LacZ staining surrounded these intensely labeled cells.
a Lineage tracing. One week after the last tamoxifen dose to induce deletion of the indicated genes, mice were fed a DDC diet for 28 days and sacrificed. LacZ and Ck19 staining of control and Tjp2 icKOHC Rosa26:LacZ mouse liver samples, showing Ck19-positive ductular structures derived from LacZ-positive hepatocytes (✽✽) and resident LacZ-negative Ck19-positive bile ducts (✽). Scale bar: 50 μm. b, c Tjp2 and Ck19 staining of control and Tjp2 icKOHC liver sections from mice fed standard or DDC diet and quantification. Scale bar: 20 μm. d Tjp2 and A6 staining of control and Tjp2 icKOHC liver sections from mice fed standard or DDC-diet. Note that in Tjp2 icKOHC animals, Tjp2 is deleted in hepatocytes, thus showing that Tjp2-negative and Ck19- or A6-positive ductular cells arise from Tjp2-deficient hepatocytes. icKOHH, tamoxifen-induced hepatocyte deletion. Scale bar: 20 μm.
In the Tjp2 icKOHC liver, Tjp2 expression is inactivated in hepatocytes but not in cholangiocytes14. Thus, if the expanded bile duct-like structures induced by DDC-diet feeding arise from Tjp2 deficient hepatocytes, they should also carry an inactivated Tjp2 gene. Bile ducts in livers of control or Tjp2 icKOHC mice fed standard chow showed, as expected, Tjp2-positive bile ducts (Fig. 4b). While bile ducts were also Tjp2-positive in control animals fed the DDC diet for 28 days, Tjp2 was absent from most of the Ck19- or A6-positive duct-like structures in Tjp2 icKOHC livers (Fig. 4b–d), corroborating the LacZ lineage tracing experiment. The data thus indicates that following inactivation of Tjp2 in hepatocytes, the DDC diet induces a strong ductular reaction, whereby new cholangiocytes arise by transdifferentiation of hepatocytes.
Increased protein levels and nuclear localization of Yap/Taz in hepatocytes lacking Tjp2
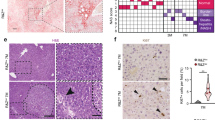
Overexpression of Yap in the liver induces transdifferentiation of hepatocytes to cholangiocytes2. Furthermore, Tjp2 associates with Yap and Taz and retains them in the cytosol, where they undergo degradation4,5,6,7. We therefore hypothesized that Tjp2 may suppress hepatocyte transdifferentiation via Yap and/or Taz. In hepatocytes isolated from control or Tjp2 cKO mice fed a standard chow, total Yap and Taz protein expression levels were strongly increased in the latter as assessed by Western blot analysis (Fig. 5a, b). Interestingly, despite the increased expression level of Yap, pS127 Yap levels were reduced in the absence of Tjp2. Immunohistochemistry and immunofluorescence microscopy confirmed stronger Yap staining in liver sections of Tjp2 icKOHC mice fed standard chow or a DDC diet for 28 days as compared to corresponding controls (Fig. 5c, d, f). Since pS127 Yap is retained in the cytosol and subject to degradation by the proteasome pathway20, the reduced pS127 Yap level in Tjp2 cKO hepatocytes is consistent with the observed higher total Yap protein levels and suggests enhanced Yap activity as compared to control hepatocytes. Indeed, while only a few cells showed nuclear colocalization of Yap and the hepatocyte marker Hnf4α21 in control mice, 3–5 times as many Hnf4α-positive hepatocytes showed nuclear localization of active (e.g. not phosphorylated on S127) Yap in Tjp2 icKOHC mice fed either the standard (Fig. 5d, e) or the DDC-supplemented (Fig. 5f, g) diet. Hepatocellular expression levels and nuclear staining intensity for active YAP were not only increased after deletion of Tjp2 (Fig. 5a–e), but they were further increased by the DDC diet, both in controls and Tjp2 icKOHC mice (Fig. 5f, g). As expected, the expression of known Yap/Taz target genes, including Sox9 which promotes cholangiocyte fate, were upregulated after Tjp2 deletion and/or DDC-diet feeding (Fig. 5h), confirming higher Yap activity. These data are consistent with the DDC diet and Tjp2 deletion influencing cellular YAP levels, with Tjp2 also playing a key role in negatively regulating Yap/Taz by retaining these Hippo pathway effectors in the cytosol of hepatocytes.
