Abstract
Despite global efforts to establish effective interventions for coronavirus disease 2019 (COVID-19) and its major complications, such as acute respiratory distress syndrome (ARDS), the treatment remains mainly supportive. Hence, identifying an effective and safe therapy for severe COVID-19 is critical for saving lives. A significant number of cell-based therapies have been through clinical investigation. In this study, we performed a systematic review of clinical studies investigating different types of stem cells as treatments for COVID-19 and ARDS to evaluate the safety and potential efficacy of cell therapy. The literature search was performed using PubMed, Embase, and Scopus. Among the 29 studies, there were eight case reports, five Phase I clinical trials, four pilot studies, two Phase II clinical trials, one cohort, and one case series. Among the clinical studies, 21 studies used cell therapy to treat COVID-19, while eight studies investigated cell therapy as a treatment for ARDS. Most of these (75%) used mesenchymal stem cells (MSCs) to treat COVID-19 and ARDS. Findings from the analyzed articles indicate a positive impact of stem cell therapy on crucial immunological and inflammatory processes that lead to lung injury in COVID-19 and ARDS patients. Additionally, among the studies, there were no reported deaths causally linked to cell therapy. In addition to standard care treatments concerning COVID-19 management, there has been supportive evidence towards adjuvant therapies to reduce mortality rates and improve recovery of care treatment. Therefore, MSCs treatment could be considered a potential candidate for adjuvant therapy in moderate-to-severe COVID-19 cases and compassionate use.
Similar content being viewed by others
Introduction
In January 2020, to raise awareness internationally and to prevent further viral spread, the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak a public health emergency1. The virus responsible for coronavirus disease 2019 (COVID-19) infected more than 128.7 million people around the world by March 20212. Despite global efforts to establish effective interventions for COVID-19, its treatment remains mainly supportive, and one of the major complications of the disease, acute respiratory distress syndrome (ARDS), poses a significant challenge3.
Coronaviruses that are responsible for severe respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) belong to the same beta coronavirus genus, and SARS and MERS resemble SARS-CoV-2 symptoms4. Novel therapeutic approaches have been developed to treat COVID-19 complications, especially ARDS, some of which exploit antiviral and immune-based mechanisms5. More recently, a significant number of cell-based therapies have been through clinical investigation, involving, most importantly, mesenchymal stem cells (MSCs) and MSC-derived conditioned media or extracellular vesicles. These therapies have multiple therapeutic targets because MSCs can release a variety of soluble mediators, but their safety and potential efficacy are still to be determined6.
Specifically, MSCs secrete keratinocyte growth factor, prostaglandin E2, granulocyte-macrophage colony-stimulating factor, and cytokines such as IL-6 and IL-13, that can influence how immune cells—both innate and adaptive—interact with the cellular environment. These soluble factors can promote alveolar macrophage phagocytosis and alter the cytokine profile released by immune cells. Such functions are expected to be effective against the respiratory infections discussed here7.
Considering the limited availability of effective therapies for COVID-19 and one of its complications ARDS, new therapeutic approaches are urgently needed. In this context, cell-based therapies can be an attractive alternative because of their accessibility and relative safety. Although the use of MSCs as immune therapy has been regarded as safe, a meta-analysis found fever as the predominant adverse event associated with MSC therapy. Notably, no acute infusion toxicities, infections, thrombotic/embolic events, or malignancy were found8. Here, we systematically reviewed studies that investigated different types of cells as a treatment for the respiratory diseases mentioned above.
Results
Study characteristics
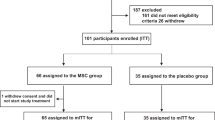
The initial search of the databases identified a total of 1347 potentially relevant records. After excluding duplicates, 1114 articles remained, and titles and abstracts of the remaining records were scanned as part of a new screening according to inclusion and exclusion criteria previously established. Four gray literatures were also analyzed. A total of 1077 articles were excluded based on the exclusion criteria. The remaining 37 articles that met the inclusion criteria were thoroughly examined. Among these, eight were excluded, and the remaining 29 were included for the review. The PRISMA flow diagram (Fig. 1) illustrates the selection process of the studies for systematic review.
Among the clinical studies, 17 of them reportedly applied cell therapy to treat COVID-19, while the remaining eight studies investigated cell therapy as a treatment for ARDS. Additionally, four clinical COVID-19 articles were included as gray literature, bringing the total of selected clinical studies to 29. None of the included articles applied cell therapy to MERS or SARS-CoV-1.
The clinical and study characteristics are described in Table 1.
Study designs and evidence levels
Among the 29 included clinical studies, there were eight case reports (27.5%), five Phase I clinical trials (17.2%), four (13.7%) pilot studies, two Phase II clinical trials (6.8%), one cohort (3.4%), and one case series (3.4%). The study designs and classification of evidence are described in Table 2.
It should be noted that eight (27.5%) studies used cell therapy as compassionate use and one (3.4%) as a proof of concept. Most studies (75%) used mesenchymal stem cell therapy in an attempt to treat COVID-19.
The articles were categorized based on levels of scientific evidence following the Oxford Center for Evidence-Based Medicine Classification9 and the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE)10.
According to the Oxford Center for Evidence-Based Medicine Classification9, of the 29 included articles, five were classified as grade A recommendations; within these, two (6.8%) were 1 A, and three (10.3%) were 1B. Among grade B recommendations, there were 14 studies. The levels of evidence were eight (27.5%) 2B and six (20.6%) 2 C. Among grade C recommendations, ten (34.4%) articles were classified as level 4 evidence.
According to the GRADE10, of the 29 included articles, two (6.8%) were considered high evidence level, three (10.3%) moderate, fourteen (48.2%) low, and ten (38.4%) minimal evidence level.
