Abstract
The field of articular cartilage repair has made significant advances in recent decades; yet current therapies are generally not evaluated or tested, at the time of pivotal trial, in patients with a variety of common comorbidities. To that end, we systematically reviewed cartilage repair clinical trials to identify common exclusion criteria and reviewed the literature to identify emerging regenerative approaches that are poised to overcome these current exclusion criteria. The term “knee cartilage repair” was searched on clinicaltrials.gov. Of the 60 trials identified on initial search, 33 were further examined to extract exclusion criteria. Criteria excluded by more than half of the trials were identified in order to focus discussion on emerging regenerative strategies that might address these concerns. These criteria included age (<18 or >55 years old), small defects (<1 cm2), large defects (>8 cm2), multiple defect (>2 lesions), BMI >35, meniscectomy (>50%), bilateral knee pathology, ligamentous instability, arthritis, malalignment, prior repair, kissing lesions, neurologic disease of lower extremities, inflammation, infection, endocrine or metabolic disease, drug or alcohol abuse, pregnancy, and history of cancer. Finally, we describe emerging tissue engineering and regenerative approaches that might foster cartilage repair in these challenging environments. The identified criteria exclude a majority of the affected population from treatment, and thus greater focus must be placed on these emerging cartilage regeneration techniques to treat patients with the challenging “red knee”.
Similar content being viewed by others
Introduction
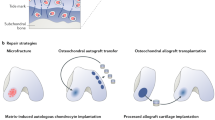
Articular cartilage injuries pose a significant clinical challenge in orthopaedics. The high prevalence of injury and lack of intrinsic tissue healing capacity leave a relatively young and healthy population on the path to degenerative osteoarthritis (OA) without intervention.1 Arthroscopic procedures reveal the presence of chondral lesions in greater than 60% of patients,2,3 and yearly incidence rates of chondral lesions nearly tripled between 1996 and 2011.4 The most common arthroscopic intervention for chondral injuries is chondroplasty, or removal of the loose pieces of cartilage. While this provides short-term symptomatic relief, the remaining cartilage is more susceptible to wear and accelerated degeneration. Another common intervention is microfracture, which involves penetrating the subchondral bone to allow bone marrow to fill the defect. This approach results in the formation of mechanically-inferior fibrocartilage tissue.5,6 Mosaicplasty, typically osteochondral autologous transplantation (OATs), is an open procedure where osteochondral plugs are harvested from non-weight bearing areas and transferred into the defect. However, this transplanted cartilage can cause donor site morbidity, does not integrate well with the existing cartilage, and has been shown to degenerate over the long term.7
More modern cartilage regeneration approaches include autologous chondrocyte implantation (ACI) and now matrix-associated autologous chondrocyte implantation (MACI), as well as autologous matrix-induced chondrogenesis (AMIC). MACI is a two-stage procedure (and an advance on the original ACI procedure), where healthy cartilage cells are harvested from the patient, expanded, seeded in a collagen matrix, and then re-implanted into the cartilage defect. AMIC, on the other hand, is a single-stage procedure where a cell-free collagen matrix is implanted into a cartilage defect in combination with microfracture. These treatments have demonstrated improved outcomes compared to traditional techniques (chondroplasty/microfracture); however, they are mostly restricted in their application and evaluation to a small fraction of the patient population with near-ideal surgical conditions.1 This sub-population of patients (with “green knees”) presents the highest probability of successful resolution of symptoms following intervention, and so are most often included in initial clinical trials. These patients are differentiated from those having “red knees”, whose cartilage pathologies are more severe or whose co-morbidities preclude them from these cartilage repair procedures.8 This “red knee” cohort is typically only left with short-term palliative treatment options, such as oral non-steroidal anti-inflammatory drugs (NSAIDs) or intra-articular injections of corticosteroids or hyaluronic acid. While these treatments do provide temporary inflammation and pain relief, bioactive factors reside in the joint for <2 months, necessitating frequent injections.9 Lacking available options for long-term repair and/or regeneration, these “red knee” patients are often neglected and almost certainly destined for total joint replacement.
To address this topic in a systematic fashion, this review first analyzes the disease burden of articular cartilage injuries, focusing on those currently deemed treatable by repair and restoration procedures. Next, we examine clinical trial exclusion criteria for cartilage lesion characteristics and patient co-morbidities that identify a “red knee” which is contra-indicated for treatment using current technologies. Patient attributes that result in exclusion by >50% of trials are used to inform the topics/conditions that should be addressed in regenerative strategies addressing a broader patient population. We then highlight recent advances in pre-clinical and translational regenerative technologies that may address these challenges in cartilage restoration, and identify areas with the most pressing need for continued development.
Disease burden and treatment trends
Cartilage injuries are extremely common, and lesions are often present in asymptomatic patients. For instance, Curl et al.2 reported that 63% (19,827/31,516) of knee arthroscopies for any indication identified chondral lesions, 32% of which had exposed bone, categorizing them as grade IV on the Outerbridge scale. Widuchowski et al.3 supported this high prevalence, reporting chondral lesions in 15,074 of 25,124 arthroscopies (60%). Of these, 9% of patients had Grade III or IV chondral lesions and were under 50 years old, meeting conservative indications for cartilage repair surgery. In both studies, the authors found that concomitant ligamentous or meniscal pathology was present in ~70% of cases, corresponding to the high prevalence of traumatic etiology in these patients. The disease burden of cartilage injuries is also growing, with the annual incidence of cartilage injuries increasing from 22/100,000 in 1996 to 61/100,000 in 2011, across all age groups and sexes. The percent of cartilage injuries treated ranged from 13.8 to 22.1% during this time period, yet only 1% of these repair procedures involved advanced techniques such as chondrocyte transplantation.4
Another study based on 25 million privately insured patients in the United States found that the yearly incidence rate of cartilage repair surgery increased from 63/100,000 in 2006 to 93/100,000 in 2011. In 2011, 70% of the procedures involved chondroplasty (smoothing the defect by removing loose strands of cartilage), 28% involved microfracture or subchondral drilling (techniques used to release marrow elements), and only 2% involved the use of advanced cartilage restoration techniques (osteochondral autograft transfer, ACI).10 In contrast, primary total knee arthroplasty (TKA) had an incidence rate of 429/100,000,11 and recent projections estimate an 85% increase in TKAs by 2030.12 The large disparity between the incidence of TKA and that of cartilage repair surgeries (Fig. 1) suggests a pressing need for more advanced cartilage repair technologies that might obviate the need for TKA and control the high socio-economic burden of end-stage knee arthritis.
Cartilage restoration procedures may also provide financial benefits for both patients and healthcare systems. A recent cost-benefit analysis with at least 5-year follow-up estimated the direct plus indirect costs of ACI to be $16,781, nearly 20% less than that of TKA ($20,568).13 Future savings provided by ACI could include avoiding multiple debridement and microfracture procedures on the same cartilage lesion and the need for a total joint replacement early in life and a subsequent revision TKA. This could circumvent the work-time lost from OA debilitation or post-surgery rehabilitation. The improvement of cartilage restoration technologies and techniques may result in shorter operative times, quicker patient recoveries, and perhaps diminished requirement for a preliminary cartilage harvest procedure, all leading to lower overall costs. Broadening the applicability of these restorative procedures and scaling up the industry of cartilage therapies would only serve to further reduce manufacturing costs, and ultimately, the financial burden on both patients and providers.