a, b Western blot analysis and quantification of Yap, Taz and pSer127 Yap expression in isolated hepatocytes from control and Tjp2 cKO mice fed normal chow. c Immunohistochemistry staining for Yap in control and Tjp2 icKOHC liver sections after a 28-day DDC-diet. Scale bar: 50 μm. d, f Immunofluorescence labeling of active (e.g. not S127 phosphorylated) Yap and HNF4α, a nuclear hepatocyte marker in liver sections of mice fed a standard (d) or a DDC- supplemented (f) diet. Scale bar: 20 μm. e, g Quantification of Yap and HNF4α-positive nuclei. h Relative mRNA expression levels of key Hippo effectors and target genes in liver of control or Tjp2 cKO mice fed normal or DDC-supplemented chow. i Western blot analysis of Yap and Wwtr1/Taz to verify their deletion in the liver of the corresponding mouse lines. j Liver to body weight ratios for the indicated mouse lines fed DDC diet for a 28-day period. k H&E staining of liver sections for the indicated mouse lines after a 28-day DDC-diet. Scale bar: 100 μm. l, m Immunohistochemistry staining for Ck19 in liver sections for the indicated mouse lines after a 28-day DDC diet and quantification of the Ck19-positive area. Scale bar: 100 μm. Data in (b, e, g, h, j, m) shown as mean ± SD, unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns=not significant (p > 0.05), with p < 0.05 considered a significant difference. (b): n = 3 or 4, (e) and (g): n = 10, (h): n = 3, (j): n = 7, (m): n = 6 mice per cohort. icKOHH, tamoxifen-induced hepatocyte deletion.
Yap and Taz are required for DDC-diet-induced ductular reaction and suppression of liver injury in hepatic Tjp2 deficient mice
To determine whether Yap/Taz are required for the DDC-diet-induced phenotype in Tjp2 icKOHC mice, we crossed Alb-CreERT2 Tjp2F/F mice with YapF/F, TazF/F or YapF/F TazF/F mice22,23, which after tamoxifen induction result in mice with an hepatocyte-inactivation of Tjp2 and Yap (Tjp2 Yap icKOHC), Tjp2 and Taz (Tjp2 Taz icKOHC) or Tjp2, Yap and Taz (Tjp2 Yap Taz icKOHC). Western blot analysis for the Hippo effectors confirmed their efficient inactivation in hepatocytes of the respective mouse strains (Fig. 5i).
While the initial decline in body weight of DDC-diet-fed Tjp2 icKOHC mice recovered from 7 days onward (Fig. 1a), this recovery was abrogated in Tjp2 Yap icKOHC mice (Supplementary Fig. 2). Likewise, the liver-to-body weight ratio increase observed in Tjp2 icKOHC animals fed DDC diet for 28 days (Fig. 1b) was stunted in the absence of Yap (Fig. 5j).
H&E staining did not reveal duct-like structures in the livers of Yap icKOHC or Tjp2 Yap icKOHC mice fed DDC diet for 28 days, indicating that the ductular reaction observed in the Tjp2 icKOHC liver requires Yap (Fig. 5k). By immunohistochemistry, Ck19-positive bile duct-like structures, observed in livers from Tjp2 icKO mice fed DDC diet for 28 days, were strongly reduced in Tjp2 Yap icKOHC and, to a lesser extent, Tjp2 Taz icKOHC tissues (Fig. 5k, l). Livers where either Yap or Taz was inactivated showed less Ck19-positive staining compared to controls and deletion of both Yap and Taz in the Tjp2 deficient background further suppressed the ductular reaction (Fig. 5m). These data indicate that both Yap and Taz contribute to the DDC-diet-induced ductular reaction observed in Tjp2 icKO mice. Given the more pronounced effect of inactivating Yap, we focused on Yap in subsequent experiments.
Overall, the improved DDC-diet-induced blood and liver biochemistry values observed in Tjp2 icKOHC mice compared to controls were abrogated if Yap was inactivated in Tjp2 icKOHC animals. Plasma (Fig. 6a) and liver (Fig. 6b) BA levels in Tjp2 Yap icKOHC mice were increased compared to control mice. While AP levels were not significantly different between Tjp2 icKOHC and Tjp2 Yap icKOHC mice (Fig. 6c), plasma ALT (Fig. 6d), AST (Fig. 6e), and bilirubin (Fig. 6f) were all elevated in the latter to levels comparable to controls. Thus, Yap is required for the development of tolerance to DDC-diet-induced liver injury observed in mice where Tjp2 has been ablated in hepatocytes.
a Plasma BA, (b) liver BA, (c) AP, (d) ALT, (e) AST, (f) Bilirubin. Data are shown as mean ± SD, unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns—not significant (p > 0.05), with p < 0.05 considered a significant difference. n = 7 mice per cohort. icKOHH, tamoxifen-induced hepatocyte deletion.