Patient characteristics
Clinical studies using cell therapy for the treatment of COVID-19 patients were performed in five different countries. Following are the total numbers of patients by country from all 29 studies: China (n = 238), the United States (n = 30), Spain (n = 13), Iran (n = 11), and Germany (n = 23). Clinical studies that investigated ARDS included patients from the United States (n = 96), the United Kingdom (n = 30), China (n = 20), Sweden (n = 5), Mexico (n = 5), and South Korea (n = 1). Age of the patients ranged from 19 to 86 years.
Intervention characteristics
Among the 29 selected clinical articles, eleven types of umbilical cord MSCs were analyzed. The cell types investigated in the studies comprised (1) human umbilical cord MSCs11,12,13,14,15,16,17,18,19,20,21, (2) cardiosphere-derived MSCs22, (3) human bone marrow MSCs (hBM-MSCs)23,24,25,26,27,28, (4) extracellular vesicles derived from BM-MSCs29, (5) autologous peripheral blood-derived mononuclear cells (PBMCs)30, (6) human adipose tissue-derived MSCs31,32, (7) allogeneic bone marrow-derived multipotent adult progenitor cells (MAPC) expanded ex vivo33, (8) ACE2-MSCs34, (9) human menstrual blood-derived MSCs35,36, (10) immunity‐and matrix-regulatory cells (IMRCs)37, and (11) MSCs derived from perinatal tissues38.
Main parameters
A total of five groups of readouts—all of them subdivided into several parameters—were identified among the clinical studies. Laboratory measurements were common to 25 out of the 29 clinical articles, representing 86.2% of our study sample. The cited parameters included standard laboratory measurements, such as lymphocyte count, COVID-19 PCR test, basic metabolic panel (BMP) and levels of procalcitonin, ferritin, angiopoietin, d-dimer, bilirubin, alanine aminotransferase (ALT), creatinine, CKMB, cardiac troponin, and immune system parameters, such as IL-6, IL-8, IL-1ɑ, IL-1β, TNF-ɑ, and IL-1. Twenty studies, which correspond to 68.9% of the included studies, investigated pulmonary function using various parameters and exams, such as chest X-ray, bronchoscopy, chest CT, lung compliance, lung injury score (LIS), and the following biomarkers: receptor for advanced glycation end-product (AGER), which is a marker for lung epithelial injury, and angiopoietin-2 (ANGPT2), a marker for endothelial injury. All studies evaluated adverse reactions, safety, and mortality after the intervention. Ten studies (34.4%) analyzed the relationship between cell therapy and the discontinuation of ventilator support. Furthermore, eight studies (27.5%) documented additional data, such as mental status, patient’s physical capacity, health-related quality of life (HRQoL) assessment, e electrocardiogram (EKG), viral load, blood type and screen, blood culture, urine culture, and body mass index (BMI).
Main outcomes
Among the 14 studies that evaluated lymphocyte counts before and after cell therapy, 12 reported a statistically significant elevation in lymphocyte numbers (85.7%). Fourteen of the seventeen studies which assessed CRP levels reported a decrease in this parameter (82.3%). As for plasma cytokines, cell therapy was found to reduce IL-6 levels in nine (52.9%) out of seventeen studies that investigated this parameter. TNF-α levels decreased in five out of seven studies (71.4%) that assessed its posttreatment values. Five out of six studies that measured ferritin reported a significant reduction after cell infusion, while six out of eight studies (75%) reported a D-dimer decrease. Remarkably, all studies that evaluated the following pulmonary parameters reported a statistically significant difference between pre- and post-cell therapy results: there was an increase in PaO2/FIO2 ratio in 12 out of 14 articles (85.7%), an increase in oxygen saturation in 50% of the articles and an improvement on lung image—chest CT or chest radiography—in 15 out of 17 studies (88.2%). Eighteen studies assessed symptoms at admission and clinical status after cell therapy, and all of them reported clinical improvement. Additionally, 11 out of 14 studies (78.5%) demonstrated discontinuation of oxygen support (intubation and ECMO) after cell infusion at a statistically significant rate. Nine studies reported a reduction in mortality, but two of them did not report a significant reduction (77.7%). Six studies evaluated and reported improvement in the duration of hospitalization. The primary outcomes and evaluated parameters are summarized in Table 3.
Evidence level: 1A and 1B studies
Studies in higher evidence level categories (1A and 1B) described some noteworthy findings. In a randomized phase 2 safety trial for patients with moderate-to-severe ARDS, Matthay et al. revealed a decrease in endothelial injury ascertained by reduced plasma concentrations of ANGPT2 in the MSC-treated patients versus the control group27. They also reported that after MSC therapy, there was a trend towards a decreased number of ventilator-free and organ failure-free days and improved oxygenation index, although not significantly27. Notably, the number of intensive care-free days was found to be statistically relevant. Additionally, they showed a reduction in the levels of CRP and ANGPT2 biomarkers27.
Shi and colleagues performed a multicenter, randomized placebo-controlled phase 2 efficacy trial with 100 severe COVID-19 patients, who either received placebo (n = 35) or umbilical cord mesenchymal stem cells (UC-MSC) infusion (n = 65) alongside the common care treatments. Supplementary oxygen was necessary for 44 (67.69%) patients from the treatment group and for 23 (65.71%) patients from the placebo group. They noticed that UC-MSC infusions could reduce the proportion of abnormal lung lesions, especially lesions with solid appearance, compared to placebo. In this study, MSCs led to a decrease in ground-glass lesions; however, it was not significant compared to the placebo group19. Both articles classified 1 A according to Oxford and GRADE systems described that cell therapy was a safe and well-tolerated alternative19,27. All three papers classified as 1B also concluded that the procedure was safe14,31,33. Zheng et al. in a single-center, randomized, double-blind, placebo-controlled trial in which 12 patients with moderate-to-severe ARDS were arbitrarily assigned to receive either allogeneic adipose-derived humans MSCs or placebo. They observed that the MSC group displayed a significant improvement in oxygenation index, compared to baseline values—but not compared to placebo. Parameters such as ventilator-free and ICU-free days and serum IL-6 and IL-8 levels did not show a difference when the baseline values were compared to predetermined time points31. Bellingan et al. reported that MSCs reduced mortality and increased ventilator-free and ICU-free days compared to placebo33. Lanzoni et al. conducted a controlled, double-blinded, randomized phase 1/2a clinical trial to determine the safety and efficacy of UC-MSC infusion in 24 patients with COVID-19 ARDS. The subjects were divided into a UC-MSC infusion (n = 12) and a placebo (n = 12) group. They noticed that UC-MSC infusion significantly contributed to improved patient survival and time of recovery. This study also reported a significant decrease in the following inflammatory cytokines after treatment: GM‐CSF (pro-inflammatory M1 macrophage phenotype inducer), IFN-γ, IL‐5, IL‐6, IL‐7, TNF-α, TNF- β, and others14.