Defining the “red knee”
Several studies have shown improved outcomes when comparing ACI, MACI, and AMIC to microfracture.14,15,16,17,18 However, these advanced restorative techniques still present increased failure rates in knees with common comorbidities (larger lesion size, “kissing lesions” on opposing articular surfaces, and pre-existing arthritic changes).19 One study evaluating MACI with long-term follow-up (5–12 years) found failure rates of 18.2 and 87.5% in complex and salvage cases, respectively, compared to a failure rate of only 4.3% in less complex cases.20 Similarly, Filardo and colleagues found that MACI treatment in osteoarthritic knees had a failure rate of 27.3% at 9-year follow-up.21 Treatment failure correlated with degenerative lesion origin, longer symptom duration, larger lesion size, older age, and prior knee surgery.22
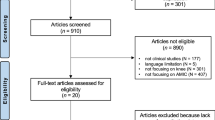
These case reports and series suggest optimal conditions for repair (i.e., ‘green knees’), and conditions in which repair is likely to be unsuccessful (i.e., ‘red knees’). This is often due to circumstances other than the cartilage defect or procedure itself. These concepts have influenced the manner in which new technologies are evaluated in clinical trials, where conditions most conducive to a successful therapy (i.e., ‘green knees’) are selected for initial trials in humans. To better understand these factors defining the ‘red knee’, we conducted a systematic review of exclusion and inclusion criteria for cartilage restoration clinical trials on “clinicaltrials.gov” using the search term “knee cartilage repair” on 1 January 2019. Studies that had been suspended, terminated, or withdrawn, as well as studies of unknown status, were excluded from the search, leaving 60 studies for review. Follow-up studies and studies using only intra-articular injections were excluded to focus on new cartilage restoration procedures. This yielded 33 clinical trials (Supplemental Table) that were used to extract inclusion and exclusion criteria for tabulation (Supplemental Methods). Common exclusion criteria are illustrated schematically in Fig. 2.
Patient age was the most common exclusion criteria used in trials, with over half the trials excluding patients over the age of 55 or under the age of 18 (Fig. 3a). The number of chondrocytes and bone marrow-derived MSCs, as well as their proliferative and matrix forming potential, may decline in adulthood.23,24,25 This might limit the healing capacity of cartilage in older patients, and thus is a common exclusion criterion. The lower limit of patient age exclusion relates to the legal age of adults capable of signing informed consent to participate in the trial in the United States. Trials in Europe were more likely to include patients in the 14–17 age range.
Graphical display of the percentage of clinical trials excluding patients with regards to a age group b lesion size, c mechanical comorbidities, and d common systemic comorbidities. Data presented as a percentage, n = 33 studies analyzed. Dashed line represents 50% exclusion cutoff for defining the “red knee” population. Exclusion criteria above this threshold are presented with black bars, and criteria below this threshold are presented with white bars. LE lower extremity; BMI body mass index
Lesion size was another common exclusion criterion, with 58% of clinical trials excluding lesions that were >8 cm2 in size (Fig. 3b). Similarly, patients with more than two lesions were excluded by 64% of trials, and patients with signs of joint-wide OA were excluded by 79% of trials. Joints requiring a large surface area of cartilage restoration are challenging for a number of reasons, including mechanical demands, graft fixation, and the large number of cells required for transplantation (in cell based therapies). Interestingly, lesions <1 cm2 were also excluded by over 50% of trials, likely due to satisfactory outcomes in small lesions treated by microfracture.26 Of note, 64% of trials also excluded lesions that had been previously treated with either microfracture or cartilage graft, and 55% of trials excluded bone lesions >7 mm in depth. These lesions might be expected to have progressive cartilage wearing and/or subchondral bone damage that could limit the repair and integration capacity of revision tissue engineering therapies applied to the same location, a controversial topic in the field at present.22,27,28
Another subset of exclusion criteria relates to scenarios that place excessive mechanical demands on the repair tissue. Over one half of the clinical trials excluded patients with body mass index (BMI) greater than 35, pathology on bilateral knees, neurologic disease of the lower extremities, the presence of kissing lesions, ligamentous instability, joint malalignment (>5° offset), and prior meniscectomy with <50% of the native meniscus remaining (Fig. 3c). These comorbidities increase the risk of excessive focal or abnormal loading and the likelihood for degeneration, and are unfortunately very common in the United States. For example, 15.5% of adults in the US currently have a BMI > 35.29 Ligamentous instability and tears (incidence: 46/100,000 person-years30), and meniscectomies (incidence: 224/100,00031) further compound the high risk of progression to OA32 and increase the likelihood of patient exclusion.
Lastly, we identified a group of systemic comorbidities related to overall health that were commonly used as exclusion criteria (Fig. 3d). Patients with systemic or local inflammatory conditions were excluded by 88% of trials. Active inflammatory conditions would be expected to interfere with any repair process, and could thus limit the efficacy of tissue engineering strategies.33,34,35,36 Similarly, 73% of trials excluded patients with systemic or local infection. Infection could bring about an inflammatory response or directly infect the repair tissue, compromising viability and functional maturation of implanted constructs.37,38 Patients with metabolic or endocrine conditions such as diabetes mellitus, parathyroid disease, and chronic kidney disease were excluded by 70% of trials. These common conditions are known to interfere with the healing process, and would likely decrease the integration and survival of implanted constructs.39,40,41,42 Similarly, patients with a history of drug or alcohol abuse were excluded by 61% of trials. These patients would also have limited healing capacity, increased inflammation, and may not adhere to the strict post-operative recovery protocol common to these trials.43,44,45,46 Other notable conditions frequently excluded were a history of cancer within 5 years (52%), immunocompromised state (45%), and vascular disease of the lower extremity (42%).
The systematic analysis found that cartilage lesions complicated by limited progenitor cell activity and healing capacity, large size, high mechanical demands, local and systemic inflammation, or other systemic diseases are often excluded from treatment, likely due to complications and poor outcomes after cartilage restoration procedures in this context. While these exclusion criteria are reasonable when first evaluating a new technology, their prevalence in the general population who might benefit from regenerative cartilage procedures is evident by the large number of patients who progress to total joint replacement. If these contraindications could be mediated by improvements in cartilage therapeutics, then the number of persons benefiting from these technologies would be dramatically increased. We identified several exclusion criteria that were used in over 50% of the surveyed clinical trials (Fig. 3—black bars). The following section focuses on emerging pre-clinical and translational research trends early in development in the field of cartilage tissue engineering and regenerative medicine, with a focus on developing technologies that may address these exclusion criteria and broaden the patient population indicated for treatment with advanced cartilage repair therapies.