DDC-diet-induced transdifferentiation of Tjp2-deficient hepatocytes into cholangiocytes requires Yap and is independent of Notch2
Since DDC diet promotes the transdifferentiation of Tjp2 deficient hepatocytes into Ck19-positive, Tjp2-negative cholangiocytes (see above), we next used lineage tracing to test if this transdifferentiation required Yap. LacZ-positive bile duct-like structures, indicative of transdifferentiation, were detected at a low frequency in livers from control DDC-diet fed Rosa26:LacZ mice but were only rarely observed in Yap cKOHC Rosa26:LacZ livers (Fig. 7a, b). A large number of LacZ-positive biliary structures in Tjp2 icKOHC Rosa26:LacZ livers was strongly reduced after Yap inactivation, and concomitant inactivation of Yap and Taz resulted in a further reduction (Fig. 7b). Similar results were obtained for the different mouse strains by monitoring transdifferentiated bile ducts based on their Ck19-positive and Tjp2-negative phenotype (Fig. 4b, d).
a, b LacZ staining of liver sections from the indicated mouse strains and quantification of LacZ-positive bile ducts. Scale bar: 100 μm. c Liver mRNA expression levels of Notch1, Notch2, and Jag1 of Tjp2 cKO mice fed a normal or DDC diet and normalized to that of controls. d Western blot analysis of Notch2 to confirm deletion in the Tjp2 Notch2 cKO liver. (tmi: transmembrane and intracellular domain, flL full-length). e, f Immunohistochemistry staining for Ck19 in liver sections from the indicated mouse lines and quantification. Scale bar: 100 μm. Data in (b, c, f) are shown as mean ± SD, unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns—not significant (p > 0.05), with p < 0.05 considered a significant difference. (b, f): n = 6, (c): n = 3 mice per cohort. icKOHH, tamoxifen-induced hepatocyte deletion.
Not only overexpression of Yap2, but also that of Notch21 can induce transdifferentiation of hepatocytes into cholangiocytes. DDC-diet feeding increased Notch1, Notch2, and Jag1 mRNA expression levels in both control and Tjp2 cKO livers (Fig. 7c). Interestingly, expression of Notch2 was higher in the liver of Tjp2 cKO mice compared to controls, independent of whether they were fed standard chow or DDC-diet, suggesting an effect of Tjp2 on Notch2 expression. To test the possibility that the observed transdifferentiation in Tjp2 cKO livers was mediated by the observed increase in Notch2 expression, we crossed Tjp2 cKO and Notch2F/F mice to generate Tjp2 Notch2 cKO animals. As shown by Western blot and immunohistochemistry, expression of Notch2 was efficiently inactivated in the liver of these mice (Fig. 7d). While Notch2 cKO mice developed less bile ducts after a 4-week DDC diet compared to controls, Tjp2 Notch2 cKO animals presented with a similar ductular reaction as observed in Tjp2 cKO mice (Fig. 7e, f), indicating that the DDC-diet-induced ductular reaction in Tjp2 cKO animals was not mediated by Notch2, and that the loss of Notch2 does not prevent the Tjp2 deficiency related ductular reaction. Therefore, both Yap and Taz, but not Notch2, mediate the enhanced DDC-induced cholangiocyte transdifferentiation of hepatocytes lacking Tjp2.
Discussion
TJP2-inactivating mutations in humans are associated with progressive familial cholestasis9,10,11. In the mouse, constitutive or inducible deletion of Tjp2 in the liver only leads to mild progressive cholestasis, but these animals are more susceptible to dietary CA. While controls tolerate a diet supplemented with 0.5% cholic acid, Tjp2 cKO mice develop severe cholestasis and liver injury14. Interestingly, we show here that mice lacking Tjp2 in hepatocytes develop tolerance to a diet supplemented with DDC via an enhanced, DDC-induced, and Yap/Taz-mediated, transdifferentiation of hepatocytes into cholangiocytes.
Tjp2 cKO mice fed a standard chow showed significantly higher plasma BA, AP, ALT, AST, and bilirubin levels, in agreement with previous data14 and consistent with mild cholestasis. When fed DDC diet for one week, these parameters were elevated as compared to control chow-fed cohorts, but, except for AP and bilirubin, comparable for DDC-diet-fed Tjp2 cKO and control mice. After 4 weeks on the DDC diet, however, the Tjp2 cKO mice presented with significantly less severe cholestasis and liver injury and better liver function as compared to corresponding controls, suggesting the development of tolerance to the detrimental effects of DDC. This adaptation was reflected in a robust expansion of Ck19, Sox9, and A6-positive, cholangiocyte-like cells. A ductular reaction in response to liver injury is a well-known phenomenon24, whereby liver progenitor cells and cholangiocytes in the periportal area expand. As expected, a ductular reaction was also observed in controls fed the DDC diet. Intriguingly, however, the DDC-diet-induced ductular reaction was not only more pronounced in the Tjp2 deficient liver, but the duct-like structures were not restricted to the periportal area as in controls but were found further in the liver parenchyma. Additional bile ducts generated by this ductular reaction likely contribute to the tolerance of Tjp2 cKO mice to prolonged DDC diet by facilitating clearance of toxic bile acids from the liver and relieving cholestasis. Consistent with the stronger ductular reaction in the Tjp2 cKO liver, cell proliferation of cholangiocytes, but not hepatocytes, was enhanced as compared to controls. However, using tamoxifen-inducible Albumin-CreERT2 and Sox9-CreERT2 mouse lines to inactivate Tjp2 in hepatocytes or cholangiocytes, respectively14, revealed that the deletion of Tjp2 in hepatocytes (e.g. Tjp2 icKOHC mice) and not in cholangiocytes (e.g. Tjp2 icKOCC mice) is responsible for the enhanced ductular reaction and the development of tolerance to the DDC-diet. A deletion of Tjp2 in periportal “hybrid” hepatocytes, which express Sox925 and can activate Sox9-Cre26, is thus unlikely to exclusively account for the observed ductular reaction.