Evidence level 2B and 2C studies
Wilson and associates conducted a study to assess the safety and the maximally tolerated dose of MSCs - up to 10 million cells/kg predicted body weight (PBW). The study was a multicenter, open-label, non-randomized, noncontrolled phase 1 clinical trial, in which nine patients were equally subdivided into three intervention groups: low dose MSCs (1 million cells/kg PBW), an intermediate dose MSCs (5 million cells/kg PBW), and the high dose MSCs (10 million cells/kg PBW). After the treatment, the concentrations of several biomarkers (IL-6, IL-8, AGER, and ANGPT2) were decreased when compared between the baseline and day 3 values31,33. However, it is not possible to relate these findings to the previously reported changes in biomarkers, due to the lack of a matched control group with the MSCs treatment group in this study.
Hashemian and collaborators performed a multicenter, open-label, non-randomized, noncontrolled trial, with 11 subjects that either received freeze/thawed UC-MSCs (n = 6) or fresh PL-MSCs (n = 11). They noted that inflammatory biomarkers (CRP, IL-6, IL-8, and TNF-α) were significantly decreased after the MSC infusions. Notably, the anti-inflammatory cytokines IL-4 and IL-10 levels were also increased in four cases. Moreover, the study reported that nine (81.81%) treated patients tolerated the MSCs infusions38.
In a pilot study, Leng et al investigated the early efficacy of MSC therapy in seven patients with COVID-19 pneumonia along with the placebo treatment in three patients. Two days after infusions, they found improvements in pulmonary function, decreased levels of CRP and inflammatory cytokines, and augmented IL-10 concentration and regulatory DC cells34.
Meng F. et al. conducted a controlled, non-randomized, phase 1 clinical trial to evaluate the safety of human umbilical cord-derived MSC infusions in 18 patients. They observed that four patients displaying the highest levels of IL-6 showed a substantial decrease within 3 days of the treatment, but no such trend was observed in patients with low plasma IL-6. Regarding the PaO2/FiO2 ratio, there were improvements in most of the severe patients. Additionally, there was a decline in concentrations of the inflammatory cytokines TNF-α, IFN-γ, monocyte chemoattractant protein 1 (MCP-1), interferon-inducible cytokine IP-10 (IP-10), IL-22, interleukin 1 receptor type 1 (IL-1RA), IL18, IL-8, and macrophage inflammatory protein 1-alpha (MIP-1)17.
In a concept study, 13 severe COVID-19 patients requiring mechanical ventilation and receiving the standard care treatment were infused with adipose tissue-derived MSCs. Within 5 days of the treatment nine (70%) patients showed clinical improvements with significant reductions in CRP, lactate dehydrogenase (LDH), D-dimer, and ferritin. Moreover, five patients demonstrated improvements concerning B-lymphocyte alongside better CD4+ and CD8+ counts, and ultimately, after the MSCs infusion seven patients were extubated within a median time of 7 days32.
Shu, L. et al. conducted a single-center open-label, randomized trial involving 41 patients. Compared to the control group (n = 29), CRP and IL-6 levels were significantly decreased from day 3 after UC-MSC infusions (n = 12)18. In this study, the small sample size and the treatment change during the trial were the major limitations18.
Tang and colleagues tested the effects of infusions of menstrual blood-derived MSCs in two severe COVID-19 patients. They reported increased lymphocytes, decreased inflammation indicators (i.e., lower CRP and IL-6 levels) after MSC infusions, and antiviral treatment36.
Another pilot study conducted by Feng et al. investigated the efficacy of UC-MSCs in 16 patients; among them, seven were categorized as critically severe and nine as severe. They reported improvements in oxygenation index, CRP, and procalcitonin levels in severe and critically severe groups. In addition, the study noted augmented levels of CD4 + cells, CD8 + T cells, and NK cell counts within 28 days of the infusion21.
In a clinical trial involving MSC infusions in 31 patients, the PaO2/FiO2 level and lymphocyte counts showed a substantial increase, whereas the CRP, PCT, IL-6, and D-dimer were significantly decreased compared to the baseline values before infusions11.
In a single-center, open-label, non-randomized, noncontrolled conducted by Singh et al. in the earliest days of the COVID-19 outbreak, six patients received allogeneic cardiosphere-derived cells after receiving an anti-IL-6 agent. The levels of Ferritin, CRP, and IL-6 were decreased after the cardiosphere-derived cell infusion22. However, the decrease cannot be attributed only to cell therapy due to the prior treatment with anti-1L-6 agent22.
In a non-randomized open-label trial, Sengupta et al. apprised the safety and efficacy of an allogeneic bone marrow MSC-secreted extracellular vesicles (ExoFloTM) in 24 severe COVID-19 patients. Three days after infusions, they noted improvements in PaO2/FiO2 ratio (p < 0.001), significant increases in the absolute counts of neutrophils, CD3+, CD4+, and CD8+ lymphocytes, and decline in CRP, ferritin, and D-dimer levels after 5 days of ExoFloTM treatment29. The major limitations of this study were the lack of randomization and blinding.