Advances in cartilage tissue engineering and regenerative therapeutics—treating the “red knee”
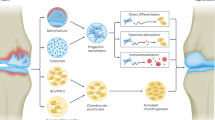
Aging is a significant concern in the field of cartilage regeneration due to its deleterious effects on stem cell density and activity, and the increase in cellular senescence with age.47,48 Cellular senescence can affect the differentiation potential, immunomodulatory abilities, and migratory capabilities of both stem cells and chondrocytes. Thus, older patients with cartilage lesions may require additional treatment measures to enhance stem cell or chondrocyte availability and activity at the lesion site. The MACI technique seeks to address these concerns by expanding autologous chondrocytes and seeding them in media designed to favor differentiation towards mature chondrocytes.49 Cell-free scaffolds (AMIC) rely on the patient’s native cells to infiltrate the graft during implantation, and are thus at an even higher risk of failure in older patients. Thus, researchers have investigated the use of growth factors to increase cell recruitment, improve cell chondrogenesis, and optimize chondrocyte populations for cartilage regeneration therapies. For example, stromal cell-derived factor 1 alpha (SDF-1α) doubled the recruitment of chondrogenic progenitor cells in a hyaluronate-fibrin hydrogel, which formed hyaline cartilage when cultured in a bovine cartilage explant model.50 Local SDF-1α release increased the recruitment of systemically infused bone marrow-derived MSCs by 700% in a mouse model of myocardial infarction (MI),51 suggesting that cartilage repair scaffolds releasing SDF-1α may perform synergistically with MSCs from marrow recruitment or intra-articular injections. Similarly, transforming growth factor-beta 3 (TGF-β3) released from cartilage repair scaffolds improved chondrogenesis in vitro and in large animal models.52,53 Additional examples of bioactive factors used in pre-clinical and clinical trials are included in Table 1, all of which suggest that tailored growth factor delivery may improve cartilage repair in aged patients.
Another technique to enhance the regenerative capacity of cells is to remove neighboring senescent cells. A recent study by Jeon and colleagues showed that senescent chondrocytes accumulate around traumatic cartilage lesions and are associated with the development of arthritis; clearance of these senescent cells, via intra-articular injection of a senolytic molecule, attenuated the development of arthritis in a mouse ACL transection model.54 Another recent study found that rejuvenating aged MSCs with SRT1720, an activator of SIRT1, significantly improved heart function and angiogenesis in a rat MI model compared to control MSCs.55 These potential therapeutics targeted towards rejuvenating, optimizing, and recruiting endogenous stem cells will likely increase the efficacy of cartilage tissue engineering techniques in the older patient population.
For patients with a single cartilage lesion greater than 8 cm2, multiple lesions, or joint wide cartilage degeneration, whole or hemi-joint tissue engineering is an attractive alternative to metal/plastic joint replacement. These ‘living’ implants could potentially last a lifetime, remodeling in response to applied load and continuously generating new matrix. Recent advances in three dimensional (3D) printing56 and rapid prototyping now allow researchers to produce anatomic 3D tissue engineered constructs.57,58 Size matching between native and engineered cartilage is critical to maintain joint mobility and function. Computer assisted design (CAD) programs can translate patient scans, via micro-computed tomography (μCT) or magnetic resonance imaging (MRI), into personalized 3D in silico molds.59 Using layer-by-layer bioprinting, Mao and colleagues’ generated an entire rabbit humeral head using a poly(ɛ-caprolactone) (PCL) and hydroxyapatite scaffold.60 These scaffolds were infused with TGF-β3 to recruit endogenous cells, and inclusion of this growth factor increased cell infiltration into the scaffold by 130%. Another anatomic tissue engineering approach by Moutos and colleagues developed a woven PCL hemispherical scaffold 22 mm in diameter, similarly shaped to the cartilage of the femoral head.61 While Mao and colleagues relied on native cells to populate their scaffold for the rabbit shoulder, Moutos and colleagues seeded their hemispherical scaffold with adipose-derived stem cells (ASCs). ASCs in these woven scaffolds released anti-cytokine factors in a sustained manner to reduce joint inflammation, and showed remarkable mechanical features, with tensile, compressive, and shear properties in the native tissue range.62 This approach could be particularly useful in cases that a large cartilage surface needs to be replaced, and there are signs of joint inflammation. In a third study, Saxena and colleagues used porcine µCT scans to create negative anatomic molds.63 Stem cells encapsulated in a hyaluronic acid hydrogel filled the negative mold, and the resulting tissue was cultured for up to 12 weeks in vitro, exhibiting excellent viability and shape retention. Overall, these studies exemplify how rapid prototyping techniques may be used to generate patient-specific tissue constructs capable of replacing expansive cartilage surfaces.
Other approaches to treat large-scale cartilage defects involve shape-filling chondro-inductive biomaterials. For example, particulated and desiccated allograft tissue, referred to as ‘BioCartilage’,64 has been used to repair cartilage in vivo in an equine cartilage large-defect model. A combination of BioCartilage, microfracture, and platelet-rich plasma showed no signs of synovial inflammation, and had superior histological scoring compared to microfracture controls. Others have also utilized devitalized tissue for cartilage repair, including Detamore and colleagues, who showed that stem cells, in the presence of devitalized cartilage microparticles, produced mechanically robust cartilage tissue.65 Taken together, there has been continued progress in the field of large-scale cartilage repair, with multiple approaches now being tested in clinically relevant large animal models. Table 2 provides examples of the various fabrication methods discussed. These approaches have the potential to address patients with large lesions that are often excluded from cartilage repair trials.
Cartilage restoration procedures have typically avoided small-size defects (<1 cm2), likely due to relatively positive outcomes with microfracture in defects of this size.66,67 However, the fibrocartilage that forms in these defects is still susceptible to long-term degeneration due to its mechanical insufficiency.6,68 Furthermore, treating partial thickness cartilage defects with microfracture may compromise the healthy intact basal layer of cartilage under the defect. Recent biomaterial technologies have been developed to address small full and partial-thickness lesions, with an emphasis on injectable therapeutics for defect-specific filling and ease of application.69,70 These injectable scaffolds typically revolve around a material solution that is either photo-polymerized71,72 or chemically cross-linked,73,74,75 allowing it to completely fill a defect before solidifying. Combining these scaffold-based injectable formulations with stem cells can also improve the treatment of small focal cartilage injuries. Since cell migration from neighboring cartilage into these small partial-thickness defects is limited,76 encapsulating cells within the injected matrix77,78 may initiate remodeling and extracellular matrix (ECM) formation immediately, facilitating and improving cartilage regeneration.
