Abstract
Terazosin is an α1-adrenergic receptor antagonist that enhances glycolysis and increases cellular ATP by binding to the enzyme phosphoglycerate kinase 1 (PGK1). Recent work has shown that terazosin is protective against motor dysfunction in rodent models of Parkinson’s disease (PD) and is associated with slowed motor symptom progression in PD patients. However, PD is also characterized by profound cognitive symptoms. We tested the hypothesis that terazosin protects against cognitive symptoms associated with PD. We report two main results. First, in rodents with ventral tegmental area (VTA) dopamine depletion modeling aspects of PD-related cognitive dysfunction, we found that terazosin preserved cognitive function. Second, we found that after matching for demographics, comorbidities, and disease duration, PD patients newly started on terazosin, alfuzosin, or doxazosin had a lower hazard of being diagnosed with dementia compared to tamsulosin, an α1-adrenergic receptor antagonist that does not enhance glycolysis. Together, these findings suggest that in addition to slowing motor symptom progression, glycolysis-enhancing drugs protect against cognitive symptoms of PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a devastating neurodegenerative disease with motor and cognitive symptoms1. A key risk factor for PD is impaired energy metabolism2,3. We have recently found that the glycolysis-enhancing drug terazosin increases energy metabolism in rodent models and in PD patients4,5. We have also found that terazosin protects against motor neurodegeneration in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model, the 6-hydroxydopamine (6-OHDA) rat model, as well as synuclein-overexpressing mice4. Strikingly, analysis of the Progression Markers Initiative (PPMI) database shows that terazosin is also associated with slowed motor symptom progression in human PD patients4. Furthermore, based on data from large administrative databases, terazosin protects against developing PD6,7. Notably, patients taking terazosin had fewer cognitive complications, as indexed by International Classification of Diseases (ICD) codes4. Despite these data, it is unclear whether terazosin is neuroprotective for cognitive manifestations of PD.
One challenge is that there are few rodent models of cognitive dysfunction in PD. Human cognition is complex and spans multiple behavioral repertoires, such as planning and reasoning, which are challenging to model in rodents. We have found that PD patients with cognitive dysfunction have increased variability during interval timing, which involves estimating an interval of several seconds8,9. Specifically, PD patients with cognitive dysfunction have higher interval timing variability compared to PD patients with preserved cognitive function. Interval timing can be readily studied in rodents10, and work from our group has demonstrated that disrupting ventral tegmental area (VTA) dopamine reliably impairs interval timing9,11,12,13 independent from motor effects11. Thus, interval timing provides an opportunity to investigate cognitive deficits in PD rodent models as a function of terazosin.
In this study, we tested the hypothesis that terazosin protects against cognitive symptoms associated with PD. We found that rodents with VTA dopamine depletion via 6-OHDA injections had higher interval timing variability, similar to PD patients with cognitive dysfunction. Subsequently, we found that mice receiving daily terazosin delivered in drinking water had decreased interval timing variability. Since terazosin is prescribed to human PD patients, we were able to combine these results with an investigation of the association between clinically prescribed terazosin and cognitive symptoms in PD in the IBM MarketScan Research Databases for Commercial Claims and Medicare Supplemental. We found that human PD patients taking terazosin or related glycolysis-enhancing medications had less risk of developing dementia compared to propensity-score-matched patients taking tamsulosin, an α1-adrenergic receptor antagonist that does not enhance glycolysis. Together, these data provide evidence that terazosin protects against cognitive symptoms, as well as motor symptoms, associated with PD.
Results
VTA 6-OHDA increases interval timing variability
We trained 35 mice starting at ~14–16 weeks of age to perform an interval timing task in which mice switched from one nosepoke to another based on internal timing cues to get a food reward (Fig. 1a)14,15,16. In these animals, we injected vehicle (0.03% ascorbic acid) as a surgical control or 6-OHDA to deplete dopamine in the VTA, which we have previously shown impairs interval timing9,11,13. We began treatment with terazosin or vehicle (dimethyl sulfoxide; DMSO) immediately and tested performance in the interval timing switch task approximately 16 days post-surgery. We normalized post-surgical behavior in the switch task to pre-surgery behavior to determine magnitude change in behavior as a result of VTA dopamine depletion and terazosin treatment. In VTA 6-OHDA mice treated with DMSO, we found no significant change in median switch time (Vehicle DMSO: median 103% (interquartile range 93–117%); relative to presurgical baseline) vs 6-OHDA DMSO: 92% (83–105%); Wilcoxon p = 0.12; Cohen’s d = 0.66), but increased timing variability as measured by the coefficient of variability (CV) relative to Vehicle DMSO controls (Vehicle DMSO: 93% (86–104%) vs 6-OHDA DMSO: 118% (116–123%); Wilcoxon p = 0.003; Cohen’s d = 1.66; Fig. 2a–c). There were no changes in the number of switch trials performed (Vehicle DMSO: 30 (18–44) vs 6-OHDA DMSO: 38 (30–45); Wilcoxon p = 0.59; Cohen’s d = 0.23), total number of rewards obtained (Vehicle DMSO: 102 (95–124) vs 6-OHDA DMSO: 106 (88–155); Wilcoxon p = 0.96; Cohen’s d = 0.27), or locomotion time (time between trial start and first left or right nosepoke in which animals have to locomote between the back and short nosepoke (Vehicle DMSO: 7.34 (6.40–7.92) seconds vs 6-OHDA DMSO: 6.58 (5.90–7.08) seconds; Wilcoxon p = 0.17; Cohen’s d = 0.63). These results were similar to the increased interval timing CV observed in PD patients associated with cognitive dysfunction8 and provide evidence that VTA dopamine depletion models aspects of cognitive dysfunction in human PD patients.