Chen et al. performing a single-center, open-label, non-randomized, noncontrolled trial comprising 25 patients, reported improved CT parameters in 16 patients after MSC infusions (64%). However, there were no changes in inflammation indices, including CRP, WBC, PCT, and IL-6 levels. Moreover, there were no changes in IgG or IgM. In contrast, the serum levels of lactate, cardiac troponin T, and creatine Kinase were elevated after the treatment23. Low statistical power due to the small sample size was a significant limitation in this study.
Häberle and associates, in a single-center, open-label, non-randomized, noncontrolled trial, enrolled 23 patients to either placebo (n = 18) or MSC treatment (n = 5). No differences in CRP and IL-6 were found between the groups. Notably, ferritin level was increased in the MSC-treated group after discharge. Notwithstanding, there was a significant reduction in neutrophils, lymphocytes, and leukocytes at discharge in the MSC treatment group compared to the placebo group28.
Evidence level 4C studies
Most studies with 4 C evidence levels were case reports, and hence the results could not be compared to findings seen in trials comprising larger cohorts of patients. It is also noteworthy that one cannot establish a cause–effect relationship from findings in case reports.
Adverse effects
All clinical studies analyzed the occurrence of adverse effects (AE) related to cell therapy. Studies whose primary endpoint was safety mainly defined the occurrence of prespecified infusion-associated AEs within 6 h of infusions in addition to cardiac arrest or death within 24 h of infusions. A total of 16 studies (55.1%) reported the occurrence of side effects, with only a minority of the studies (24.1%) attributing the side effects directly to the use of cell therapy. Approximately 41% of the studies reported that the observed side effects were not linked to the MSC treatment. Twenty-seven (93.1%) studies did not report the AEs during the administration of MSCs. The AE are also detailed in Table 4.
AE related to cell infusion
As for the side effects observed at the time of infusions or within hours after infusions, one study reported hypoxemia, hypotension and/or hypertension, and muscle spasms, but they were easily controlled and did not acutely alter the patients’ medical conditions16. A clinical trial observed transient facial flushing and fever immediately after the infusion, which resolved spontaneously17. One clinical trial detected diarrhea and a rash in the chest area, but it resolved31. Another article reported a single Common Terminology Criteria for Adverse Events (CTCAE) grade-1 infusion-related reaction, which settled without intervention33. One patient experienced transient shivering, which occurred once in two cases and disappeared in less than 1 h38.
AE related to cell therapy
A study reported worsening of bradycardia in a subject who previously had bradycardia and required transient vasopressor treatment14. One pilot study observed liver dysfunction, heart failure, and allergic rash after treatment, but fortunately, all patients survived—the report did not specify the possible reason for the occurrence of these adverse reactions. Nevertheless, the serum levels of lactate, serum cardiac troponin, and creatine kinase were significantly increased after MSCs therapy, reinforcing the cautious use of MSC infusions in patients with previous metabolic acidosis and coronary heart disease14.
AE not related to cell therapy
Adverse events unrelated to cell therapy included progressively increased creatinine, epistaxis, and hematuria; a patient was also diagnosed with lower-extremity arterial thrombosis16. Another patient experienced hypoxemia, which was thought to be caused by the progression of COVID-19 based on previously existing symptoms17. One patient experienced pneumothorax, which recovered spontaneously under conservative treatment, and it was judged by the site investigators and found to be unrelated to MSCs intervention19. Feng et al. reported two patients with bacterial pneumonia and septic shock as complications of severe COVID-1921. In a case series, one patient developed nosocomial pneumonia with fever several days after MSC infusions, but it was not related to cell therapy as per the report. Nonetheless, it remains to be addressed whether MSC infusions increase the risk of infectious complications in COVID-19 patients with ARDS, although no such increases were seen earlier in MSC clinical trials involving immune-competent recipients25. Wilson et al. reported multiple embolic infarcts, which were thought to be present before MSC infusions based on a previous MRI scan26. Another trial observed a worsening hypoxic state, a respiratory failure requiring intubation, pulmonary embolism, and acute renal failure. The reactions were reviewed by an independent Data Safety Monitoring Board (DSMB), which concluded that the symptoms were unrelated to the therapeutic intervention29. One subject experienced pneumonia due to a methicillin-resistant Staphylococcus aureus, and another patient developed a fungal infection by Candida spp32.
Mortality
There were no reported deaths directly linked to stem cell administration. Death was observed in 17 studies, and a total of 79 patients died out of 472, including placebo and patients who received MSCs. It was reported that 47 (14%) deaths occurred among 330 patients receiving MSC infusions, while the placebo groups had 32 (23%) deaths out of 142 patients. Five studies described deaths caused by complications of COVID-1911,16,18,21,29. One patient experienced repeated infections by multidrug-resistant pathogens and eventually suffered septic shock and died13. A phase II trial reported two deaths in the MSC-treated group, secondary to acute respiratory failure; both were reviewed and declared as unrelated to MSC infusions14.
Iglesias et al. reported two deaths; the first patient developed acute renal failure and the second cardiomyopathy and liver failure16. A pilot trial observed two deaths caused by pneumonia and septic shock, which were lethal complications of COVID-19 and occurred independently of MSC treatment21. A phase I trial noted one death in the MSC treatment group and two deaths in the placebo group, but none of the deaths were considered to be related to MSC infusions by the clinical investigators and were consistent with the patients existing disease processes26.