An array of exclusion criteria (BMI > 35, partial meniscectomy, ligamentous instability, malalignment) are related to mechanical comorbidities that current treatments do not address. Due to these mechanical circumstances, the cartilage in these knees is subject to complex stresses that are significantly greater in magnitude than those the healthy “green” knee would experience. Cartilage repair strategies must account for these stresses early in recovery to avoid failure following implantation and patient remobilization. Therapies that rely on scaffold-free cellular approaches, such as ACI, are not designed to handle these mechanical burdens early post-surgery, and thus, utilizing scaffolds with improved bulk mechanical properties could increase efficacy of treatment and promote earlier return to normal activity in these knees. To review the mechanical properties of cartilage scaffolds over the years, a systematic review was performed (Supplemental Methods). Initial scaffold attempts used simple sponges and hydrogels with moduli (both instantaneous and equilibrium) in the tens of kilopascals (kPa), while the modulus of native cartilage is 20–50 times higher. More recently, groups have utilized a variety of fabrication (weaving,62,79 3D printing,56,80,81 casting57,63) and cross-linking (UV photoinitiator,82,83 EDC crosslinking,83,84,85 dehydrothermal treatment83) techniques to improve the mechanical properties of the time-zero scaffold (Fig. 4—red squares and triangles). For example, Valonen and colleagues developed a 3D-woven poly(ε-caprolactone) (PCL) scaffold with an aggregate modulus of 550 kPa, well within the range of native articular cartilage.86 Other groups have created fiber-reinforced hydrogels, and achieved stiffness values greater than 400 kPa,80,87 resulting in replacement scaffolds for defects that require greater mechanical support. An important consideration is balancing the mechanics of these scaffolds with their resorption and ability to form new cartilage tissue.88,89 A scaffold should provide architecture and support for initial load-bearing, but exhibit a degradation profile that allows infiltrated cells to respond and form neo-cartilage tissue. Alternatively, in vitro culture of constructs52,90 can promote increased deposition and organization of ECM, further elevating mechanical properties of these scaffolds (Fig. 4—green squares and triangles). While more expensive, these lab-grown in vitro engineered replacements have the potential to withstand loadbearing immediately upon implantation, as long as adequate fixation of the grafts can be achieved.
Modulus values (kPa) as a function of time (Jan 2001 to Jan 2018). Squares and triangles represent instantaneous and equilibrium moduli, respectively. Red, green, and blue points represent time-zero scaffold, cultured construct, and mechanical assessments from in vivo studies, respectively. Survey of PubMed literature utilizing search terms “Cartilage”, “Scaffold”, and “Modulus”. Studies with inadequate description of testing methods were excluded
Another important mechanical exclusion criterion in cartilage repair procedures is the presence of kissing lesions. Due to the continuous contact and articulation between two kissing implants, surface frictional and shear properties are of vital importance. Likewise, ligamentous instability and malalignment can exacerbate stresses parallel to the articular surface, increasing shear forces experienced by the implant and repair tissue.91,92,93 Therefore, along with bulk compressive mechanical properties, any cartilage replacement should minimize friction and maximize shear resistance to prevent wear or implant dislodgement. One study successfully increased lubricin (lubricating protein) concentration in superficial zone chondrocytes;94 this advance could be applied to cartilage scaffolds in order to reduce the coefficient of friction at the articulating surface. Another modality to decrease friction, that also provides shear resistance, is fabricating scaffolds with an aligned superficial zone.95,96 For example, Accardi and colleagues97 found that varying the alignment of electrospun nanofibers had a positive effect on implant shear properties. The group additionally varied electrospinning speed during fabrication to tune fiber organization through the scaffold depth to provide a shear-resistant superficial layer. These adaptations could be considered in knees requiring supplemental mechanical stability.
A previous attempt at cartilage repair is often an exclusion criterion for a subsequent cartilage regenerative procedure. This is driven by the likelihood of subchondral bone changes as a consequence of failed repair, particularly if the first attempt involved disruption of the tidemark for marrow recruitment. Without sufficient subchondral load support, subsequent cartilage treatment approaches may be predisposed to failure, and thus, the entire osteochondral unit must be considered in this cohort of patients. One potential intervention, currently used clinically, is osteochondral allograft transplantation (OATS).98,99 These grafts can provide symptom relief and success as a salvage procedure following failed cartilage repair. However, issues with graft survivability, disease transmission, and integration have motivated tissue engineers to develop newer composite scaffolds that guide localized regeneration of the cartilage and bone layers of an osteochondral unit.100,101 Clinically, the TruFit Plug (Smith & Nephew, San Antonio TX) is one of the only synthetic osteochondral scaffolds that has been evaluated in patients,102,103 with a subchondral phase containing calcium sulfate for bone regeneration and an articulating phase that relied on marrow stimulation for cartilage regeneration. While short-term results showed clinical improvement in patient MRI scores, improvement over conventional microfracture/OATS procedures has not been proven. In order to improve outcomes, biphasic scaffolds can be created with a softer upper layer containing chondrogenic factors (e.g., TGF-β, chondroitin sulfate) and a stiffer lower layer, often loaded with calcium phosphate, hydroxyapatite, or bone morphogenetic protein (BMP), to provide structural support of the above cartilage layer and to promote osteogenic tissue formation and boney integration.53,104,105,106 Bi-layered scaffolds derived from articular cartilage ECM and growth plate ECM can be used to regenerate osteochondral tissue with better recapitulation of the native architecture.107
To avoid the interfacial shear stress that are experienced between two such distinct layers, recent studies have developed gradient scaffolds, using the same growth factors and scaffold materials as a biphasic scaffold, but with a smooth transition between layers.108,109 For example, Di Luca et al.109 used a brush functionalization technique to create a gradient of TGF-β3, decreasing in concentration from the articulating surface to the subchondral region, and likewise created a reverse gradient of BMP-2. Other studies have utilized growth factor gradients, for example via microsphere incorporation.108,110 Furthermore, a transitional zone between cartilage and bone layers has been achieved via dispersion mixing with syringe pump systems, selective laser sintering, and pore shape gradients.111,112,113,114 The field of osteochondral tissue engineering has matured substantially and progress towards clinically applicable replacements will be vital for the large portion of the population currently excluded from clinical trials.
Patients with inflammation of the joint are often excluded from cartilage repair attempts given that catabolic cytokines and proteinases (MMPs) present in the synovial fluid degrade the native ECM, and would similarly degrade any implanted tissue, predisposing treatment failure. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) are two important inflammatory cytokines that not only lead to cartilage matrix destruction, but also prevent chondrogenic differentiation of mesenchymal stem cells.115 These cytokines can be systemically upregulated, or produced by synoviocytes, chondrocytes, or meniscal cells.116 Also, co-morbidities, such as obesity, can elevate levels of pro-inflammatory cytokines, exacerbating the effects of a joint injury.117
To control the inflammatory environment and reduce proteolysis, intra-articular injection is clinically appealing. Such a treatment could be administered following a traumatic joint injury to prevent cartilage ECM proteolysis in the presence of pro-inflammatory cytokines (IL-1, IL-6, IL-8, and TNF-ɑ) and MMPs. To address this possibility, in a number of animal118,119 and human studies,120 high doses of anti-catabolic glucocorticoids, which inhibit the activation of MMPs121 and the expression inflammatory cytokines,122 have been administered. Dexamethasone (DEX) inhibits inflammation and cartilage damage by influencing both synoviocytes and chondrocytes.119,123 However, given the dynamic environment of the knee and the low residence time of small molecules in the synovial space, delivery and retention of molecules to positively impact cartilage regeneration before being cleared remains a challenge. Targeted intra-articular delivery of DEX can be achieved by using small positively charged nanoparticles bound to DEX to form electrostatic interactions between the cationic particle and the anionic cartilage matrix.124 Dendrimer-based nanocarriers were also recently shown to penetrate full-thickness cartilage explants, and be retained in a native joint environment.125 These nanoparticles may be powerful carriers for a glucocorticosteroid treatment.
