Abstract
Acute myocardial infarction and chronic heart failure rank among the major causes of morbidity and mortality worldwide. Except for heart transplantation, current therapy options only treat the symptoms but do not cure the disease. Stem cell-based therapies represent a possible paradigm shift for cardiac repair. However, most of the first-generation approaches displayed heterogeneous clinical outcomes regarding efficacy. Stemming from the desire to closely match the target organ, second-generation cell types were introduced and rapidly moved from bench to bedside. Unfortunately, debates remain around the benefit of stem cell therapy, optimal trial design parameters, and the ideal cell type. Aiming at highlighting controversies, this article provides a critical overview of the translation of first-generation and second-generation cell types. It further emphasizes the importance of understanding the mechanisms of cardiac repair and the lessons learned from first-generation trials, in order to improve cell-based therapies and to potentially finally implement cell-free therapies.
Similar content being viewed by others
Introduction
Myocardial infarction (MI) mortality decrease1 has contributed with an aging population to the rise of heart failure (HF) incidence.1 After MI, cardiomyocyte death triggers wall thinning, ventricular dilatation, and fibrosis that can cause left ventricular (LV) dysfunction and HF.2 HF counts 30 million patients1 and a ~50% death rate within 5 years post diagnosis.3 Pharmacological therapies and revascularization techniques (e.g., percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG)) have improved patient survival and quality of life, but cannot stop or reverse HF. The heart can ultimately be supported by left ventricular assist devices or replaced by transplantation, but organ shortage, high costs, and complex postoperative management limit these strategies. Hence, novel curative treatments are needed.
Stem cell therapy has been proposed for heart repair and regeneration. The exact mechanisms of cardiac repair by transplanted cells are merely unknown. Two main hypotheses exist: (1) direct cardiomyogenic/vasculogenic differentiation, and (2) indirect stimulation of the reparative response through paracrine effects.4
Different cell types are under evaluation regarding their regenerative potential. First-generation cell types including skeletal myoblasts (SMs), bone marrow mononuclear cells (BMMNCs), hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), and mesenchymal stem cells (MSCs) were initially introduced. Despite promising preclinical studies, first-generation approaches displayed heterogeneous clinical outcomes.4, 5 Variations between trials may be attributed to differences in design (cell preparation, delivery route, timing, dose, endpoints, and follow-up (FU) methods). Well-conducted recent meta-analyses reviewed the efficacy of (mostly first-generation) cell-based approaches and came to divergent conclusions.6,7,8
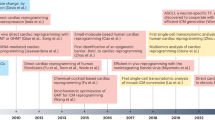
Nevertheless, the field partially switched to second-generation cell types including lineage-guided cardiopoietic cells, cardiac stem/progenitor cells (CSCs/CPCs), and pluripotent stem cells (Fig. 1).
Evolution of translational cardiac regenerative therapies. First-generation cell types such as SMs, BMMNCs, HSCs, EPCs, and MSCs demonstrated feasibility and safety with, however, heterogeneous outcomes and limited efficacy in the clinical setting. In order to better match the target organ, second-generation cell therapies propose the use of cpMSCs, CSCs/CPCs, and CDCs, and pluripotent stem cells such as ESCs and iPSCs. Next-generation therapies for cardiac repair are directed toward cell enhancement (e.g., biomaterials, 3D cell constructs, cytokines, miRNAs) and cell-free concepts (e.g., growth factors, non-coding RNAs, extracellular vesicles, and direct reprograming)
This article provides a critical overview of the translation of first-generation and second-generation cell types with a particular focus on controversies and debates. It also sheds light on the importance of understanding the mechanisms of cardiac repair and the lessons learned from first-generation trials, in order to improve cell-based therapies and to potentially finally implement cell-free therapies.
First-generation cell types
Skeletal myoblasts
With the goal of remuscularizing the injured heart and based on the inference that force-generating cells would function in the cardiac milieu and increase cardiac contractility, SMs figured among the first cell types to be tested. They can be obtained in high number from autologous skeletal muscle satellite cells by expansion in vitro, can be activated in response to muscle damage in vivo, and are resistant to ischemia.9
SMs in preclinical trials
Initial studies in small and large animals were encouraging, with SMs participating at heart muscle formation.10, 11 However, SMs were shown to not electrophysiological couple to native cardiomyocytes in rodents.12, 13 Indeed, N-cadherin and connexin-43 expression was downregulated after transplantation.12 SMs did not differentiate into cardiomyocytes in rodents,14 but could surprisingly differentiate into myotubes in sheep,15 although these findings could not be replicated. Small and large animal trials were nonetheless further conducted and displayed an improvement of LV function.15,16,17 The involved mechanisms were, however, not understood.
SMs in clinical trials
Despite the mixed outcomes in preclinical trials, SMs were rapidly translated into the clinics with phase-I trials in both MI and HF.18,19,20,21,22,23 Although the transplantation of autologous SMs displayed an arrhythmogenic potential in a phase-I trial of severe ischemic cardiomyopathy (ICM),24 SMs were further implanted in the randomized phase-II MAGIC study (97 patients with severe LV dysfunction).25 However, an increased risk of ventricular arrhythmias potentially due to missing junctional proteins26 stopped SMs investigation. The risk of ventricular arrhythmias is relevant now that pluripotent cell-derived cardiomyocytes aim at re-attempting heart remuscularization.
Bone marrow (BM)-derived cells
Moving away from remuscularization, strategies using stem cells aimed at direct/indirect regeneration. The main stem cell source for these early studies was the BM. Investigated cell types were mostly BMMNCs and their subpopulations including HSCs. Blood-circulating EPCs, probably originating from the BM, were also adopted. BMMNCs have constituted a most often used cell source due to their safety, high availability,27 and facile isolation. HSCs can be isolated via surface markers such as CD34 and CD133. EPCs can simply be harvested from a blood sample.