One patient had a fatal cardiopulmonary arrest; however, it was attributed to a preexisting history of coronary artery disease, and the DSMB judged that the death was likely not related to MSC infusions27. In another clinical trial, one patient in the MSC infusions group died of multiple organ failure, which was concluded as not related to MSC infusions by the clinical investigators and was consistent with the patient’s disease progression31. Jungebluth et al. reported multisystem organ failure after MSC infusions, possibly secondary to disseminated fungal infection and intra-abdominal sepsis30. Among the two patients who died, one was due to gastrointestinal bleeding and another to secondary pneumonia, unassociated with MSC therapy32. Another trial reported five deaths after cell infusions, two were intubated, two had signs of sepsis, the fifth patient had a cardiac arrest, and none of the deaths were considered connected to cell therapy38. The mortality after MSC infusions is described in Table 5.
Discussion
The present systematic review evaluated the available results of stem cell therapy for treating patients with COVID-19 and ARDS. By July 2021, according to the US National Institutes of Health (NIH) ClinicalTrials.gov database, 417 clinical investigations of cell therapies against SARS-CoV-2 have been conducted39. The database search for this systematic review was conducted in March 2021. Following the inclusion criteria evaluation, 29 articles were eventually included. The vast number of trials under progress shows that cell therapy has been suggested as a beneficial alternative to treating COVID-19 and its complications ARDS. Among the 417 registered trials, 203 were recruiting participants, five were enrolling participants by invitation, 32 were active, having already passed the recruitment phase, and 63 were completed. Of these 63, six trials already presented results. The remaining 114 trials were not in progress or had an unknown status. Novel therapies for COVID-19 are being explored with the primary objective of ameliorating the rates of morbidity and mortality by reducing lung injury, improving host immunity, and decreasing inflammation. Previous clinical trials have suggested that cell therapy is safe and leads to multiple beneficial effects in diverse respiratory conditions40,41,42,43,44,45. Our evaluation of the literature also suggested similar findings for cell-based therapy to treat patients affected by COVID-19 and ARDS.
In a recent report, Kim and Knoepfler evaluated stem cell (including MSCs) and NK cell therapy results from two clinical trial registries comprising 79 cell therapy trials for COVID-19. Among these, 67.1% were randomized, 25.3% were double-blinded, 34.2% were placebo-controlled, and only 22.8% met all three criteria, namely randomization, double-blinding, and placebo controls46. In the present systematic review, we included 29 published articles in which six (20.7%) were randomized, four (13.8%) were double-blinded, eight (27.6%) were placebo-controlled, and four (13.8%) met all three criteria. Overall, the results of cell therapy trials for COVID-19 have not reached a high impact, as trials meeting the three primary criteria, randomization, double-blinding, and placebo controls have been minimal.
MSCs are extensively used in cell therapy since their efficacy and safety have been shown in several diseases in preclinical and clinical trials, with significant efficacy in inflammatory and immunologic conditions47,48,49,50,51,52. Most of the cell therapy studies reviewed here utilized MSCs as donor cells in patients with COVID-19 and ARDS. The most common sources of MSCs used to treat respiratory pathologies are bone marrow, umbilical cord blood, adipose tissue, and endothelial progenitor cells53. MSCs are characterized by their immunomodulatory functions combined with their regenerative and proliferative ability to help severely affected COVID-19 patients. These cells secrete a variety of paracrine factors that may facilitate immunomodulation and anti-inflammatory effects, which have been hypothesized as possible mechanisms contributing to improved lung function and regeneration in respiratory diseases54,55. As seen in most of the analyzed studies, the beneficial effects of MSC intervention in COVID-19 and ARDS appeared to be linked to improvements in the host immune system and inflammatory response. Such a conclusion is based on increased lymphocyte counts and decreased CRP and pro-inflammatory cytokine levels, facilitating lung repair.
Regarding the clinical outcomes analyzed in this study, three outcome domains were identified, which were further subdivided into several parameters: immune response, pulmonary function, and systemic response. Although most cases of COVID-19 are mild to moderate in severity, ~15% of cases evolve to severe pneumonia, and ~5% progress to ARDS and multiple organ failure. The progression to a worse presentation is related chiefly to cytokine upregulation and exaggerated inflammatory response. In severe ailing patients, a hyperimmune reaction termed cytokine storm results in critical illness and end-organ dysfunction, with a high mortality rate22. Findings from the included articles indicate a possible positive impact of cell therapy on crucial immunological and inflammatory processes that lead to organ injury in SARS-CoV-2-infected patients. Laboratory measurements were common to 25 of the 29 included clinical articles, representing 86.2% of published reports. Among the clinical laboratory findings, 18 studies demonstrated improvements in immunomodulatory responses or inflammation markers. Therefore, reversing or even attenuating the cytokine storm appeared to be a lifesaver in patients with severe COVID-1911.
The primary site of SARS-CoV-2 infection is the respiratory system. The virus is responsible for injuring the lungs and causing impaired alveolar oxygenation, hypoxemia, and acidosis. Dysregulated pulmonary functions following SARS-CoV-2 infection should be considered, as it can progress to death or permanent lung injury if the patient recovers56. Therefore, notwithstanding the miscellaneous nature of measures exploited in different studies, 23 studies (79%) evaluated pulmonary functions. All these studies evaluated pulmonary functions using a variety of parameters, such as PaO2/FIO2, oxygen saturation ratio, lung imaging—chest CT or chest radiography, symptoms at admission and clinical status after cell therapy, discontinuation of oxygen support (intubation and ECMO) after cell infusion and reduction in mortality. In these studies, noteworthy improvements were reported between pre- and post-cell therapy status.
The studies categorized as evidence levels 1A and 1B reported that clinical outcomes could be influenced by cell preparation techniques leading to considerable differences in MSC viability27. Bellingan and colleagues also reported increased intensive care-free days, as well as a reduction in mortality33. Lanzoni and associates showed a considerable reduction in the concentration of pro-inflammatory factors. However, the small sample size and modified inclusion criteria during the trial are some of the limitations in these studies14.