In addition to localized glucocorticosteroid delivery, IL-1 receptor antagonist (IL-1Ra), a naturally occurring inhibitor of IL-1 activity, is another potential avenue for intra-articular anti-inflammatory therapeutics. There are currently multiple IL-1 targeting drugs on the market including Anakinra, a modified version of the human IL-Ra protein, and Canakinumab, a monoclonal antibody targeted at IL-1β. However, neither of these drugs has improved OA symptoms in human clinical trials to date, likely because of the dynamic joint environment.126,127 For either glucocorticosteroid or IL-1Ra delivery to be successful, the delivery mechanism (nanoparticles, scaffolds, etc.) is an extremely important design consideration. To improve the efficacy of IL-1Ra, researchers have tethered the protein to nanoscale block polymers to target cartilage.128,129 The IL-1Ra tethered polymeric nanoparticles were stable, non-toxic, and effective at blocking the IL-1 signaling pathway. In another study, IL-1Ra transgenes were incorporated into a woven scaffold.61 As a result, the stem cells in the scaffold released IL-1Ra in a sustained fashion over the course of 28 days in culture. Gene delivery of IL-1Ra through a regenerative scaffold or direct delivery of IL-1Ra via nanoparticle carriers are both promising options for cartilage repair in patients with post-surgical inflammation and other chronic inflammatory conditions.
Conclusions and future outlook
Articular cartilage injuries are prevalent in a large portion of the population, and their incidence is only increasing. Cartilage regeneration technology has the potential to repair these lesions and prevent their progression to debilitating OA requiring total joint arthroplasty. Yet, the number of knee cartilage repair and regeneration procedures performed annually is dwarfed by the volume of total knee replacements. Current cartilage regeneration treatments approved for clinical use (MACI, AMIC) have shown satisfactory results in an optimal subset of patients with “green knees”. However, clinical trials assessing the efficacy of these techniques exclude the majority of the patient population with “red knees”, patients with large lesion size, high mechanical demands, older age, inflammation, infection, and other systemic diseases. Broadening the eligibility criteria of new trials would make the results more representative of the entire patient population. Following the footsteps of modern oncology research,130 cartilage regeneration trials should strive for inclusiveness. Exclusion criteria should have clear rational focusing on potential toxicities rather than efficacy concerns. Easier patient recruitment will allow analysis of larger and more representative populations, while subgroup analysis may help elucidate efficacy in a more selective “green knee” population. For example, including patients with common comorbidities would better highlight which conditions compromise cartilage restorative techniques and which do not. An alternative is to conduct secondary clinical trials in specific excluded populations, as is currently being done with MACI in pediatric patients.131
Translational research in the field of cartilage tissue engineering is working to improve treatment options (Fig. 5) to address comorbidities and reduce the number of excluded patients with cartilage lesions. Growth factors and other drugs released from scaffold materials can be used to recruit and rejuvenate cartilage progenitor cells in elderly patients. Scaffold material and fabrication technique can be tuned to provide greater surface area coverage and mechanical support. Targeted delivery of anti-inflammatory drugs may also improve scaffold integration and maturation in patients with inflammatory comorbidities.
Even more important than research in this field is the advancement from pre-clinical to clinical trials. The high cost, stringent regulatory oversight, and decades involved in the execution of rigorous clinical trials create barriers for advancement of this technology. Collaboration between large research groups and clinicians is key to the safe and successful progression of cartilage regeneration therapy, with the ultimate goal of providing all patients who have a cartilage injury with the opportunity for repair to conserve their joint rather than replace it.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The inclusion and exclusion criteria data that support the findings of this study are available from ClinicalTrials.gov “https://clinicaltrials.gov/”. The synthesized raw data is also available upon request.
References
Smith, G. D., Knutsen, G. & Richardson, J. B. & of Orthopaedics Robert Jones, P. A clinical review of cartilage repair techniques. J. Bone Jt. Surg. 87, 445–449 (2005).
Curl, W. W. et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 13, 456–460 (1997).
Widuchowski, W., Widuchowski, J. & Trzaska, T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14, 177–182 (2007).
Mor, A. et al. Trends in arthroscopy-documented cartilage injuries of the knee and repair procedures among 15-60-year-old patients. Scand. J. Med. Sci. Sport. 25, e400–e407 (2015).
Gomoll, A. Microfracture and augments. J. Knee Surg. 25, 009–016 (2012).
Carey, J. L. Fibrocartilage following microfracture is not as robust as native articular cartilage. J. Bone Jt. Surg.-Am. 94, e80–e81 (2012).
Devitt, B. M., Bell, S. W., Webster, K. E., Feller, J. A. & Whitehead, T. S. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee (2017). https://doi.org/10.1016/j.knee.2016.12.002
van der Linden, M. H., Saris, D. B. F., Bulstra, S. K. & Buma, P. Treatment of cartilaginous defects in the knee: recommendations from the Dutch Orthopaedic Association. Ned. Tijdschr. Geneeskd. 157, A5719 (2013).
Patel, J. M., Saleh, K. S., Burdick, J. A. & Mauck, R. L. Bioactive factors for cartilage repair and regeneration: improving delivery, retention, and activity. Acta Biomater. (2019). https://doi.org/10.1016/j.actbio.2019.01.061
McCormick, F. et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthrosc. - J. Arthrosc. Relat. Surg. 30, 222–226 (2014).
Inacio, M. C. S., Paxton, E. W., Graves, S. E., Namba, R. S. & Nemes, S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthr. Cartil. 25, 1797–1803 (2017).
Sloan, M., Premkumar, A. & Sheth, N. P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. - Am. 100, 1455–1460 (2018).
Aae, T. F., Randsborg, P.-H., Lurås, H., Årøen, A. & Lian, Ø. B. Microfracture is more cost-effective than autologous chondrocyte implantation: a review of level 1 and level 2 studies with 5 year follow-up. Knee Surg., Sports. Traumatol. Arthrosc. 26, 1044–1052 (2018).
Kon, E. et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Am. J. Sports Med. 37, 33–41 (2009).
Crawford, D. C., DeBerardino, T. M. & Williams, R. J. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions. J. Bone Jt. Surg.-Am. 94, 979–989 (2012).
Wylie, J. D., Hartley, M. K., Kapron, A. L., Aoki, S. K. & Maak, T. G. What is the effect of matrices on cartilage repair? A systematic review. Clin. Orthop. Relat. Res. 473, 1673–1682 (2015).
Basad, E. et al. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg., Sport. Traumatol. Arthrosc. 18, 519–527 (2010).
Volz, M., Schaumburger, J., Frick, H., Grifka, J. & Anders, S. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int. Orthop. 41, 797–804 (2017).