BM-derived cells in preclinical trials
BMMNCs were among the first cells to be tested in large animals despite inconsistent reports on their mechanism of action. Differentiation into cardiomyocytes was first observed in rodents,28, 29 but was criticized later.30 Large animal preclinical studies yielded promising results with, however, mixed outcomes.31,32,33,34 Regarding HSCs, one of the few existing large animal studies found no evidence of myocardial differentiation of CD34+ HSCs, but showed increased angiogenesis/vasculogenesis, potentially due to paracrine effects on the host vasculature.35 Few large animal and clinical studies were conducted with EPCs and their results were mixed.36,37,38
BM-derived cells in clinical trials
Although BM-derived cells showed encouraging preliminary results, efficacy outcomes were heterogeneous in clinics.4 While some clinical trials showed modest but significant improvement of cardiac function,39,40,41 others did not find significant beneficial effects of cell therapy.42,43,44,45
In acute MI, first-generation cell-based clinical trials were all performed using intracoronary delivery of autologous BMMNCs.46,47,48,49,50 HEBE43, 51 and TOPCARE-AMI52 also investigated other progenitor cells. While certain trials (TOPCARE-AMI,52 REPAIR-AMI,53,54,55 BOOST,56, 57 and FINCELL)41 have shown improvement in LV ejection fraction (LVEF) in cell-treated groups compared to controls, others did not display any significant change (ASTAMI,58 BONAMI,59 Leuven-AMI,60 and HEBE)43, 51 at early FU. At a long-term 5-year FU, the beneficial effect of cell therapy persisted in TOPCARE-AMI61 but not in BOOST,62 which was the first published clinical trial to assess BMMNC injection compared to controls in 60 patients. The REPAIR-AMI trial (NCT00279175) was the largest European cell therapy study of autologous BM-derived progenitor cells with 204 patients. Patients underwent BM aspiration 3–6 days after successful PCI following MI. Either BMMNCs or placebo were infused via intracoronary delivery the following day. Quantitative LV angiography was performed to measure the change in global LVEF between baseline and 4-month FU. At 4 months, the cell-treated group displayed a significantly higher increase in LVEF compared to placebo.53 This improvement in LVEF was sustained at 2-year FU,55 thus contradicting the results of BOOST, where this effect was lost after 18 months.57 The HEBE trial is another large European trial where 200 patients were randomized to treatment with BMMNCs, peripheral blood mononuclear cells (PBMCs) or placebo. No difference between cell-treated groups and control group was shown at 4 months by cardiac MRI (cMRI).43 The 5-year FU after AMI displayed a significantly higher frequency of major clinical cardiovascular adverse events in the PBMC group compared to placebo.51
Similar results to the early FU of HEBE are shown by the SWISS-AMI trial (NCT00355186), where 192 MI patients were assigned to one control and two BMMNC treatment groups. BMMNC groups received intracoronary administration of autologous BMMNC at 5–7 days or 3–4 weeks after MI.63 Cell infusion at either early or late time points did not significantly improve LV function at 4 months as measured by cMRI.64 At 12 months, BMMNC treatment did not improve LV function compared to control. An important drop-out rate limited the results.65
To put an end to the ongoing controversies and to further elucidate the clinical value of intracoronary-delivered autologous BMMNCs, the ongoing BAMI trial (NCT01569178) aims to examine the time from randomization to death for an average time frame of 3 years. This multicenter, randomized, controlled, phase-III study is investigating safety and reduction of all-cause mortality in patients with reduced LV function (LVEF ≤ 45%) after successful PCI following MI. About 3000 patients will be enrolled. While BAMI is expected to either definitely confirm BM cell therapy efficacy or disprove it, the trial is criticized because it may not teach anything new about the mechanism of either outcome.
To tackle the problem of the optimal timing for cell administration, two phase-II trials were performed in MI patients. In TIME (NCT00684021) and LateTIME (NCT00684060), 150 × 106 cells were delivered at 3/7 days66 and 2–3 weeks67 post PCI, respectively. These randomized, double-blind, placebo-controlled trials involved 12066 and 8767 patients, respectively. At 6 months, no significant increase in LVEF was observed in the BMMNC group compared to placebo in both trials.68, 69 Overall, TIME and LateTIME did not find significant benefit of early vs. late BMMNC treatment.68, 69
BM-derived cells have also been investigated in HF,70,71,72,73,74,75,76,77 yielding mixed outcomes as in MI. The phase-II FOCUS-CCTRN trial (NCT00824005) studied the effect of transendocardial NOGA® delivery of 100 × 106 autologous BMMNCs or placebo to 92 patients. BMMNCs did not significantly improve maximal oxygen consumption, LV end-systolic volume (ESV), or reversibility on single-photon emission computed tomography compared to placebo after 6 months.44 These results contradict the positive outcomes previously obtained by the TOPCARE-CHD trial, where intracoronary BMMNC-treated HF patients showed a 2.9% increase in LVEF at 3 months compared to baseline.40, 78 All together, the outcomes of therapy using BM-derived and progenitor cells were heterogeneous and rather disappointing whether it was in MI or HF. Possible reasons may be low cell engraftment and limited differentiation potential,5 suggesting that the modest improvements yielded by cell therapy may be due to paracrine mechanisms rather than direct regeneration.
Mesenchymal stem cells
MSCs are multipotent, plastic-adherent stromal cells that can differentiate into different cell types including adipocytes, chondrocytes, and osteocytes.79 Although controversially discussed, differentiation into cardiomyocytes was shown in experimental studies.80 MSCs may be found in all postnatal organs and the presence of MSCs was shown in mouse hearts.81, 82 Cells positive for W8B2 antigen highly expressing mesenchymal markers have recently been discovered in the human heart.83 Human MSCs have most often been isolated from the BM (BM-MSCs) but can also be obtained from adipose tissue, synovial tissue, umbilical cord, and peripheral blood.80 Besides autologous usages, MSCs were also considered in allogeneic therapies due to their high expansion rate and immunomodulatory properties. Interestingly, MSCs were originally considered immuno-privileged, due to their cytokine secretion and surface antigen expression,80 but conflicting reports from preclinical studies have questioned this property.84 Allogeneic MSCs may lose their immune privilege upon differentiation,85 thus leading to earlier clearance compared to autologous MSCs.86
MSCs in preclinical trials
MSCs have extensively been investigated in vivo.87,88,89,90,91 Preclinical trials have shown that adipose tissue-derived MSCs represent an auspicious cell source with therapeutic potential for cardiac repair.92, 93 BM-derived MSCs were promising in numerous preclinical trials. Regarding efficacy, MSCs were administered to pigs with encouraging outcomes.94,95,96,97
MSCs in clinical trials
Approaches using MSCs are studied with promising results,74, 98,99,100,101,102 but their efficacy needs to be further validated. Preliminary studies on ICM were performed in the POSEIDON trial (NCT01087996). This phase-I/II randomized non-controlled pilot study compared the safety and efficacy of transendocardial delivery of autologous vs. allogeneic BM-MSCs in 30 patients. Three different cell doses (20, 100, and 200 million cells) were tested in both treatment groups. Surprisingly, the lowest dose yielded the best outcomes in terms of LV volumes and LVEF. Moreover, despite its small size, POSEIDON has given preliminary evidence of comparable safety and efficacy between autologous and allogeneic MSCs.103, 104 Larger controlled trials are needed to further investigate MSC efficacy.
Meta-analyses of cell therapy in MI and HF
Meta-analyses of preclinical trials
A compelling meta-analysis of large animal models of ischemic heart disease (IHD) analyzed 52 studies and 888 animals. In addition to confirming the safety of cell therapy, a difference of 7.5% in LVEF at FU compared to controls was found.105 Although BMMNCs and MSCs were the most used cell types, trends suggested that BMMNCs were less effective than other cell types.
A new meta-analysis of large animal studies (82 studies with 1415 animals) in the context of autologous and allogeneic cell therapy for IHD106 showed a significant difference of 8.3% in LVEF and a significant decrease in end-diastolic volume (EDV) between treated and control animals. Similar differences in LVEF were observed for both autologous and allogeneic therapies.