The DDC-diet liver injury model has been widely used to study the transdifferentiation of hepatocytes into cholangiocytes18,19. Lineage tracing using a LacZ reporter confirmed abundant Ck19-positive ductular structures in the Tjp2 icKOHC liver that originate from LacZ-positive hepatocytes. In addition, since Tjp2 is selectively ablated in hepatocytes (and not in cholangiocytes) in the Tjp2 icKOHC mouse, the fact that the DDC-diet-induced LacZ and Ck19-positive ductular cells were also devoid of Tjp2 further corroborates their origin from hepatocytes. In contrast, the more modest ductular reaction that was observed in DDC-diet fed control or Tjp2 icKOCC mice was predominantly derived from existing cholangiocytes.
Hepatocyte transdifferentiation is regulated by Hippo and Notch signaling1,2. Although the expression of several effectors of Notch signaling was upregulated in Tjp2 cKO mice, deletion of Notch2 did not abrogate the DDC-diet-induced ductular reaction, suggesting that in this model, this process is independent of Notch2 signaling. Hippo signaling regulates the identity of cholangiocytes and overexpression of the Hippo effector Yap induces the transdifferentiation of hepatocytes into cholangiocytes2. Since pS127 Yap is targeted for degradation, the reduced pS127 Yap levels are consistent with the higher total Yap (and Taz) levels in Tjp2 cKO mice. Total Yap and active Yap (e.g. not phosphorylated at S127) levels were also increased in DDC-diet-fed Tjp2 cKO and Tjp2 icKOHC mice compared to controls. While predominantly present at the hepatocyte cell border in DDC-diet-fed control mice, active Yap accumulated in the hepatocyte nucleus in the corresponding Tjp2 icKOHC animals.
Concomitant inactivation of Tjp2 and Yap, Taz, or both, confirmed that the DDC-diet-induced ductular reaction in the absence of Tjp2 was dependent on the Hippo effectors, with the deletion of Yap showing a more pronounced suppression of the ductular reaction. Although the increase in plasma BA and AP was less pronounced, blood and liver biochemistry parameters in Tjp2 Yap icKOHC mice deteriorated to levels comparable to those in control or Yap icKOHC animals. This observation indicates that the DDC-diet-induced ductular reaction in the absence of Tjp2 may enhance the clearance of bile, thereby improving liver function and relieving cholestasis. Porphyrin plugs were still present in Tjp2 Yap icKOHC livers, suggesting that the altered porphyrin clearance in the absence of Tjp2 is Yap independent.
Although the inactivation of Tjp2 in hepatocytes leads to stabilization and an increase of Yap and Taz protein levels, concomitant DDC exposure is required to induce the massive ductular reaction. In control mice, the DDC diet also induces a ductular reaction which, however, is limited and restricted to the periportal area. The excessive transdifferentiation of hepatocytes in Tjp2 icKOHC mice is suppressed by Yap deletion to levels comparable to control animals, with the residual ductular reaction now restricted to the periportal area. The limited periportal ductular reaction in control animals was also reduced after the deletion of Yap and, in both controls and Tjp2 icKOHC mice, was further suppressed after the additional inactivation of Taz. These observations indicate that both Yap and Taz contribute to hepatocyte transdifferentiation, that this is a physiological response to the DDC diet, and that Tjp2 negatively regulates this process. Yap and Taz directly interact via their C-terminal PDZ binding motifs with the 1st PDZ domain of Tjp2 and mostly using MDCK cells, Tjp2 has been shown to repress Yap/Taz activity by preventing their nuclear translocation4,5,6,7,8. In vivo, such a regulatory role is less well established, but has been inferred from a correlation between reduced Tjp2/ZO-2 expression and increased Yap nuclear localization in a rat model of experimental compensatory renal hypertrophy and in liver steatosis of obese Zucker rats7,8. The present work thus establishes that Tjp2 negatively regulates Yap/Taz in vivo by suppressing DDC-induced hepatocyte transdifferentiation in the mouse. We postulate that the relative molar ratios of Yap and Tjp2 regulate Yap activity. Indeed, also overexpression of Yap in the liver can induce hepatocyte transdifferentiation2, possibly because the available Tjp2 can no longer retain the excessive Yap in the cytosol and/or present it to Lats1 for phosphorylation.