Wylie, J. D., Hartley, M. K., Kapron, A. L., Aoki, S. K. & Maak, T. G. Failures and reoperations after matrix-assisted cartilage repair of the knee: a systematic review. Arthroscopy. 32, 386–392 (2016).
Brix, M. O. et al. Treatment of full-thickness chondral defects with Hyalograft C in the knee. Am. J. Sports Med. 42, 1426–1432 (2014).
Filardo, G. et al. Matrix-assisted autologous chondrocyte transplantation for cartilage regeneration in osteoarthritic knees. Am. J. Sports Med. 41, 95–100 (2013).
Filardo, G. et al. Clinical profiling in cartilage regeneration. Am. J. Sports Med. 42, 898–905 (2014).
Lotz, M. & Loeser, R. F. Effects of aging on articular cartilage homeostasis. Bone 51, 241–248 (2012).
Stolzing, A., Jones, E., McGonagle, D. & Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 129, 163–173 (2008).
Brady, K., Dickinson, S. C. & Hollander, A. P. Changes in chondrogenic progenitor populations associated with aging and osteoarthritis. Cartilage 6, 30S–35SS (2015).
Mithoefer, K., Mcadams, T., Williams, R. J., Kreuz, P. C. & Mandelbaum, B. R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am. J. Sports Med. 37, 2053–2063 (2009).
Grumet, R. C., Bajaj, S. & Cole, B. J. Chapter 27 - Failed Cartilage Repair. Insa. Scott Surg. Knee 221–228.e1 (2011). https://doi.org/10.1016/B978-1-4377-1503-3.00027-5
Pestka, J. M. et al. Revision surgery after cartilage repair: data From the German Cartilage Registry (KnorpelRegister DGOU). Orthop. J. Sport. Med. 6, 2325967117752623 (2018).
Flegal, K. M., Carroll, M. D., Kit, B. K. & Ogden, C. L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307, 491 (2012).
Gianotti, S. M., Marshall, S. W., Hume, P. A. & Bunt, L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J. Sci. Med. Sport 12, 622–627 (2009).
Abrams, G. D. et al. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am. J. Sports Med. 41, 2333–2339 (2013).
Roos, H. et al. Knee osteoarthritis after meniscectomy prevalence of radiographic changes after twenty-one years, compared with matched controls. ARTHRITIS Rheum. 41, 687–693 (1098).
Lotz, M. Cytokines in cartilage injury and repair. Clin. Orthop. Relat. Res. 391(Suppl), S108–S115 (2001).
Guilak, F. et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin. Orthop. Relat. Res. 423, 17–26 (2004).
Goldring, M. B. et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell. Mater. 21, 202–220 (2011).
Mohanraj, B. et al. Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro-inflammatory cytokines. J. Orthop. Res. 36, 2901–2910 (2018).
San-Mi, Y., Hyung-Ki, P., Sung-Jin, C., Jae-Chil, C. & Ra-Sun, K. The current analysis of the risk factors for bone graft infection after cranioplasty. Korean J. Neurotrauma 9, 57–63 (2013).
Pashneh-Tala, S., MacNeil, S. & Claeyssens, F. The tissue-engineered vascular graft—past, present, and future. Tissue Eng. Part B Rev. 22, 68–100 (2016).
Humphers, J. M., Shibuya, N., Fluhman, B. L. & Jupiter, D. The impact of glycosylated hemoglobin and diabetes mellitus on wound-healing complications and infection after foot and ankle surgery. J. Am. Podiatr. Med. Assoc. 104, 320–329 (2014).
Noordin, S. & Glowacki, J. Parathyroid hormone and its receptor gene polymorphisms: implications in osteoporosis and in fracture healing. Rheumatol. Int. 36, 1–6 (2016).
Kudo, S., Mizuta, H., Otsuka, Y., Takagi, K. & Hiraki, Y. Inhibition of chondrogenesis by parathyroid hormone in vivo during repair of full-thickness defects of articular cartilage. J. Bone Miner. Res. 15, 253–260 (2010).
Ketteler, M., Rothe, H., Krüger, T., Biggar, P. H. & Schlieper, G. Mechanisms and treatment of extraosseous calcification in chronic kidney disease. Nat. Rev. Nephrol. 7, 509–516 (2011).
Oladeji, L. O. et al. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am. J. Sports Med. 45, 2797–2803 (2017).
Roy, S. et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J. Neuroimmune Pharmacol. 6, 442–465 (2011).
Fukushima, W. et al. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 468, 2715–2724 (2010).
Azar, M. M., Springer, S. A., Meyer, J. P. & Altice, F. L. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 112, 178–193 (2010).
Turinetto, V., Vitale, E. & Giachino, C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 17, E1164 (2016).
Fehrer, C. & Lepperdinger, G. Mesenchymal stem cell aging. Exp. Gerontol. 40, 926–930 (2005).
Gigante, A., Bevilacqua, C., Ricevuto, A., Mattioli-Belmonte, M. & Greco, F. Membrane-seeded autologous chondrocytes: Cell viability and characterization at surgery. Knee Surg., Sport. Traumatol. Arthrosc. 15, 88–92 (2007).
Yu, Y. et al. Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 67, 1274–1285 (2015).
Purcell, B. P., Elser, J. A., Mu, A., Margulies, K. B. & Burdick, J. A. Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials 33, 7849–7857 (2012).
Kim, I. L. et al. Fibrous scaffolds with varied fiber chemistry and growth factor delivery promote repair in a porcine cartilage defect model. Tissue Eng. Part A 21, 2680–2690 (2015).
Re’em, T., Witte, F., Willbold, E., Ruvinov, E. & Cohen, S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater. 8, 3283–3293 (2012).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Liu, X. et al. SRT1720 promotes survival of aged human mesenchymal stem cells via FAIM: a pharmacological strategy to improve stem cell-based therapy for rat myocardial infarction. Cell Death Dis. 8, e2731 (2017).
Mouser, V. H. M. et al. Three-dimensional bioprinting and its potential in the field of articular cartilage regeneration. Cartilage 8, 327–340 (2017).
Ballyns, J. J. et al. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng. Part A 14, 1195–1202 (2008).
Rowland, C. R., Colucci, L. A. & Guilak, F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials 91, 57–72 (2016).
Rengier, F. et al. 3D printing based on imaging data: review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 5, 335–341 (2010).
Lee, C. H. et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376, 440–448 (2010).
Moutos, F. T. et al. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl Acad. Sci. USA 113, E4513–E4522 (2016).
Moutos, F. T., Freed, L. E. & Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 6, 162–167 (2007).
Saxena, V. et al. Anatomic mesenchymal stem cell-based engineered cartilage constructs for biologic total joint replacement. Tissue Eng. Part A 22, 386–395 (2016).
Fortier, L. A. et al. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am. J. Sports Med. 44, 2366–2374 (2016).
Beck, E. C. et al. Chondroinduction from naturally derived cartilage matrix: a comparison between devitalized and decellularized cartilage encapsulated in hydrogel pastes. Tissue Eng. Part A 22, 665–679 (2016).
Asik, M., Ciftci, F., Sen, C., Erdil, M. & Atalar, A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthrosc. J. Arthrosc. Relat. Surg. 24, 1214–1220 (2008).