Controversies in meta-analyses of clinical trials
Several meta-analyses have assessed cell therapy in clinical trials. In order to improve the analysis of the safety and efficacy of cell therapy in MI, the first multinational database of individual patient data (IPD) (ACCRUE, NCT01098591) was established.6 ACCRUE contains unbiased data with uniform clinical definitions and parameters. This allows the examination of specific patient subgroups and the identification of predictive factors for the improvement of cell therapy. One thousand two hundred fifty-two IPD from 12 randomized trials of intracoronary cell therapy after MI were analyzed. Although the results showed cell therapy safety, they did not display any efficacy compared to controls and no predictive factors could be identified. Timing/dose of cell therapy and baseline EF did not influence the results. Although the study investigated mainly first-generation cell types, the main limitation was the variety in cell types. The database is still growing but cannot replace large randomized trials, such as the current BAMI trial.6
Interestingly, while several publication-based meta-analyses report an effect of BM-derived cell therapy,107, 108 another recent meta-analysis of cell therapy in MI has further showed no difference between cell-treated and control-groups when the LV parameters were assessed by cMRI.8, 109 Indeed, the endpoints and the FU method can also influence the outcomes. The DAMASCENE study has evidenced that the change in EF might be a problematic endpoint, as a higher number of discrepancies in trial reporting is associated with a better change in EF. It was indeed found that factual discrepancies are present in autologous BM cells trials and that trials having >30 discrepancies showed a mean EF effect size of 7.7%, while trials having no discrepancies (only 5 trials over 49 examined trials) showed a mean EF effect size of −0.4%.110 However, the DAMASCENE study has also been challenged as misleading111 and a meta-analysis has shown significant cell therapy efficacy when the discrepant trials were excluded.112
In HF, a meta-analysis including 31 randomized controlled trials (RCTs) with 1521 patients assessed the safety and efficacy of autologous cell therapy.7 A comparison was performed between cell treatment and placebo/controls. Cell therapy was associated with a significant decrease in mortality and rehospitalization during long-term FU. Furthermore, cell treatment improved LVEF significantly but modestly. HF symptoms, exercise capacity, and quality of life ameliorated significantly. Nevertheless, only half of the examined trials reported blinding and half did not report methods of allocation concealment, thus considerably increasing the performance/selection bias.7 The difference between cell treatment and control groups was indeed eliminated when only double-blind trials were included.7 Therefore, further larger RCTs are necessary to confirm clinical long-term efficacy in HF.
Second-generation cell types
Motivated by the inconsistencies of first-generation cell types, the field has shifted toward the use of other cell types. Second-generation therapies aim at orienting non-resident stem cells, such as MSCs and pluripotent stem cells, toward cardiac differentiation. CSCs/CPCs may further better match the target organ, as they are derived directly from the heart.
Only few experimental studies compared first-generation and second-generation cell types. They found that cardiac-committed cells displayed an improved therapeutic effect as assessed by improved engraftment, cardiac function, angiogenesis, and scar size.113,114,115,116,117
Cardiopoietic MSCs (cpMSCs)
Guided cardiopoiesis using cardiogenic growth factors priming has been introduced and advanced into the clinics. One of the cell sources used for guided cardiopoiesis is autologous BM-MSCs. Cytosolic expression of cardiac transcription factors is induced by simultaneous activation with TGF-β, BMP-4, and Activin-A along with retinoic acid, while their nuclear translocation is prompted by IGF-1 and IL-6.1 FGF-2 and thrombin are further used to maintain cell cycle activity.1
cpMSCs in preclinical trials
The use of cpMSCs in a murine model of chronic ICM has shown therapeutic benefit.118 Large animal trials were missing so far. A first report of safety and efficacy of intramyocardial delivery of human cpMSCs into immunosuppressed pigs with post-infarction LV dysfunction has recently shown promising results,119 including higher EF and reduced infarct size compared to controls. The low cell retention suggested the involvement of paracrine mechanisms in neo-angiogenesis and recruitment of endogenous progenitors. These findings need to be validated in long-term studies.
cpMSCs in clinical trials
Despite the absence of large animal studies, cpMSCs were rapidly introduced into the clinics. The multicenter randomized phase-II C-CURE trial (NCT00810238) investigated the transendocardial injection of cardiopoietic BM-MSCs. A non-significant increase in LVEF compared to baseline was shown in the cell treatment group but not in the control group. Besides indicating clinical feasibility/safety at 2-year FU, the trial claims to display signs of efficacy.120 Of note, two phases were included in the initial design of C-CURE: a safety/feasibility phase and a potential later efficacy phase. However, the trial was limited to the first phase based on advice from regulatory authorities.121
Based on C-CURE data, a progressive translation into the two phase-III CHART trials was initiated. CHART-1 (NCT01768702) is an ongoing controlled multicenter, randomized clinical trial, evaluating cpMSCs in ischemic HF. The trial randomized 315 patients and 271 patients were analyzed for efficacy (120 received cpMSCs and 151 sham control). However, the CHART-1 trial failed to meet its primary efficacy endpoint at 39 weeks.122 The authors identified post hoc a responsive patient subgroup based on baseline HF severity (LV EDV of 200–370 ml). The CHART-2 trial (NCT02317458) will target these type of patients. This further poses the question of which cell type to use depending on patient/pathology.
Cardiac stem/progenitor cells
CSCs/CPCs are derived directly from biopsies of the target organ, and therefore supposedly ensure a perfect match.4 CSCs/CPCs are multipotent, clonogenic, and express stem cell markers such as Sca1123, 124 and c-kit.124, 125 Sca1+ CPC exosomes can inhibit cardiomyocytes apoptosis.126 Of note, there is a lack of an agreed Sca1 equivalent in humans. Several studies have associated c-kit with cardiomyocyte biology.127,128,129,130,131 Although it was shown in rodents that c-kit+ CSCs/CPCs could differentiate into cardiomyocytes,125, 132, 133 this was challenged by lineage-tracing analysis studies that suggested that this phenomenon occurs at a purported functionally insignificant rate.134,135,136,137
Cells derived from cardiac explants can form cardiospheres, which can be dissociated to yield cardiosphere-derived cells (CDCs). All these cell types are thought to possess enhanced regeneration capacity through the stimulation of endogenous cardiac cells and/or paracrine mechanisms. Of interest is also the combination of CSCs/CPCs with MSCs to achieve a synergistic effect.138, 139
CSCs/CPCs in preclinical trials
CSCs/CPCs are studied in the preclinical setting.140,141,142 Interestingly, a recent report showed that overexpression of Pim1 kinase enhanced the cardiac repair potential of human c-kit+ CSCs transplanted into an MI swine model.143 CDCs were administered to pigs with encouraging efficacy outcomes.144, 145 Allogeneic CDCs were transplanted via intracoronary delivery at escalating doses between 5 and 10 million cells in an MI pig model.146 This study showed safety/feasibility and significant cardioprotection with reduced infarct size, microvascular obstruction, and adverse remodeling compared to controls.
Recently, a compelling meta-analysis of CSC therapy in preclinical MI studies has showed an LVEF improvement of 10.7% in cell-treated animals compared to controls.147
Interestingly, MSCs/CSCs combination has yielded encouraging results.138, 139 Transendocardially delivered MSCs and c-kit+ CSCs have showed positive synergistic effects in a swine model after ischemia/reperfusion injury.139 However, these results await further confirmation from additional studies.