It is currently unclear if during transdifferentiation to cholangiocytes, hepatocytes transiently acquire an oval cell (e.g. A6-positive) identity. Yap has been implicated in the activation of oval cell proliferation and DDC-diet-fed mice showed an expansion of A6-positive cells27. While restricted to the periportal area in control mice, A6-positive cells spread over the entire liver in Tjp2 cKO mice fed a DDC diet. Interestingly, despite higher Yap/Taz levels in the liver of Tjp2 cKO mice, standard chow or CA14 supplemented diet did not result in the extra-periportal ductular reaction and hepatocyte transdifferentiation14 that was observed in Tjp2 cKO mice fed a DDC-diet. These suggest that Yap/Taz activity or output are regulated or require additional factors, which themselves are modulated, directly or indirectly, by DDC or its metabolites. DDC activates the constitutive androstane receptor (CAR)28. Indeed, the CAR agonist TCPOBOP leads to nuclear accumulation of Yap in mouse hepatocytes29. CAR-mediated hepatocyte proliferation, but not the induction of drug-metabolizing enzymes, requires Yap30. DDC-diet-induced hepatic injury also activates a neonatal splicing program by suppressing the translation of Esrp2 mRNA, which rewires the Hippo pathway31. Although Tjp2 deletion and DDC diet feeding independently increase Yap protein levels, the contribution of both may be required to induce hepatocyte transdifferentiation. It will thus be interesting to further elucidate the crosstalk between Tjp2 and DDC in modulating Yap/Taz activity in the injured liver.
In summary, Tjp2 suppresses Yap-mediated transdifferentiation of hepatocytes into cholangiocytes in response to DDC-diet-induced liver injury. Mice with an ablation of Tjp2 in hepatocytes develop tolerance to DDC-diet-induced liver injury through an enhanced, Yap dependent, transdifferentiation of hepatocytes to cholangiocytes. The resulting duct-like structures could contribute to the development of tolerance of these mice to the DDC diet, presumably by facilitating the clearance of bile from the liver.
Methods
Mouse strains, genotyping
Animal experimentation was approved by the relevant IACUC (A*STAR IACUC, Protocol #201558) and carried out under specific pathogen-free conditions. Tjp2 was deleted in liver (Tjp2 Alb-Cre, Tjp2 cKO, liver-specific conditional knock-out), hepatocytes (Tjp2 Alb-CreERT2, after tamoxifen-induced deletion Tjp2 icKOHC), cholangiocytes (Tjp2 Sox9-CreERT2, after tamoxifen-induced deletion Tjp2 icKOCC)14. Mouse lines with tamoxifen-induced conditional deletion of the specified genes in hepatocytes (Alb-CreERT2) or cholangiocytes (Sox9-CreERT2) are referred to as “icKOHH” and “icKOCC”, respectively. Sox9-CerERT2 (C57BL/6-Sox9em1(cre/ERT2)Tchn/J) mice and the Rosa26:LacZ (Rosa26:Lox-STOP-Lox-LacZ) reporter line (B6;129S4-Gt(ROSA)26Sortm1Sor/J)32, used for lineage tracing, were obtained from Jackson laboratory. To delete Yap or WWtr1/Taz in hepatocytes, Tjp2F/F and Alb-CreERT2 mice were crossed with YapF/F mice23 (generously obtained from Duojia Pan, Department of Molecular Biology and Genetics, Howard Hughes Medical Institute, Johns Hopkins University School of Medicine, Baltimore, USA, via Stefano Piccolo, University of Padova, Italy) and/or TazF/F mice22 (generously provided by Stefano Piccolo, University of Padova, Italy), or Notch2F/F (Jackson Laboratory) mice to generate YapF/F Alb-CreERT2, TazF/F Alb-CreERT2, Tjp2F/F YapF/F Alb-CreERT2, Tjp2F/F TazF/F Alb-CreERT2, and Tjp2F/F YapF/F TazF/F Alb-CreERT2 mice. Tamoxifen was used to delete the floxed Tjp2, Yap, Taz, or Notch2 gene to obtain Tjp2 Yap icKOHC, Tjp2 Taz icKOHC or Tjp2 Yap Taz icKOHC mice, respectively. To delete Notch2 in mouse liver, Tjp2F/F, Albumin-Cre mice, and Notch2F/F (Jackson Laboratory) mice were crossed to generate Notch2F/FAlb-Cre and Tjp2F/FNotch2F/FAlb-Cre mice (Notch2 cKO and Tjp2 Notch2 cKO after gene deletions). To induce the deletion of the floxed alleles, 8-week-old mice were injected intraperitoneally (100 mg/kg) with tamoxifen (20 mg/ml in sunflower oil, Sigma-Aldrich) for 5 consecutive days and only used for further experimentation 7 days or later after the last tamoxifen injection. Littermate mice lacking Cre were used as controls and a previous characterization of other possible control