Behery, O., Siston, R. A., Harris, J. D. & Flanigan, D. C. Treatment of cartilage defects of the knee. Clin. J. Sport Med. 24, 21–30 (2014).
Kreuz, P. C. et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 14, 1119–1125 (2006).
Ren, K., He, C., Xiao, C., Li, G. & Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 51, 238–249 (2015).
Yu, F. et al. An injectable hyaluronic acid/PEG hydrogel for cartilage tissue engineering formed by integrating enzymatic crosslinking and Diels–Alder “click chemistry”. Polym. Chem. 5, 1082–1090 (2014).
Uttayarat, P. et al. Photopolymerization of hydrogels for cartilage tissue engineering. in 2015 8th Biomedical Engineering International Conference (BMEiCON) 1–3 (IEEE, 2015). https://doi.org/10.1109/BMEiCON.2015.7399526
Papadopoulos, A. et al. Injectable and photopolymerizable tissue-engineered auricular cartilage using poly(ethylene glycol) dimethacrylate copolymer hydrogels. Tissue Eng. Part A 17, 161–169 (2011).
Chen, F. et al. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 166, 31–44 (2017).
Chen, F. et al. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 6, 20014 (2016).
Jin, R. et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 30, 2544–2551 (2009).
Chang, C., Lauffenburger, D. A. & Morales, T. I. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthr. Cartil. 11, 603–612 (2003).
Lü, S. et al. Injectable and self-healing carbohydrate-based hydrogel for cell encapsulation. ACS Appl. Mater. Interfaces 7, 13029–13037 (2015).
Loebel, C., Rodell, C. B., Chen, M. H. & Burdick, J. A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 12, 1521–1541 (2017).
Friedman, J. M. et al. Comparison of fixation techniques of 3d-woven poly(ε-caprolactone) scaffolds for cartilage repair in a weightbearing porcine large animal model. Cartilage 194760351770095 (2017). https://doi.org/10.1177/1947603517700953
Visser, J. et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 6, 6933 (2015).
Hung, K. C., Tseng, C. S., Dai, L. G. & Hsu, Shui Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 83, 156–168 (2016).
Bian, L. et al. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 34, 413–421 (2013).
Rowland, C. R., Lennon, D. P., Caplan, A. I. & Guilak, F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials 34, 5802–5812 (2013).
Ko, C.-S., Huang, J.-P., Huang, C.-W. & Chu, I.-M. Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J. Biosci. Bioeng. 107, 177–182 (2009).
Davidenko, N. et al. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 25, 131–142 (2015).
Valonen, P. K. et al. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(ɛ-caprolactone) scaffolds. Biomaterials 31, 2193–2200 (2010).
Kundanati, L. et al. Fabrication and mechanical characterization of hydrogel infused network silk scaffolds. Int. J. Mol. Sci. 17, E1631 (2016).
Akalp, U., Bryant, S. J. & Vernerey, F. J. Tuning tissue growth with scaffold degradation in enzyme-sensitive hydrogels: a mathematical model. Soft Matter 12, 7505–7520 (2016).
Ng, K. W., Kugler, L. E., Doty, S. B., Ateshian, G. A. & Hung, C. T. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthr. Cartil. 17, 220–227 (2009).
Bian, L. et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425–6434 (2011).
Almekinders, L. C., Pandarinath, R. & Rahusen, F. T. Knee stability following anterior cruciate ligament rupture and surgery. The contribution of irreducible tibial subluxation. J. Bone Jt. Surg. Am. 86–A, 983–987 (2004).
Shelburne, K. B., Pandy, M. G. & Torry, M. R. Comparison of shear forces and ligament loading in the healthy and ACL-deficient knee during gait. J. Biomech. 37, 313–319 (2004).
Sell, T. C. et al. Predictors of proximal tibia anterior shear force during a vertical stop-jump. J. Orthop. Res 25, 1589–1597 (2007).
Iwasa, K. & Reddi, A. H. Optimization of methods for articular cartilage surface tissue engineering: cell density and transforming growth factor beta are critical for self-assembly and lubricin secretion. Tissue Eng. Part C. Methods 23, 389–395 (2017).
Lin, H.-Y., Tsai, W.-C. & Chang, S.-H. Collagen-PVA aligned nanofiber on collagen sponge as bi-layered scaffold for surface cartilage repair. J. Biomater. Sci. Polym. Ed. 28, 664–678 (2017).
Camarero-Espinosa, S., Rothen-Rutishauser, B., Weder, C. & Foster, E. J. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 74, 42–52 (2016).
Accardi, M. A. et al. Effects of fiber orientation on the frictional properties and damage of regenerative articular cartilage surfaces. Tissue Eng. Part A 19, 2300–2310 (2013).
Gracitelli, G. C., Meric, G., Pulido, P. A., McCauley, J. C. & Bugbee, W. D. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage 6, 98–105 (2015).
Bugbee, W. D., Pallante-Kichura, A. L., Görtz, S., Amiel, D. & Sah, R. Osteochondral allograft transplantation in cartilage repair: graft storage paradigm, translational models, and clinical applications. J. Orthop. Res. 34, 31–38 (2016).
Chen, G., Sato, T., Tanaka, J. & Tateishi, T. Preparation of a biphasic scaffold for osteochondral tissue engineering. Mater. Sci. Eng. C. 26, 118–123 (2006).
Bi, L. et al. Fabrication and characterization of a biphasic scaffold for osteochondral tissue engineering. Mater. Lett. 65, 2079–2082 (2011).
Verhaegen, J. et al. TruFit plug for repair of osteochondral defects-where is the evidence? Systematic review of literature. Cartilage 6, 12–19 (2015).
D’Ambrosi, R., Giacco, F., Ragone, V. & Ursino, N. Arthroscopic treatment of osteochondral knee defects with resorbable biphasic synthetic scaffold: clinical and radiological results and long-term survival analysis. Int. Orthop. (2018). https://doi.org/10.1007/s00264-018-4270-7
Levingstone, T. J. et al. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 32, 149–160 (2016).
Mohan, N. et al. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng. Part A 17, 2845–2855 (2011).
Mohan, N., Gupta, V., Sridharan, B., Sutherland, A. & Detamore, M. S. The potential of encapsulating ‘raw materials’ in 3D osteochondral gradient scaffolds. Biotechnol. Bioeng. 111, 829–841 (2014).
Kelly, D. J. et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 188, 63–73 (2018).
Mohan, N. et al. Microsphere-based gradient implants for osteochondral regeneration: a long-term study in sheep. Regen. Med. 10, 709–728 (2015).
Di Luca, A., Van Blitterswijk, C. & Moroni, L. The osteochondral interface as a gradient tissue: from development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res. Part C. Embryo Today Rev. 105, 34–52 (2015).
Gupta, V., Mohan, N., Berkland, C. J. & Detamore, M. S. Microsphere-based scaffolds carrying opposing gradients of chondroitin sulfate and tricalcium phosphate. Front. Bioeng. Biotechnol. 3, 96 (2015).