CSCs/CPCs in clinical trials
New controversies have also emerged with the second-generation era. Two major phase-I trials assessed cardiac-derived cells for the first time in the clinics. The randomized SCIPIO trial investigated the safety and efficacy of intracoronary c-kit+ CSC therapy in 33 ICM patients (20 treated and 13 controls). About 113 days after CABG, 1 × 106 autologous cells were injected.148 The cell-treated group displayed a significant increase in LVEF at 4 and 12 months.149 After CSC injection, decreases in infarct size of 22.7 and 30.2% were measured at 4 and 12 months, respectively.148, 149 Nevertheless, this trial is subject to an expression of concern by The Lancet.150
The CADUCEUS trial (NCT00893360) is a randomized study of the safety and preliminary efficacy of intracoronary delivery of autologous CDCs in patients with LV dysfunction after MI. CDCs were applied in 17 patients 1.5–3 months after MI with a varying dose of 12.5–25 × 106 cells. Eight patients were assessed as standard care patients.151, 152 No tumor formation, major adverse cardiac events, or deaths were observed after 6 months. While CDC treatment resulted in favorable structural changes (scar mass, viable heart mass, regional contractility, and systolic wall thickening) compared to controls, no changes in EDV, ESV, and LVEF were observed at 6 months.151 At 1-year FU, signs of efficacy were displayed, as measured by reduced scar size and improvement in regional function compared to controls.152 Although CADUCEUS is a well-performed study, its small size prevents judging efficacy.
Further ongoing studies are evaluating the safety and efficacy of CSCs/CPCs. The first study to address the safety and efficacy of allogeneic CDC therapy in phase-II is the ALLSTAR trial (NCT01458405). Enrollment is completed according to the sponsor. Allogeneic CSCs are also currently tested in the CAREMI trial (NCT02439398).
Another interesting hybrid approach is presented by the preliminary phase-I ALCADIA trial (NCT00981006) with autologous CDCs and controlled release of basic fibroblast growth factor to treat ICM. Results have shown increased LVEF and decreased scar size 6 months after treatment.153, 154 However, ALCADIA is a small study (n = 6) without control group and further trials are needed.
To further advance the preclinical CSCs/MSCs combinatorial approach, the phase-II, randomized, placebo-controlled CONCERT-HF trial (NCT02501811) is recruiting participants to investigate the feasibility/safety and effect of autologous BM-MSCs and c-kit+ CSCs delivered by transendocardial injection in ICM subjects.
Although initial clinical results from studies investigating CSCs/CPCs are promising, demonstrating feasibility/safety and signs of efficacy, these cell types must be further assessed in larger cohorts and their mechanisms of cardiac repair must be fully elucidated.
Pluripotent stem cells
Pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), constitute another source for guided cardiac differentiation. ESCs can differentiate into any cell type found in the adult organism. Human ESC-derived cardiomyocytes express cardiac transcription factors and display adult cardiomyocyte phenotype and beating activity in vitro.155 However, common concerns include ethical issues and safety, since residual undifferentiated cells could induce teratoma formation.
iPSCs constitute a potential alternative to ESCs as they display similar characteristics while avoiding the ethical debate. iPSCs are obtained from adult somatic cells by forcing the re-expression of key reprogramming genes. However, many questions and safety issues such as tumor formation remain to be clarified.
Pluripotent stem cells in preclinical trials
Preclinical studies with pluripotent stem cells yielded mixed results depending on the animal model. Differences between rodents and large animals were repeatedly noted. Cardiac-committed mouse ESCs were successfully implanted into sheep, resulting in improved LV function.156 Similar results have also been observed in rodents, but with teratoma formation.155 Human ESC-derived cardiomyocytes were implanted in macaques.157 The infarcted heart was “remuscularized” and cardiomyocytes underwent progressive but incomplete maturation over 3 months. The grafts were vascularized and electrically coupled. Although the electrical coupling was enhanced compared to studies of the same group in rodents, non-fatal ventricular arrhythmias not observed in rodents could be detected in monkeys.157 It should be noted that the findings of this study have been challenged in the literature.158
In several preclinical trials, human ESC-derived CPCs have also been transplanted into small and large animal models of MI, showing improved cardiac function. Cell embedding into a fibrin patch has further improved cell engraftment and efficacy, thus leading to clinical trials.159
Cell sheets made of human iPSC-derived cardiomyocytes were delivered into a swine ICM model.160 Despite low long-term cell survival, no teratoma was observed and cardiac function was improved. Recently, human iPSCs have been differentiated into the three cardiac lineages. Their transplantation in a pig MI model showed cell engraftment and improved cardiac function without ventricular arrhythmias.161 Issues such as rejection and teratoma formation need to be further addressed before advancement into clinics.
Pluripotent stem cells in clinical trials
Witnessed with both apprehension and curiosity by the scientific community, human ESC-based therapy has also recently advanced into the clinics. The proof-of-concept ESCORT trial (NCT02057900) is testing ESC-derived CPCs (CD15+ Isl-1+ progenitors) embedded into a fibrin scaffold. The patch was delivered for the first time into a patient with advanced ischemic HF.162 While preliminary outcomes are promising and show the feasibility of producing clinical-grade ESC-derived CPCs, the forthcoming results of the study are necessary to draw a conclusion.162
Cell enhancement and cell-free approaches: the next generation?
Cell enhancement approaches
Several strategies are investigated to ameliorate the poor performance of transplanted cells. They mainly consist in improving cell retention, survival, coupling, and differentiation. To improve cell retention, scaffold-based and scaffold-free approaches can be used. Scaffolds for cardiac cell therapy include decellularized matrices, injectable biomaterials, and cardiac patches made of synthetic or natural hydrogels.163, 164 Scaffold-free tissue-based constructs such as cell sheets and microtissues also exist.165,166,167 Bispecific antibodies can also be used to link cells to the injured heart.168 The CELLWAVE trial (NCT00326989) used shock-wave therapy to promote homing of BMMNCs in HF patients.169 Survival and angiogenesis can be improved by using pro-survival and angiogenic cytokines or by modification of specific genes.170, 171 Overexpression of N-cadherin and connexin-43 could improve coupling. Cells can also be pre-conditioned in hypoxic conditions172 and differentiation can be enhanced with microRNAs (miRNAs).173, 174
Cell-free approaches
Based on the hypothesis that cell therapy mainly functions through paracrine mechanisms, new strategies propose to skip the cells and only use the supposedly paracrine factors. These approaches mainly include the administration/regulation of growth factors and non-coding RNAs. Following the rationale of in situ modification, direct reprogramming aims to convert scar fibroblasts into cardiomyocyte-like cells.
Administration/regulation of growth factors
Examples of investigated growth factors are the vascular endothelial growth factor (VEGF), the granulocyte-colony stimulating factor (G-CSF), and erythropoietin (Epo). VEGF gene therapy failed to improve perfusion of ischemic myocardium in the NORTHERN clinical trial.175 G-CSF did not display significant improvement in myocardial function compared to placebo in the clinics.176,177,178 While administration of Epo displayed encouraging results with preservation of cardiac function in infarcted mice,179 the phase-III REVIVAL-3 trial (NCT00390832) showed no improvement in LVEF or infarct size compared to placebo at 6 month FU.180 A lack of reduction in infarct size was also documented by other clinical studies with shorter FU times.181, 182 The poor outcomes of growth factor-based approaches may be due to inappropriate dosages and/or the lack of organ selectivity, among others.