mouse lines showed no significant differences among these in key parameters related to liver function and injury14. Male animals were used, but female mice showed comparable results. For genotyping, genomic DNA isolated from tail clippings was amplified using the following primer pairs to detect the wild-type or floxed alleles: Yap: P1: 5’-CCA TTT GTC CTC ATC TCT TAC TAA C-3’ & P2: 5’-GAT TGG GCA CTG TCA ATT AAT GGG CTT-3’ (wild-type: 498 bps, floxed: 597 bps); Taz: P1: 5’-TCT TCC AAG GTG CTT CAG AGA-3’ & P2: 5’-ATT TAG GCC AAA GTC GCT CA-3’ (wild-type: 238 bps, floxed: 312 bps); Notch2: P1: 5’-CAA CCC CAG ATA GGA AGC AG-3’ & P2: 5’-GAG CCT TTT CCC CAT ATT CC-3’ (wild-type: 202 bps, floxed: 240 bps). Genotyping for the Tjp2 gene was done using primer-1 (5’-GTT CCT ATC CTG TTA GTT GGT AGT CC-3’) and primer-2 (5’-AAA GGG TCT CAT GTA GGT CAA GC-3’), yielding a 265 bp (wild-type allele) or a 422 bp (conditional mutant allele).
Messenger RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total messenger RNA (mRNA) was extracted from whole liver and processed for quantitative reverse-transcription polymerase chain reaction using a QuantStudioTM 3 Real-Time PCR System (Applied Biosystems) and specific primers (Supplementary Table).
Hepatocyte isolation and culture
Mice were anaesthetized using xylazine and ketamine as approved in the IACUC protocol. The abdomen was cut open and the inferior vena cava (IVC) was exposed. Perfusion with warm Kreb Ringer solution containing 50 mM EGTA was carried out using a catheter inserted into the mouse IVC. Once the blood was removed from the portal vein (PV) and the liver turned pale, the perfusion buffer was exchanged with digestion buffer containing Kreb Ringer solution with calcium chloride and LiberaseTM enzyme (Sigma). The digestion was carried out until the liver became soft and friable, indicating that digestion was completed. With the gall bladder removed, the liver was excised and transferred to a petri dish with the remaining digestion buffer to release the individual hepatocytes from the liver lobes. Liberase activity was inhibited by transferring the hepatocytes from the dish to a 50 ml tube containing culture media (RPMI containing 10% FCS and antibiotics) and the hepatocytes were gently washed twice with culture media. The number and viability of the hepatocytes were checked and cells plated for experiments.
DDC diet
Mice were fed with chow diet supplemented with 0.1% 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC: Cat #D80002, Sigma) for 28 days. When treated with tamoxifen, mice were provided with the DDC diet one week after the last tamoxifen dose.
Serum and tissue biochemical analyses
Kits were used to determine bilirubin, serum alanine aminotransferase (ALT), alkaline phosphatase (AP) and aspartate aminotransferase (AST) (Teco Diagnostics), or plasma total BA (Diazyme
Laboratories) levels. For liver BA levels, 100 mg liver tissue was ground in liquid nitrogen, suspended in 1 mL water, sonicated, centrifuged, and BA levels were determined in the supernatant. Hydroxyproline was measured with the Hydroxyproline Assay Kit (Sigma, Catalog: MAK008). Briefly,10 mg liver tissue was homogenized and mixed with 100 µl water. Samples were hydrolyzed by the addition of 100 µl of concentrated (12 M) HCL for 3 h at 120 °C. After mixing and centrifugation at Mix and 10,000 x g for 3 minutes, the absorbance of the supernatant at a wavelength of 560 nm was measured. The numbers of mice per cohort analyzed are given in the respective figure legends, and samples were analyzed in 2 or 3 different and independent experimental runs.
Immunofluorescence staining
Liver samples were embedded in OCT and sectioned at a thickness of 5 µm. Sections were stained with primary antibodies against ZO-2 (Tjp2) (rabbit; Cat #71–1400, Invitrogen, 1:100 dilution), Ck19 (rat; Troma III, Developmental Studies Hybridoma Bank DSHB, 1:20 dilution), A6 (rat; A6 BCM, DSHB, 1:20 dilution), Yap (rabbit; ab205270, Abcam, 1:50 dilution), HNF4α (mouse; MA1-199, Invitrogen, 1:100 dilution) and compatible fluorescently labeled secondary antibodies (Invitrogen, 1:200 dilution). Images were taken from at least 3 independent mice using a Zeiss LSM800 confocal microscope and Zen v3.4 (blue edition) software. Representative images are shown.