Several studies with evidence level B demonstrated a decrease in pro-inflammatory biomarkers after cell therapy intervention11,17,18,22,29,34,36,38, improved lung function34, reduced B-lymphocyte counts11,21,28,29,32 and better oxygenation levels11,16,17,21,23,31,33,34. However, these studies have significant limitations, including the lack of details on MSC origin or isolation34, the small sample size18,21,32,38, variability in MSC doses administered to patients31,33, irregular regimens of MSC administration32, insufficient cells for all the randomized patients assigned to receive cell infusions resulting in their assignment to the control group18, lack of a control group11,21,23, variability of treatments the patients received prior to cell infusions22, and the study design classified as retrospective23. Regarding 4 C evidence level studies, most of them were case reports, and hence, the extrapolation of these results to larger cohorts of patients will be difficult24.
Steroids act through similar proposed immune mechanisms as MSCs, the predominant cell type employed for infusions in the evaluated articles. Corticosteroid treatment for COVID-19 has proven to reduce mortality of patients on respiratory support57. However, some studies have discussed a few possible AE caused by the prolonged use of steroids, including hypertension, fluid retention, and increased risk of infection58. In addition, critically ill patients can have signs of hypercortisolism due to suppression of the neuroendocrine system, leading to the adrenal deficiency that could enhance mortality risk59, but it would differ based on individual variability in pharmacokinetics. The studies performing stem cell intervention reported restoration of lung function possibly through immunomodulation with just two transfusions, raising the debate that cell therapy could be beneficial in cases where patients do not respond well to standard treatment with steroids due to individual variability.
When proposing new therapeutic approaches, mortality is an important consideration, and this was one of the principal outcomes we reviewed in the present study. As per the reports, there were no deaths directly linked to cell therapy. Deaths were reported in 17 studies, and a total of 79 patients died. In all cases, it was suggested by the authors and committees that none were related to MSC infusions, but few studies were unsure about the cause of death. The studies also suggested that adverse events following MSC infusions in COVID-19 patients are rare or milder when present. The reported adverse events included facial flushing, transient fever, and hypoxia, but these were primarily attributed to the progression of COVID-19 and not directly to cell infusions17. Furthermore, cardiac arrest was reported 2 h after the infusion of the vehicle or cells in control and treated groups. These events were attributed to the infusion protocol (catheterization) rather than the MSC infusions per se14. Additionally, a study using MSCs for the treatment of ARDS reported a decrease in the mortality rate when patients received the therapy within 28 days. However, after 60 days, 38% of patients died. Still, the researchers concluded that the treatment was able to moderate the severe form of the disease and that the mortality in the treated group was not statistically higher than in the control group. They found that patients who received treatment with MSCs had numerically higher disease severity scores before the proposed treatment27. Besides, some of the collateral effects, including increased levels of lactate, serum cardiac troponin, and creatine kinase after MSC infusions, which resulted in liver dysfunction and heart failure, need to be investigated to determine their possible correlation with cell therapy and caution for patients with metabolic acidosis or coronary heart disease23. One study addressed the need for studies that correlate MSCs with an increased risk of infections; however, no such increase was reported in clinical trials25. All AE were controlled, indicating that cell therapy for treating COVID-19 and ARDS is likely safe.
Since the emergency pandemic situation broke out only recently, there was not enough time to produce a larger number of clinical trials with higher evidence levels, enrolling larger cohorts of patients and randomized controlled and multicenter studies involving cell-based therapy in severely affected COVID-19 patients. Randomized controlled trials have been pivotal to testing the safety and efficacy of a variety of interventions60. The novelty regarding the appraisal of safety and efficacy of stem cell infusions as an adjuvant treatment for COVID-19 is another critical issue that possibly explains the large number of low evidence studies discussed in this review.
In particular, we highlighted the efficacy of MSC therapy to halt the cytokine storm caused by SARS-CoV-2 infection, evident from the downregulated expression of pro-inflammatory cytokines and an increased concentration of an anti-inflammatory cytokine. Recently, COVID-19 was identified as a multi-organ infection with many symptoms linked to persistent manifestations and long-term effects on the cardiovascular and nervous systems after acute COVID-1961. In this context, MSC therapy could also help treat the long-term effects of COVID-19 infection, especially those emerging from chronic inflammation.
The number of patients recruited for the clinical studies was low since some trials were explored as a proof of concept and employed as compassionate use. Because the SARS-CoV-2 outbreak is still recent, there has not been enough time to produce extensive randomized clinical trials with a larger number of patients to understand better the effects of stem cell therapy on COVID-19-induced ARDS. Additionally, some of the analyzed studies are still in initial phases, and hence, our evaluation of findings was on the preliminary outcomes. Likewise, some studies did not use a control group, making the evaluation of the efficacy of MSC infusions difficult. Moreover, studies changed the course of treatment during the trial due to the lack of MSCs. Additionally, causes of death were unclear in some cases and were considered as unrelated to MSC infusions in others without adequate analysis and discussion on the issue. Therefore, additional clarifications are needed on how the cause of death was inferred as unrelated to cell infusions.
Furthermore, several aspects of cell therapy have not been satisfactorily elucidated. These include the best source of MSCs, the optimal dose of MSCs, the frequency of IV administration, and the therapeutic window for cell therapy intervention after COVID-19 infection to provide maximal protection to the lungs and other organs. It is also essential to consider that, according to Oxford and GRADE evaluation, most studies included here did not meet the highest level of evidence (A), and most of them were characterized as very low to moderate levels. Furthermore, it is too early to speculate on the exact mechanism by which stem cell therapy improves pulmonary complications, although this systematic review strived to provide provocative discussions.
Increased mortality worldwide due to SARS-CoV-2 infection and the emergence of new variants of the coronavirus emphasize the need to develop new therapeutic strategies for treating complications of COVID-19 and associated respiratory diseases such as ARDS. Treating COVID-19 complications is challenging since no treatment regimen has been entirely successful until now. Hence, identifying an effective and safe therapy for patients with severe COVID-19 is crucial for saving lives.