Administration/regulation of non-coding RNAs
Non-coding RNAs include miRNAs and long non-coding RNAs. They may represent possible therapeutic targets due to their abundance in the cardiovascular system and their potential function in heart physiology and disease.183 miRNAs have been mainly investigated in mice but also in large animals. In a porcine MI model, local and selective inhibition of miR-92a resulted in enhanced angiogenesis and prevention of adverse remodeling.184 miRNAs are found at the intracellular level but also in extracellular vesicles, such as exosomes. Exosomes also contain mRNAs, proteins and lipids, and are thought to play a role in cell–cell communication and in cardiovascular physiology.185 They are currently investigated as diagnostic markers and their roles may also encompass cardioprotection.186 Human CPC-derived extracellular vesicles have displayed a decrease in cardiomyocyte apoptosis and an increase in angiogenesis and LVEF in acute MI rats.187 Following positive results in vitro and in rodents,188, 189 CDC-derived exosomes were shown to decrease infarct size and preserve LVEF in a recent preclinical study in acute and chronic porcine MI.190 Interestingly, this effect was observed in acute MI with intramyocardial but not with intracoronary injection. Mouse ESC-derived exosomes displayed enhanced cardiac function and repair in infarcted mice.191 Nevertheless, it is challenging to separate the relative contributions of regeneration vs. salvage of existing myocardial tissue.
Extracellular vesicles have raised a great interest. However, several open issues remain to be addressed before they can fully supplant cell-based therapies. For instance, the type of donor cells, the type and size of vesicles, their content and their potential immunogenicity need to be investigated in further detail.
Direct reprogramming
To achieve direct reprogramming, a specific cocktail of transcription factors192,193,194 or miRNAs195 can be used. A recent report has shown the feasibility of direct reprogramming of human fibroblasts into cardiomyocyte-like cells using only small molecules.196 Direct reprogramming of murine fibroblasts into cardiomyocyte-like cells was shown in vitro and in vivo192,193,194 and has opened the way to large animal studies. The field of direct reprogramming is still at its infancy and has to cope with several issues before it can reach clinical translation. Vectors need to safely and efficiently transfect the heart cells without triggering the immune response, which could clear the vectors and the transfected cells. Only fibroblasts and no other neighboring cells should be targeted. Moreover, direct reprogramming approaches need to be tested with human cells and large animal models before they can reach the clinics.
Conclusion
Cell therapy holds potential to tackle MI and HF. Issues such as the cell type, cell number, delivery route, timing, FU periods, and endpoints remain unsolved. The field has rapidly evolved to address in particular the ideal cell type. The first attempts of heart remuscularization with SMs were abandoned due to ventricular arrhythmias. Then, the rationale of direct/indirect regeneration by stem cells was adopted. While first-generation cells such as BM-derived cells and MSCs gave overall promising results in preclinical studies, they yielded heterogeneous efficacy outcomes in the clinics. The reasons include differences in trial design and a too rapid translation despite a lack of understanding of the biological mechanisms. Prominent meta-analyses have reached contradictory results about cell therapy efficacy. Large clinical trials such as BAMI will hopefully settle the discussion. Motivated by the desire to match the target organ, second-generation approaches currently investigate cpMSCs, CSCs/CPCs, and pluripotent stem cells. While encouraging results were displayed in both the preclinical and clinical settings, controversies exist. Notably, cpMSCs entered the clinics without previous large animal trials and CHART-1, which did not meet its primary efficacy endpoint, was initiated without a clear evidence of efficacy in a phase-II trial. CSCs/CPCs are promising but their mode of action should be further investigated. Pluripotent stem cells have made their entrance into the clinics despite a lack of uniformity between small and large animal studies. Their advancement is now followed with both interest and apprehension. And yet, it is crucial to learn from first-generation trials and to gain a better understanding of the mode of action of transplanted cells. Future preclinical trials should not only test safety and efficacy endpoints, but rather specific hypotheses on mechanisms of efficacy.5 The cell type should be carefully selected and fully characterized in terms of viability, function, optimal dose, and timing of administration.5 This knowledge could then also be applied in cell-enhancement strategies. A systematic analysis of the cell secretome could further profit to the translation of cell-free approaches. Besides cell-free techniques, the next-generation approaches in the cell therapy evolution might include the combinatorial cell delivery concept,138 the repeated sequential administration of cells,197 and the use of modified cells for enhanced repair.143 Regardless of the cell type, the main challenges of cell therapy are still the overall poor cell retention and high degree of cell death after transplantation. Until these problems are overcome, the full potential of cell therapy will likely never be realized.
References
Terzic, A. & Behfar, A. Stem cell therapy for heart failure: ensuring regenerative proficiency. Trends Cardiovasc. Med. 26, 395–404 (2016).
Sutton, M. G. & Sharpe, N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101, (2981–2988 (2000).
Mozaffarian, D. et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation 133, E38–E360 (2016).
Behfar, A., Crespo-Diaz, R., Terzic, A. & Gersh, B. J. Cell therapy for cardiac repair—lessons from clinical trials. Nat. Rev. Cardiol. 11, 232–246 (2014).
Madonna, R. et al. Position paper of the European society of cardiology working group cellular biology of the heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J.. doi:10.1093/eurheartj/ehw113 (2016).
Gyongyosi, M. et al. Meta-analysis of cell-based CaRdiac studies (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 116, 1346–1360 (2015).
Fisher, S. A., Doree, C., Mathur, A. & Martin-Rendon, E. Meta-analysis of cell therapy trials for patients with heart failure. Circ. Res. 116, 1361–1377 (2015).
Gyongyosi, M., Wojakowski, W., Navarese, E. P., Moye, L. A. & Investigatorsu, A. Meta-analyses of human cell-based cardiac regeneration therapies: controversies in meta-analyses results on cardiac cell-based regenerative studies. Circ. Res. 118, 1254–1263 (2016).
Menasche, P. Skeletal myoblasts and cardiac repair. J. Mol. Cell. Cardiol. 45, 545–553 (2008).
Murry, C. E., Wiseman, R. W., Schwartz, S. M. & Hauschka, S. D. Skeletal myoblast transplantation for repair of myocardial necrosis. J. Clin. Invest. 98, 2512–2523 (1996).
Taylor, D. A. et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med. 4, 929–933 (1998).
Reinecke, H., MacDonald, G. H., Hauschka, S. D. & Murry, C. E. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J. Cell. Biol. 149, 731–740 (2000).
Roell, W. et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 450, 819–824 (2007).
Reinecke, H., Poppa, V. & Murry, C. E. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J. Mol. Cell. Cardiol. 34, 241–249 (2002).
Ghostine, S. et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation 106, I131–I136 (2002).
Jain, M. et al. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation 103, 1920–1927 (2001).
Gavira, J. J. et al. Repeated implantation of skeletal myoblast in a swine model of chronic myocardial infarction. Eur. Heart J. 31, 1013–1021 (2010).
Menasche, P. et al. Myoblast transplantation for heart failure. Lancet 357, 279–280 (2001).
Siminiak, T. et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am. Heart J. 148, 531–537 (2004).
Dib, N. et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy—four-year follow-up. Circulation 112, 1748–1755 (2005).
Siminiak, T. et al. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trials. Eur. Heart J. 26, 1188–1195 (2005).