Immunohistochemistry
Paraffin blocks were sectioned at a thickness of 5 µm. For immunohistochemistry, antigens were retrieved by steaming the slides for 20 min in a 2100 Retriever (Pick Cell Laboratories). The slides were then stained with primary antibodies against Ki67 (rabbit; Cat #9129 Cell Signaling, 1:100 dilution), Ck19 (rat; Troma III, DSHB, 1:20 dilution), A6 (rat; A6-BCM, DSHB, 1:20 dilution), Sox9 (rabbit; Cat #ab185230 Abcam, 1:50 dilution), Yap (mouse; Cat #12395 Cell Signaling, 1:50 dilution) and compatible fluorescently labeled secondary antibodies (Invitrogen, 1:200 dilution).
Histology
Freshly dissected livers were fixed in 4% paraformaldehyde overnight, processed, and embedded in paraffin. 5 μm sections were stained with H&E or Sirius red and imaged with a Zeisscam
camera on a Zeiss Axio microscope. Five mice per cohort and images from at least 2 slides for each mouse were analyzed and used for quantification. Representative images are shown.
LacZ staining
Livers were dissected seven days after the last tamoxifen injection, frozen in optimal cutting temperature compound, and 10 μm-thick sections cut and mounted on slides. After fixation in formalin for 10 minutes, LacZ staining was carried out (NovaUltra kit) per the manufacturer’s protocol.
Slides were counterstained with Nuclear Faster Red for 3–5 min. Three mice per cohort and at least 5 sections for each mouse were analyzed. Representative images are shown.
Edu labeling
Edu, dissolved in DMSO (1 mg/10 µl) and further diluted in PBS (1 mg/100 µl), was injected intraperitoneally (1 mg/g body weight) 1 h before sacrificing the mice. Images were taken from at least 3 independent mice using a Zeiss LSM800 confocal microscope and Zen v3.4 (blue edition) software. Representative images are shown.
Western blotting
Fresh liver samples were frozen in liquid nitrogen, crushed into powder and lysed for 15 min on ice in lysis buffer (50-mM Tris-HCl, pH7.5, 100 mM NaCl, 1 mM MgCl2, and 0.5% Triton X-100, supplemented with protease inhibitor cocktail and one PhosSTOP tablet per 10 ml [Cat.# 04 906 837 01, Roche]). Lysates were sonicated and centrifuged (13,000 × g for 15 min) at 4 °C. Supernatants were collected and equal amounts of protein were fractionated by SDS-polyacrylamide gel electrophoresis and subjected to Western blotting using antibodies against Alas-1 (rabbit; ab154860, Abcam), Alad (rabbit; ab151754, Abcam), Ck19 (rat; Troma III, DSHB), Collagen I (rabbit; Cat #NB600-408, NOVUS Biologicals), Hbms1 (rabbit; ab129092, Abcam), FC (mouse; sc377377, Santa Cruz), Laminin1-2 (rabbit; ab11575, Abcam), αSMA (rabbit; ab5694, Abcam), Yap/Taz (rabbit; Cat #8418, Cell Signaling), pSer127 Yap (rabbit; Cat #13008, Cell Signaling), Notch2 (rabbit; Cat #5732, Cell Signaling), Vinculin (mouse; Cat #V9131, Sigma). Primary and secondary antibodies were diluted 1:1000 and 1:3000, respectively. Samples from at least 3 independent mice were analyzed using ImageJ v15.3 and images of representative blots are shown. Gels and blots in a specific panel derive from the same experiment and were processed in parallel. Unprocessed and uncropped scans of blots shown in Figs. 1h and 5a are shown in Supplementary Fig. 3.
Statistical analysis
Experimental design, the number of mice per cohort and replicates are described in detail under the respective methods and in the figure legends. The data, expressed as the mean ± SD, displayed normal distribution and variance between groups. Prism software (v. 9.3.1, GraphPad) was used for statistical analysis by unpaired Student’s t test. *p < 0.05; **p < 0.005, ***p < 0.0005, ns—not significant (p > 0.05), with p < 0.05 considered a significant difference.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jeliazkova, P. et al. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology 57, 2469–2479 (2013).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Haymond, A. et al. Protein painting, an optimized MS-based technique, reveals functionally relevant interfaces of the PD-1/PD-L1 complex and the YAP2/ZO-1 complex. J. Biol. Chem. 294, 11180–11198 (2019).
Oka, T. et al. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem. J. 432, 461–472 (2010).