The use of cell therapy, especially MSCs, to treat COVID-19 appears promising based on the observations and findings in published studies. MSC therapy has shown promise to suppress cytokine storms, prevent the overactivation of the immune system, and repair the lung injury caused by SARS-CoV-2 infection. While the mechanisms of action of these cells are not yet fully understood, the beneficial effects promoted by MSCs are noteworthy. Thus, considering the current global pandemic situation, cell-based therapy could be considered an alternative treatment to containing the public health crisis, such as outbreaks in hospitals and care units and the collapse of medical infrastructure. Additionally, vaccines are already reducing the overall COVID-19 cases in many countries. However, cell-based therapy could also be used to treat the long-term sequelae caused by SARS-CoV-2 infection in patients, especially those related to chronic inflammation. For example, one in eight survivors of COVID-19 is diagnosed with neurological and psychiatric conditions, which may comprise confusion, problems in concentrating and memory recall, or depression. Nevertheless, more extensive double-blind, placebo-controlled, and multicenter clinical trials with a larger cohort of patients are necessary to validate the safety and efficacy of this therapeutic strategy.
Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement62 and was registered at the International Register of Prospective Systematic Reviews under identification number CRD42021248263.
Search strategy
A systematic search was conducted in MEDLINE (via PubMed), Embase, and Scopus. Comparable elements of research were used for all databases with some adaptations to each database search system. The descriptors comprised “COVID-19”, “SARS-CoV-2”, “Coronavirus”, “2019-nCoV”, “SARS”, “MERS”, “acute respiratory distress syndrome”, and “cell therapy.” Data were obtained from published articles from January 2000 to March 2021. There were no language restrictions.
Study selection
Three authors (L.L.L., L.P., and S.C.B.) independently assessed potentially eligible studies for their suitability for inclusion in this review. Disagreements were resolved by a fourth reviewer (GZ). During the screening of titles and abstracts, relevant papers were defined if they mentioned aspects of cell therapy applied as a therapeutic possibility for patients with COVID-19, ARDS, SARS, or MERS. Abstracts were analyzed according to the inclusion criteria, and all studies that met these criteria were included for full article reading.
Studies were included if they pertained to any stem cell therapy applied as a therapeutic alternative for patients with COVID-19, ARDS, SARS, or MERS with original data. The exclusion criteria were (1) review articles (including critical letters and systematic reviews), (2) studies with no mention of cell therapy, (3) studies relating to cell therapy applied to unspecified respiratory diseases, and (4) experimental studies. Figure 1 displays a flowchart of study selection and inclusion.
Data extraction
Regarding the appraisal of the data from all included studies, five tables were structured to summarize each study’s characteristics and findings, main parameters and outcomes, study design and classification of evidence, AE, and mortality. Table 1 shows clinical studies detailing the title, authors, investigated disease, donor cell type, route of cell administration, participant characteristics, n of the study, inclusion criteria, analyzed parameters, and primary outcomes. Table 2 summarizes the study design and classification of evidence from each study assessed using the Oxford Centre for Evidence-Based Medicine and the Grading of Recommendation Assessment Development and Education (GRADE) systems. Table 3 summarizes the key evaluated parameters separated into three domains: laboratory, pulmonary, and other parameters. Table 4 summarizes the AE encountered in different studies. Table 5 summarizes the mortality data described in different studies.
Data availability
The data are available from the corresponding author upon reasonable request.
References
Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
COVID-19 Map - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html.
Siemieniuk, RAC. et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 370, m2980 (2020).
Zhong, H. et al. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol. Res. 157, 104872 (2020).
Khoury, M. et al. Cell-based therapies for coronavirus disease 2019: proper clinical investigations are essential. Cytotherapy 22, 602–605 (2020).
Khoury, M. et al. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J 55, 2000858 (2020).
Liu, S. et al. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res. Ther. 11, 169 (2020).
Thompson, M. et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine 19, 100249 (2020).
Durieux, N., Vandenput, S. & Pasleau, F. [OCEBM levels of evidence system]. Rev. Med. Liege 68, 644–649 (2013).
Guyatt, G. H., Oxman, A. D., Schünemann, H. J., Tugwell, P. & Knottnerus, A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. https://doi.org/10.1016/j.jclinepi.2010.09.011 (2011).
Guo, Z. et al. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care 24, 420 (2020).
Zhang, Y. et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 11, 207 (2020).
Chang, Y. et al. Intratracheal administration of umbilical cord blood-derived mesenchymal stem cells in a patient with acute respiratory distress syndrome. J. Korean Med. Sci. 29, 438–440 (2014).
Lanzoni, G. et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. https://doi.org/10.1002/sctm.20-0472 (2021).
Liang, B. et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine 99, e21429 (2020).
Iglesias, M. et al. Mesenchymal stem cells for the compassionate treatment of severe acute respiratory distress syndrome due to COVID 19. Aging Dis. 12, 360–370 (2021).
Meng, F. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct. Target. Ther. 5, 172 (2020).
Shu, L. et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 11, 361 (2020).
Shi, L. et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 6 (2021).
Peng, H. et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res. Ther. 11, 291 (2020).
Feng, Y. et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif. 53, e12947 (2020).
Singh, S. et al. Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res. Cardiol. 115, 36 (2020).
Chen, X. et al. Mesenchymal stem cell therapy in severe COVID-19: a retrospective study of short-term treatment efficacy and side effects. J. Infect. https://doi.org/10.1016/j.jinf.2020.05.020 (2020)
Simonson, O. E. et al. Five-year follow-up after mesenchymal stromal cell-based treatment of severe acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 202, 1051–1055 (2020).