Hagege, A. A. et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation 114, I108–I113 (2006).
Gavira, J. J. et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J. Thorac. Cardiovasc. Surg. 131, 799–804 (2006).
Menasché, P. et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 41, 1078–1083 (2003).
Menasche, P. et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117, 1189–1200 (2008).
Abraham, M. R. et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ. Res. 97, 159–167 (2005).
Chen, C. H., Sereti, K. I., Wu, B. M. & Ardehali, R. Translational aspects of cardiac cell therapy. J. Cell. Mol. Med. 19, 1757–1772 (2015).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Kudo, M. et al. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J. Mol. Cell. Cardiol. 35, 1113–1119 (2003).
Murry, C. E. et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668 (2004).
Bel, A. et al. Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution. Circulation 108, II247–II252 (2003).
Moelker, A. D. et al. Reduction in infarct size, but no functional improvement after bone marrow cell administration in a porcine model of reperfused myocardial infarction. Eur. Heart J. 27, 3057–3064 (2006).
de Silva, R. et al. Intracoronary infusion of autologous mononuclear cells from bone marrow or granulocyte colony-stimulating factor-mobilized apheresis product may not improve remodelling, contractile function, perfusion, or infarct size in a swine model of large myocardial infarction. Eur. Heart J. 29, 1772–1782 (2008).
Graham, J. J. et al. Long-term tracking of bone marrow progenitor cells following intracoronary injection post-myocardial infarction in swine using MRI. Am. J. Physiol. Heart Circ. Physiol. 299, H125–H133 (2010).
Zhang, S. et al. Host vascular niche contributes to myocardial repair induced by intracoronary transplantation of bone marrow CD34+progenitor cells in infarcted swine heart. Stem Cells 25, 1195–1203 (2007).
Kawamoto, A. et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107, 461–468 (2003).
Dubois, C. et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J. Am. Coll. Cardiol. 55, 2232–2243 (2010).
Zhang, S. et al. Comparison of various niches for endothelial progenitor cell therapy on ischemic myocardial repair: coexistence of host collateralization and Akt-mediated angiogenesis produces a superior microenvironment. Arterioscler. Thromb. Vasc. Biol. 32, 910–923 (2012).
Schachinger, V. et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J. Am. Coll. Cardiol. 44, 1690–1699 (2004).
Assmus, B. et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 355, 1222–1232 (2006).
Huikuri, H. V. et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur. Heart J. 29, 2723–2732 (2008).
Tendera, M. et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur. Heart J. 30, 1313–1321 (2009).
Hirsch, A. et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur. Heart J. 32, 1736–1747 (2011).
Perin, E. C. et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. J. Am. Med. Assoc. 307, 1717–1726 (2012).
Nasseri, B. A. et al. Autologous CD133+bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur. Heart J. 35, 1263–1274 (2014).
Bartunek, J. et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation 112, I178–I183 (2005).
Dohmann, H. F. R. et al. Multicenter double blind trial of autologous bone marrow mononuclear cell transplantation through intracoronary injection post acute myocardium infarction—MiHeart/AMI study. Trials 9, 41–41 (2008).
Yousef, M. et al. The BALANCE study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 53, 2262–2269 (2009).
Hamshere, S. et al. A randomised double-blind control study of early intracoronary autologous bone marrow cell infusion in acute myocardial infarction (REGENERATE-AMI). BMJ Open 4, e004258 (2014).
Choudry, F. et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trialdagger. Eur. Heart J. 37, 256–263 (2016).
Delewi, R. et al. Long term outcome after mononuclear bone marrow or peripheral blood cells infusion after myocardial infarction. Heart 101, 363–368 (2015).
Assmus, B. et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 106, 3009–3017 (2002).
Schachinger, V. et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221 (2006).
Schachinger, V. et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur. Heart J. 27, 2775–2783 (2006).
Assmus, B. et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 3, 89–96 (2010).
Wollert, K. C. et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148 (2004).
Meyer, G. P. et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (bone marrow transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113, 1287–1294 (2006).
Lunde, K. et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 355, 1199–1209 (2006).
Roncalli, J. et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur. Heart J. 32, 1748–1757 (2011).
Janssens, S. et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367, 113–121 (2006).
Leistner, D. M. et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin. Res. Cardiol. 100, 925–934 (2011).
Meyer, G. P. et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur. Heart J. 30, 2978–2984 (2009).
Surder, D. et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the Swiss Multicenter Intracoronary Stem Cells Study in Acute Myocardial Infarction (SWISS-AMI). Am. Heart J. 160, 58–64 (2010).
Surder, D. et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 127, 1968–1979 (2013).
Sürder, D. et al. Effect of bone marrow-derived mononuclear cell treatment, early or late after acute myocardial infarctionnovelty and significance. Circ. Res. 119, 481–490 (2016).
Traverse, J. H. et al. Rationale and design for TIME: a phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am. Heart J. 158, 356–363 (2009).
Traverse, J. H. et al. LateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex. Heart Inst. J. 37, 412–420 (2010).
Traverse, J. H. et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. J. Am. Med. Assoc. 306, 2110–2119 (2011).
Traverse, J. H. et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. J. Am. Med. Assoc. 308, 2380–2389 (2012).
Hendrikx, M. et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation 114, I101–I107 (2006).
Fischer-Rasokat, U. et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ. Heart Fail. 2, 417–423 (2009).
Perin, E. C. et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). Am. Heart J. 161, 1078–1087 (2011). e1073.
Donndorf, P., Kaminski, A., Tiedemann, G., Kundt, G. & Steinhoff, G. Validating intramyocardial bone marrow stem cell therapy in combination with coronary artery bypass grafting, the PERFECT phase III randomized multicenter trial: study protocol for a randomized controlled trial. Trials 13, 99 (2012).
Heldman, A. W. et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. J. Am. Med. Assoc. 311, 62–73 (2014).
Patila, T. et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: a prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J. Heart Lung Transplant. 33, 567–574 (2014).
Hamshere, S. et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur. Heart J. 36, 3061–3069 (2015).
Martino, H. et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). Eur. Heart J. 36, 2898–2904 (2015).
Assmus, B. et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD registry. Circ. Res. 100, 1234–1241 (2007).
Caplan, A. I. & Dennis, J. E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084 (2006).
Williams, A. R. & Hare, J. M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 109, 923–940 (2011).
Chong, J. J. H. et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 9, 527–540 (2011).
Asli, N., Xaymardan, M. & Harvey, R. Epicardial origin of resident mesenchymal stem cells in the adult mammalian heart. J. Dev. Biol. 2, 117–137 (2014).
Zhang, Y. et al. Cardiac repair with a novel population of mesenchymal stem cells resident in the human heart. Stem Cells 33, 3100–3113 (2015).
Kinkaid, H. Y., Huang, X. P., Li, R. K. & Weisel, R. D. What’s new in cardiac cell therapy? Allogeneic bone marrow stromal cells as “universal donor cells”. J. Card. Surg. 25, 359–366 (2010).
Lohan, P. et al. Changes in immunological profile of allogeneic mesenchymal stem cells after differentiation: should we be concerned? Stem Cell Res. Ther. 5, 99 (2014).