Remue, E. et al. TAZ interacts with zonula occludens-1 and −2 proteins in a PDZ-1 dependent manner. FEBS Lett. 584, 4175–4180 (2010).
Oka, T., Schmitt, A. P. & Sudol, M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene 31, 128–134 (2012).
Dominguez-Calderon, A. et al. ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Mol. Biol. Cell 27, 1581–1595 (2016).
González-González, L. et al. ZO-2 favors Hippo signaling, and its re-expression in the steatotic liver by AMPK restores junctional sealing. Tissue Barriers, 1994351, https://doi.org/10.1080/21688370.2021.1994351 (2021).
Carlton, V. E. et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat. Genet. 34, 91–96 (2003).
Sambrotta, M. et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat. Genet. 46, 326–328 (2014).
Ge, T., Zhang, X., Xiao, Y., Wang, Y. & Zhang, T. Novel compound heterozygote mutations of TJP2 in a Chinese child with progressive cholestatic liver disease. BMC Med Genet 20, 18 (2019).
Bull, L. N. & Thompson, R. J. Progressive familial intrahepatic cholestasis. Clin. Liver Dis. 22, 657–669 (2018).
Itoh, M., Terada, M. & Sugimoto, H. The zonula occludens protein family regulates the hepatic barrier system in the murine liver. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 165994 (2021).
Xu, J. et al. Protective functions of ZO-2/Tjp2 expressed in hepatocytes and cholangiocytes against liver injury and cholestasis. Gastroenterology 160, 2103–2118 (2021).
Li, C. Z. et al. Human iPSC-derived hepatocyte system models cholestasis with tight junction protein 2 deficiency. JHEP Reports https://doi.org/10.1016/j.jhepr.2022.100446 (2022).
Xu, J. et al. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol. Cell Biol. 28, 1669–1678 (2008).
Kikuchi, S. et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat. Genet. 31, 320–325 (2002).
Schaub, J. R. et al. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018).
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Parviz, F. et al. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 34, 292–296 (2003).
Azzolin, L. et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010).
Sato, K. et al. Ductular reaction in liver diseases: pathological mechanisms and translational significances. Hepatology 69, 420–430 (2019).
Font-Burgada, J. et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779 (2015).
Han, X. et al. Lineage tracing reveals the bipotency of SOX9(+) hepatocytes during liver regeneration. Stem Cell Rep. 12, 624–638 (2019).
Lee, K. P. et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl Acad. Sci. USA 107, 8248–8253 (2010).
Yamazaki, Y., Moore, R. & Negishi, M. Nuclear receptor CAR (NR1I3) is essential for DDC-induced liver injury and oval cell proliferation in mouse liver. Lab Invest. 91, 1624–1633 (2011).
Gao, Y. et al. Constitutive androstane receptor induced-hepatomegaly and liver regeneration is partially via yes-associated protein activation. Acta Pharm. Sin. B 11, 727–737 (2021).
Bhushan, B. et al. Yap is crucial for CAR-driven hepatocyte proliferation, but not for induction of drug metabolism genes in mice. Hepatology, https://doi.org/10.1002/hep.31521 (2020).
Bangru, S. et al. Alternative splicing rewires Hippo signaling pathway in hepatocytes to promote liver regeneration. Nat. Struct. Mol. Biol. 25, 928–939 (2018).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Acknowledgements
The authors are grateful to Stefano Piccolo (University of Padova) and Duojia Pan (Johns Hopkins University School of Medicine) for generously providing floxed Taz and Yap mice. The Advanced Molecular Pathology Laboratory (AMPL) at the Institute of Cell and Molecular Biology (IMCB) kindly provided technical expertise and support for histology processing. This study was supported by grant OFIRG15nov120 from the National Medical Research Council, Singapore, and by the Agency for Science, Technology and Research (A*STAR), Singapore.
Author information
Authors and Affiliations
Contributions
Study concept and design: J.X., W.H.; data acquisition and analysis: J.X., P.J.K. A.G.M.O., C.M.F.G., S.M.A.; data analysis and interpretation: J.X., P.J.K., A.G.M.O., C.M.F.G., S.M.A., W.H.; drafting of the manuscript: J.X., W.H.; revision of manuscript J.X., W.H.; statistical analysis: J.X.; obtained funding: W.H.; study supervision: W.H.; approval of the final manuscript: all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, J., Kausalya, P.J., Ong, A.G.M. et al. ZO-2/Tjp2 suppresses Yap and Wwtr1/Taz-mediated hepatocyte to cholangiocyte transdifferentiation in the mouse liver. npj Regen Med 7, 55 (2022). https://doi.org/10.1038/s41536-022-00251-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-022-00251-6
This article is cited by
-
YAP-mediated trophoblast dysfunction: the common pathway underlying pregnancy complications
Cell Communication and Signaling (2023)