Simonson, O. E. et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 4, 1199–1213 (2015).
Wilson, J. G. et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 3, 24–32 (2015).
Matthay, M. A. et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 7, 154–162 (2019).
Häberle, H. et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J. Intensive Care Med. https://doi.org/10.1177/0885066621997365 (2021).
Sengupta, V. et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 29, 747–754 (2020).
Jungebluth, P. et al. Autologous peripheral blood mononuclear cells as treatment in refractory acute respiratory distress syndrome. Respiration 90, 481–492 (2015).
Zheng, G. et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 15, 39 (2014).
Sánchez-Guijo, F. et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 25, 100454 (2020).
Bellingan, G. et al. Primary analysis of a phase 1/2 study to assess multistem® cell therapy, a regenerative advanced therapy medicinal product (ATMP), in acute respiratory distress syndrome (MUST-ARDS). Am. J. Respir. Crit. Care Med. 199, (2019).
Leng, Z. et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 11, 216–228 (2020).
Lu, J., Xie, Z.-Y., Zhu, D.-H. & Li, L.-J. Human menstrual blood-derived stem cells as immunoregulatory therapy in COVID-19: a case report and review of the literature. World J. Clin. Cases 9, 1705–1713 (2021).
Tang, L. et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 14, 664–673 (2020).
Wu, J. et al. First case of COVID-19 infused with hESC derived immunity- and matrix-regulatory cells. Cell Prolif. 53, e12943 (2020).
Hashemian, SMR. et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res. Ther. 12, 91 (2021).
COVID-19 Studies from the World Health Organization Database - ClinicalTrials.gov.
Chambers, D. C. et al. Mesenchymal stromal cell therapy for chronic lung allograft dysfunction: results of a first-in-man study. Stem Cells Transl. Med. 6, 1152–1157 (2017).
Glassberg, M. K. et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase I safety clinical trial. Chest 151, 971–981 (2017).
Geiger, S., Hirsch, D. & Hermann, FG. Cell therapy for lung disease. Eur. Respir. Rev. 26, 170044 (2017).
Chambers, D. C. et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 19, 1013–1018 (2014).
Keller, C. A. et al. Feasibility, safety, and tolerance of mesenchymal stem cell therapy for obstructive chronic lung allograft dysfunction. Stem Cells Transl. Med. 7, 161–167 (2018).
Cruz, F. F. & Rocco, P. R. M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 14, 31–39 (2020).
M, K. & PS, K. Anticipated impact of stem cell and other cellular medicine clinical trials for COVID-19. Regen. Med. 16, 525–533 (2021).
Connick, P. et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 11, 150–156 (2012).
Chan, M. C. W. et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl Acad. Sci. USA 113, 3621–3626 (2016).
Zhao, Q., Ren, H. & Han, Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. https://doi.org/10.1016/j.jocit.2014.12.001 (2016).
Volarevic, V. et al. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors 43, 633–644 (2017).
Kamen, D. L. et al. CT-04 safety and efficacy of allogeneic umbilical cord-derived mesenchymal stem cells (MSCs) in patients with systemic lupus erythematosus: results of an open-label phase I study. Lupus Sci. Med. https://doi.org/10.1136/lupus-2018-lsm.76 (2018).
Mahendiratta, S. et al. Stem cell therapy in COVID-19: pooled evidence from SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS: a systematic review. Biomed. Pharmacother. 137, 111300 (2021).
Behnke, J. et al. MSC based therapies-New perspectives for the injured lung. J. Clin. Med. 9, 682 (2020).
Najar, M. et al. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy 18, 160–171 (2016).
Prockop, D. J. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy 19, 1–8 (2017).
Wang, F., Kream, R. M. & Stefano, G. B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. https://doi.org/10.12659/MSM.928996 (2020).
JAC, S. et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324, 1330–1341 (2020).
Mattos-Silva, P. et al. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir. Physiol. Neurobiol. 280, 103492 (2020).
Scaroni, C., Armigliato, M. & Cannavò, S. COVID-19 outbreak and steroids administration: are patients treated for Sars-Cov-2 at risk of adrenal insufficiency? J. Endocrinol. Investig. 43, 1035–1036 (2020).
Evidence-based medicine: how COVID can drive positive change. Nature 593, 168 (2021).
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. https://doi.org/10.1038/s41591-021-01283-z (2021).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Tao, J. et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J. Infect. Dev. Ctries. 14, 1138–1145 (2020).
Acknowledgements
The authors are supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). A.K.S. is supported by a grant from the National Institutes of Health Grant (R01NS106907-01).
Author information
Authors and Affiliations
Contributions
G.Z., designed the manuscript. G.Z., L.P., L.L.L., and S.C.B. performed the literature search and wrote the original draft. I.M.G., D.R.M., and A.K.S. critically revised the manuscript. J.C.C. designed, critically revised, and supervised the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zanirati, G., Provenzi, L., Libermann, L.L. et al. Stem cell-based therapy for COVID-19 and ARDS: a systematic review. npj Regen Med 6, 73 (2021). https://doi.org/10.1038/s41536-021-00181-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-021-00181-9
This article is cited by
-
Stem Cell Extracellular Vesicles as Anti-SARS-CoV-2 Immunomodulatory Therapeutics: A Systematic Review of Clinical and Preclinical Studies
Stem Cell Reviews and Reports (2024)
-
Ventilator-Induced Lung Injury as a Dynamic Balance Between Epithelial Cell Damage and Recovery
Annals of Biomedical Engineering (2023)
-
Decidual stromal cells for the treatment of severe COVID-19 ARDS
Intensive Care Medicine (2023)
-
Safety and efficiency of stem cell therapy for COVID-19: a systematic review and meta-analysis
Global Health Research and Policy (2022)
-
Predicting multipotency of human adult stem cells derived from various donors through deep learning
Scientific Reports (2022)