Huang, X. P. et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 122, 2419–2429 (2010).
Zeng, L. et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 115, 1866–1875 (2007).
Hashemi, S. M. et al. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur. Heart J. 29, 251–259 (2008).
Gyongyosi, M. et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ. Cardiovasc. Imaging 1, 94–103 (2008).
Dixon, J. A. et al. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation 120, S220–S229 (2009).
Jameel, M. N. et al. Long-term functional improvement and gene expression changes after bone marrow-derived multipotent progenitor cell transplantation in myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 298, H1348–H1356 (2010).
Valina, C. et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 28, 2667–2677 (2007).
Mazo, M. et al. Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical swine model of myocardial infarction. Cell Transplant. 21, 2723–2733 (2012).
Schuleri, K. H. et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur. Heart J. 30, 2722–2732 (2009).
Quevedo, H. C. et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 106, 14022–14027 (2009).
Hatzistergos, K. E. et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 107, 913–922 (2010).
Williams, A. R. et al. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J. Am. Heart Assoc. 2, e000140 (2013).
Houtgraaf, J. H. et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 59, 539–540 (2012).
Lee, J. W. et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J. Korean Med. Sci. 29, 23–31 (2014).
Perin, E. C. et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE trial. Am. Heart J. 168, 88–95 (2014). e82.
Mathiasen, A. B. et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 36, 1744–1753 (2015).
Perin, E. C. et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res. 117, 576–584 (2015).
Hare, J. M. et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. J. Am. Med. Assoc. 308, 2369–2379 (2012).
Suncion, V. Y. et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: an analysis from the percutaneous stem cell injection delivery effects on neomyogenesis (POSEIDON) randomized trial. Circ. Res. 114, 1292–1301 (2014).
van der Spoel, T. I. et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc. Res. 91, 649–658 (2011).
Jansen of Lorkeers, S. J. et al. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies. Circ. Res. 116, 80–86 (2015).
Abdel-Latif, A. et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch. Intern. Med. 167, 989–997 (2007).
Jiang, M. et al. Randomized controlled trials on the therapeutic effects of adult progenitor cells for myocardial infarction: meta-analysis. Expert Opin. Biol. Ther. 10, 667–680 (2010).
de Jong, R., Houtgraaf, J. H., Samiei, S., Boersma, E. & Duckers, H. J. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ. Cardiovasc. Interv. 7, 156–167 (2014).
Nowbar, A. N. et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. Br. Med. J. 348, g2688 (2014).
Moye, L. DAMASCENE and meta-ecological research: a bridge too far. Circ. Res. 115, 484–487 (2014).
Afzal, M. R. et al. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circ. Res. 117, 558–575 (2015).
Rossini, A. et al. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc. Res. 89, 650–660 (2011).
Oskouei, B. N. et al. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl. Med. 1, 116–124 (2012).
Li, T.-S. et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 59, 942–953 (2012).
Zheng, S.-X. et al. Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction. Stem Cell Rev. Rep. 9, 339–349 (2013).
Citro, L. et al. Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction. PLoS ONE 9, doi:ARTN e116281 10.1371/journal.pone.0116281 (2014).
Behfar, A. et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J. Am. Coll. Cardiol. 56, 721–734 (2010).
Emmert, M. Y. et al. Safety and efficacy of cardiopoietic stem cells in the treatment of post-infarction left-ventricular dysfunction—from cardioprotection to functional repair in a translational pig infarction model. Biomaterials 122, 48–62 (2017).
Bartunek, J. et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 61, 2329–2338 (2013).
Bartunek, J. et al. Reply. J. Am. Coll. Cardiol. 62, 2454–2456 (2013).
Bartunek, J. et al. Cardiopoietic cell therapy for advanced ischemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. doi:10.1093/eurheartj/ehw543 (2016).
Oh, H. et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 100, 12313–12318 (2003).
Messina, E. et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 95, 911–921 (2004).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Xiao, J. et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 7, e2277 (2016).
Cimini, M. et al. c-kit dysfunction impairs myocardial healing after infarction. Circulation 116, I77–I82 (2007).
Tallini, Y. N. et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl. Acad. Sci. USA 106, 1808–1813 (2009).
Jesty, S. A. et al. c-kit+precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA. 109, 13380–13385 (2012).
Ye, L. et al. Aging kit mutant mice develop cardiomyopathy. PLoS ONE 7, e33407 (2012).
Hatzistergos, K. E. et al. cKit+cardiac progenitors of neural crest origin. Proc. Natl. Acad. Sci. USA 112, 13051–13056 (2015).
Hosoda, T. et al. Clonality of mouse and human cardiomyogenesis in vivo. Proc. Natl. Acad. Sci. USA 106, 17169–17174 (2009).
Ellison, G. M. et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 (2013).
van Berlo, J. H. et al. c-kit+cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014).
Sultana, N. et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat. Commun. 6, 8701 (2015).
Liu, Q. et al. Genetic lineage tracing identifies in situ kit-expressing cardiomyocytes. Cell. Res. 26, 119–130 (2016).
van Berlo, J. H. & Molkentin, J. D. Most of the dust has settled cKit(+) progenitor cells are an irrelevant source of cardiac myocytes in vivo. Circ. Res. 118, 17–19 (2016).
Williams, A. R. et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127, 213–223 (2013).
Karantalis, V. et al. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J. Am. Coll. Cardiol. 66, 1990–1999 (2015).
Bolli, R. et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128, 122–131 (2013).
Gallet, R. et al. Intracoronary delivery of self-assembling heart-derived microtissues (cardiospheres) for prevention of adverse remodeling in a pig model of convalescent myocardial infarction. Circ. Cardiovasc. Interv. 8, doi:10.1161/CIRCINTERVENTIONS.115.002391 (2015).
Jansen of Lorkeers, S. J. et al. Xenotransplantation of human cardiomyocyte progenitor cells does not improve cardiac function in a porcine model of chronic ischemic heart failure. Results from a randomized, blinded, placebo controlled trial. PLoS ONE 10, e0143953 (2015).
Kulandavelu, S. et al. Pim1 kinase overexpression enhances ckit+cardiac stem cell cardiac repair following myocardial infarction in swine. J. Am. Coll. Cardiol. 68, 2454–2464 (2016).
Johnston, P. V. et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120, 1075–1083, 1077 p following 1083 (2009).
Lee, S. T. et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J. Am. Coll. Cardiol. 57, 455–465 (2011).
Kanazawa, H. et al. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ. Heart Fail. 8, 322–332 (2015).
Zwetsloot, P. P. et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ. Res. 118, 1223–1232 (2016).
Bolli, R. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011).
Chugh, A. R. et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126, S54–S64 (2012).
The Lancet, E. Expression of concern: the SCIPIO trial. Lancet 383, 1279 (2014).
Makkar, R. R. et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012).
Malliaras, K. et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (cardiosphere-derived autologous stem cells to reverse ventricular dysfunction). J. Am. Coll. Cardiol. 63, 110–122 (2014).
Takehara, N., Ogata, T., Nakata, M., Kami, D., Nakamura, T. Matoba, S. et al. The ALCADIA (Autologous Human Cardiac-Derived Stem Cell To Treat Ischemic Cardiomyopathy) Trial. Hiroaki Matsubara Kyoto Prefectual Univ of Medicine, Kyoto, Japan.