Dormer, N. H. et al. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J. Biomed. Mater. Res. A 100, 162–170 (2012).
Di Luca, A. et al. Osteochondral regeneration: tuning cell differentiation into a 3D scaffold presenting a pore shape gradient for osteochondral regeneration. Adv. Healthc. Mater. 5, 1832 (2016).
Du, Y. et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 137, 37–48 (2017).
Tang, G. et al. Preparation of PLGA scaffolds with graded pores by using a gelatin-microsphere template as porogen. J. Biomater. Sci. Polym. Ed. 23, 2241–2257 (2012).
Wehling, N. et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 60, 801–812 (2009).
Favero, M. et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: a coculture study. J. Cell. Physiol. 234, 11176–11187 (2018).
Wang, T. & He, C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 44, 38–50 (2018).
Ishida, Y. & Heersche, J. N. M. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J. Bone Miner. Res. 13, 1822–1826 (1998).
Huebner, K. D., Shrive, N. G. & Frank, C. B. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J. Orthop. Res. 32, 566–572 (2014).
Moghadam-Kia, S. & Werth, V. P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 49, 239–248 (2010).
Richardson, D. W. & Dodge, G. R. Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treated articular chondrocytes. Inflamm. Res. 52, 39–49 (2003).
Uddin, M. N., Siddiq, A., Oettinger, C. W. & D’Souza, M. J. Potentiation of pro-inflammatory cytokine suppression and survival by microencapsulated dexamethasone in the treatment of experimental sepsis. J. Drug Target. 19, 752–760 (2011).
Roach, B. L. et al. Dexamethasone release from within engineered cartilage as a chondroprotective strategy against interleukin-1α. Tissue Eng. Part A 22, 621–632 (2016).
Bajpayee, A. G., Quadir, M. A., Hammond, P. T. & Grodzinsky, A. J. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthr. Cartil. 24, 71–81 (2016).
Geiger, B. C., Wang, S., Padera, R. F., Grodzinsky, A. J. & Hammond, P. T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 10, eaat8800 (2018).
Mb, C. Canakinumab in the treatment of erosive hand osteoarthritis: a case series. J. Case Rep. Stud. 3, 503 (2015).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009).
Whitmire, R. E. et al. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials 33, 7665–7675 (2012).
Agarwal, R. et al. Synthesis of self-assembled IL-1Ra-presenting nanoparticles for the treatment of osteoarthritis. J. Biomed. Mater. Res. Part A 104, 595–599 (2016).
Kim, E. S. et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J. Clin. Oncol. 35, 3737–3744 (2017).
Vericel Corporation. A study of MACI in patients aged 10 to <17 years with symptomatic chondral or osteochondral defects of the knee (PEAK). ClinicalTrials.gov (2018). https://clinicaltrials.gov/ct2/show/NCT03588975?term=maci+child&recrs=abdef&rank=1. Accessed 27 Dec 2018.
Lu, S. et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 35, 8829–8839 (2014).
Cheng, Z. et al. 3D printing hydrogel with graphene oxide is functional in cartilage protection by influencing the signal pathway of Rank/Rankl/OPG. Mater. Sci. Eng. C 82, 244–252 (2018).
Crecente-Campo, J., Borrajo, E., Vidal, A. & Garcia-Fuentes, M. New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation. Eur. J. Pharm. Biopharm. 114, 69–78 (2017).
Lohmander, L. S. et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 66, 1820–1831 (2014).
Howard, D., Wardale, J., Guehring, H. & Henson, F. Delivering rhFGF-18 via a bilayer collagen membrane to enhance microfracture treatment of chondral defects in a large animal model. J. Orthop. Res. 33, 1120–1127 (2015).
Sundararaj, S. K. C., Cieply, R. D., Gupta, G., Milbrandt, T. A. & Puleo, D. A. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J. Tissue Eng. Regen. Med. 9, E202–E209 (2015).
Florine, E. M. et al. Delivering Heparin-Binding Insulin-Like Growth Factor 1 with Self-Assembling Peptide Hydrogels. Tissue Eng. Part A 21, 637–646 (2015).
Zhang, F., Leong, W., Su, K., Fang, Y. & Wang, D.-A.A transduced living hyaline cartilage graft releasing transgenic stromal cell-derived factor-1 inducing endogenous stem cell homing in vivo. Tissue Eng. Part A 19, 1091–1099 (2013).
Lee, B. INVOSSA, a first-in-class of cell and gene therapy for osteoarthritis treatment: the phase III trial. Osteoarthr. Cartil. 26, S43–S44 (2018).
Fedorovich, N. E. et al. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng. Part C Methods 18, 33–44 (2011).
Younesi, M., Goldberg, V. M. & Akkus, O. A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration. Acta Biomater. (2016). https://doi.org/10.1016/j.actbio.2015.11.024.
Ford, A. C. et al. A modular approach to creating large engineered cartilage surfaces. J. Biomech. 67, 177–183 (2018).
Acknowledgements
Research reported in this publication was supported by the University of Pennsylvania and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. Additional support was provided by grants from the American Orthopaedic Society for Sports Medicine and the National Institutes of Health (R01 EB008722). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or other funding agencies.
Author information
Authors and Affiliations
Contributions
All listed authors had substantial contribution to the conceptualization, writing, and editing of this article. A.R.M. performed the systematic review, and wrote the Abstract, Introduction, Disease Burden and Treatment Trends, Defining the “Red Knee”, Aging, and Conclusions sections. J.M.P. performed the systematic mechanical property review, and wrote the Small Lesions, Increased Mechanical Demands, and Subchondral Bone Damage sections. H.M.Z. wrote the Large Lesions and Systemic Disease/Inflammation sections. J.L.C. provided clinical insight into disease burden, exclusion criteria, and clinical trial review. R.L.M. supervised the development of all stages of this work, and identified many of the regenerative approaches addressed, and revised and approved the submission.
Corresponding author
Ethics declarations
Competing interests
J.L.C. receives research support from AlloSource, Anika Therapeutics, and Vericel Corporation. J.L.C. is also a paid consultant for Vericel Corporation. These relationships did not influence the subject matter of this review. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martín, A.R., Patel, J.M., Zlotnick, H.M. et al. Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations. npj Regen Med 4, 12 (2019). https://doi.org/10.1038/s41536-019-0074-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-019-0074-7
This article is cited by
-
From cells to organs: progress and potential in cartilaginous organoids research
Journal of Translational Medicine (2023)
-
3D Bioprinting Strategies for Articular Cartilage Tissue Engineering
Annals of Biomedical Engineering (2023)
-
Towards Clinical Translation of In Situ Cartilage Engineering Strategies: Optimizing the Critical Facets of a Cell-Laden Hydrogel Therapy
Tissue Engineering and Regenerative Medicine (2023)
-
Clinical and quality-of-life outcomes of a combined synthetic scaffold and autogenous tissue graft procedure for articular cartilage repair in the knee
Journal of Orthopaedic Surgery and Research (2022)
-
Systematic study of single-cell isolation from musculoskeletal tissues for single-sell sequencing
BMC Molecular and Cell Biology (2022)