Telukuntla, K. S., Suncion, V. Y., Schulman, I. H. & Hare, J. M. The advancing field of cell-based therapy: insights and lessons from clinical trials. J. Am. Heart Assoc. 2, e000338 (2013).
Sanganalmath, S. K. & Bolli, R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 113, 810–834 (2013).
Menard, C. et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet 366, 1005–1012 (2005).
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Anderson, M. E., Goldhaber, J., Houser, S. R., Puceat, M. & Sussman, M. A. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ. Res. 115, 335–338 (2014).
Menasche, P. et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. Eur. Heart J. 36, 743–750 (2015).
Kawamura, M. et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–S37 (2012).
Ye, L. et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761 (2014).
Menasche, P. et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 36, 2011–2017 (2015).
Georgiadis, V., Knight, R. A., Jayasinghe, S. N. & Stephanou, A. Cardiac tissue engineering: renewing the arsenal for the battle against heart disease. Integr. Biol. 6, 111–126 (2014).
Hastings, C. L. et al. Drug and cell delivery for cardiac regeneration. Adv. Drug Deliv. Rev. 84, 85–106 (2015).
Emmert, M. Y. et al. Transcatheter based electromechanical mapping guided intramyocardial transplantation and in vivo tracking of human stem cell based three dimensional microtissues in the porcine heart. Biomaterials 34, 2428–2441 (2013).
Emmert, M. Y. et al. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials 34, 6339–6354 (2013).
Günter, J. et al. Microtissues in cardiovascular medicine: regenerative potential based on a 3D microenvironment. Stem Cells Int. 2016, 20 (2016).
Zhao, T. C. et al. Targeting human CD34(+) hematopoietic stem cells with anti-CD45 x anti-myosin light-chain bispecific antibody preserves cardiac function in myocardial infarction. J. Appl. Physiol. 104, 1793–1800 (2008).
Assmus, B. et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. J. Am. Med. Assoc. 309, 1622–1631 (2013).
Samper, E., Diez-Juan, A., Montero, J. A. & Sepulveda, P. Cardiac cell therapy: boosting mesenchymal stem cells effects. Stem Cell Rev. Rep. 9, 266–280 (2013).
Liu, X. B., Wang, J. A., Ji, X. Y., Yu, S. P. & Wei, L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res. Ther. 5, doi:ARTN 111 10.1186/scrt499 (2014).
Yu, S. P., Wei, Z. & Wei, L. Preconditioning strategy in stem cell transplantation therapy. Transl. Stroke Res. 4, 76–88 (2013).
Sluijter, J. P. G. et al. MicroRNA-1 and-499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscl. Throm. Vas. 30, 859–868 (2010).
Hosoda, T. et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation 123, 1287–1296 (2011).
Stewart, D. J. et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol. Ther. 17, 1109–1115 (2009).
Engelmann, M. G. et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization—final results from the G-CSF-STEMI (granulocyte colony-stimulating factor ST-segment elevation myocardial infarction) trial. J. Am. Coll. Cardiol. 48, 1712–1721 (2006).
Ripa, R. S. et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation 113, 1983–1992 (2006).
Kang, H. J. et al. Intracoronary infusion of the mobilized peripheral blood stem cell by G-CSF is better than mobilization alone by G-CSF for improvement of cardiac function and remodeling: 2-year follow-up results of the myocardial regeneration and angiogenesis in myocardial infarction with G-CSF and intra-coronary stem cell infusion (MAGIC Cell) 1 trial. Am. Heart J. 153, e231–e238 (2007). 237.
Zafiriou, M. P. et al. Erythropoietin responsive cardiomyogenic cells contribute to heart repair post myocardial infarction. Stem Cells 32, 2480–2491 (2014).
Ott, I. et al. Erythropoietin in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a randomized, double-blind trial. Circ. Cardiovasc. Interv. 3, 408–413 (2010).
Najjar, S. S. et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. J. Am. Med. Assoc. 305, 1863–1872 (2011).
Prunier, F. et al. Single high-dose erythropoietin administration immediately after reperfusion in patients with ST-segment elevation myocardial infarction: results of the erythropoietin in myocardial infarction trial. Am. Heart J. 163, 200–207 e201 (2012).
Bar, C., Chatterjee, S. & Thum, T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 134, 1484–1499 (2016).
Bellera, N. et al. Single intracoronary injection of encapsulated antagomir‐92a promotes angiogenesis and prevents adverse infarct remodeling. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 3, e000946 (2014).
Emanueli, C., Shearn, A. I. U., Angelini, G. D. & Sahoo, S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vasc. Pharmacol. 71, 24–30 (2015).
Barile, L., Moccetti, T., Marban, E. & Vassalli, G. Roles of exosomes in cardioprotection. Eur. Heart J. doi:10.1093/eurheartj/ehw304 (2016).
Barile, L. et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 103, 530–541 (2014).
Ibrahim, A. G., Cheng, K. & Marban, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2, 606–619 (2014).
Tseliou, E. et al. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. Coll. Cardiol. 66, 599–611 (2015).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. doi:10.1093/eurheartj/ehw240 (2016).
Khan, M. et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117, 52–64 (2015).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Song, K. H. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012).
Jayawardena, T. M. et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465–1473 (2012).
Cao, N. et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. doi:10.1126/science.aaf1502 (2016).
Tokita, Y. et al. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ. Res. 119, 635–651 (2016).
Acknowledgements
We thank Carol De Simio-Hilton, Department of Surgical Research, University Hospital Zurich, Switzerland, for excellent graphical support on Fig. 1. This study was funded by institutional grants of the University of Zurich. M.Y.E. was supported by the Swiss Heart Foundation and the Olga-Mayenfisch Foundation.
Author information
Authors and Affiliations
Contributions
Conception of review: E.C., P.W., M.Y.E., and S.P.H. Writing of review: E.C. and M.Y.E. Literature review and acquisition/analysis of data: E.C., F.S.P., P.W., J.G., J.S., A.B., and M.Y.E. Administrative, technical, or supervisory tasks: P.W., S.P.H., and M.Y.E. Drafting and/or revision of the manuscript for critical content: E.C., P.W., and M.Y.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cambria, E., Pasqualini, F.S., Wolint, P. et al. Translational cardiac stem cell therapy: advancing from first-generation to next-generation cell types. npj Regen Med 2, 17 (2017). https://doi.org/10.1038/s41536-017-0024-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-017-0024-1
This article is cited by
-
Cardiac progenitor cell therapy: mechanisms of action
Cell & Bioscience (2024)
-
Near-infrared-II fluorescence/magnetic resonance double modal imaging of transplanted stem cells using lanthanide co-doped gadolinium oxide nanoparticles
Analytical Sciences (2024)
-
Preparation of Cell-Seeded Heart Patch In Vitro; Co-Culture of Adipose-Derived Mesenchymal Stem Cell and Cardiomyocytes in Amnion Bilayer Patch
Cardiovascular Engineering and Technology (2022)
-
Therapeutic Applications of Stem Cells and Extracellular Vesicles in Emergency Care: Futuristic Perspectives
Stem Cell Reviews and Reports (2021)