a Experimental timeline created with BioRender.com. DMSO: dimethyl sulfoxide. b Interval timing switch task highlighting optimal performance during long trials. Trials are initiated at the back nosepoke. Identical cues are delivered for both short and long trials, which are randomly delivered. On short trials, mice are rewarded for the first response after 6 s at the designated short nosepoke (left or right). On long trials, mice start by responding at the designated short nosepoke. When there is no reward after 6 s, mice switch to the designated long nosepoke (contralateral to designated short nosepoke) and wait 18 s for reward delivery. This time to switch from the short to long nosepoke is a time-based decision as in other interval timing tasks. Switch time is defined as the time of last response at the short nosepoke before responses start at the long nosepoke, and only switch trials are analyzed.
a Cumulative distribution function of switch times. b Switch time coefficient of variability (CV) and c mean switch time. Data from 10 VTA Vehicle mice treated with dimethyl sulfoxide (DMSO; in black), 8 VTA 6-hydroxydopamine (6-OHDA) mice treated with DMSO (in red), and 11 VTA 6-OHDA mice treated with terazosin (TZ; in green). Each dot represents a single mouse, and the horizontal line represents the median value. All data presented are approximately 16 days post-surgery and normalized according to pre-surgical behavior. * p < 0.05.
Terazosin protects against VTA 6-OHDA timing deficits
Terazosin is neuroprotective in preclinical rodent models of PD-related motor dysfunction4. We investigated whether terazosin protects against the effects of VTA 6-OHDA in interval timing. Strikingly, we found that VTA 6-OHDA mice treated with terazosin had decreased interval timing variability relative to VTA 6-OHDA mice treated with DMSO (6-OHDA DMSO: 118% (116–123%) vs 6-OHDA terazosin: 97% (88–108%); Wilcoxon p = 0.005; Cohen’s d = 1.53), but no change in median switch time (6-OHDA DMSO: 92% (83–105%) vs 6-OHDA terazosin: 101% (92–106%); Wilcoxon p = 0.40; Cohen’s d = 0.35; Fig. 2a–c). There were no changes in the number of switch trials performed (6-OHDA DMSO: 38 (30–45) vs 6-OHDA terazosin: 25 (16–51); Wilcoxon p = 0.68; Cohen’s d = 0.09), or total number of rewards obtained (6-OHDA DMSO: 106 (88–155) vs 6-OHDA terazosin: 113 (97–128); Wilcoxon p = 1.0; Cohen’s d = 0.07). Of note, terazosin treatment did not affect interval timing variability in mice without dopamine depletion (Vehicle DMSO: 93% (86–104%) vs Vehicle terazosin 110% (97–131%); Wilcoxon p = 0.15; Cohen’s d = 0.96). These data support our hypothesis that terazosin is protective in rodents modeling aspects of cognitive dysfunction in PD.
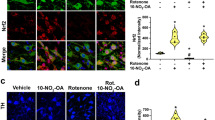
We also analyzed tyrosine hydroxylase positive (TH+) immunofluorescence in the VTA as an indirect measure of dopamine depletion (Fig. 3a–c) since terazosin has been shown to protect substantia nigra TH levels in rats4. VTA 6-OHDA mice had markedly decreased TH+ fluorescence in the VTA (Vehicle DMSO: 1776 (1577–2009) arbitrary units (AU) vs 6-OHDA DMSO: 673 (623–779) AU; Wilcoxon p = 0.00005; Cohen’s d = 3.07; Fig. 3d). Terazosin can be neuroprotective against dopamine depletion4, but we found no statistically significant effects of terazosin on TH+ fluorescence in the VTA of dopamine-depleted mice (6-OHDA DMSO: 673 (623–779) vs 6-OHDA terazosin: 1071 (653–1094); Wilcoxon p = 0.09; Cohen’s d = 0.79; Fig. 3d). To establish a relationship between the change in VTA TH+ immunofluorescence and behavioral results, we correlated fluorescence values with changes in switch time CV following surgery and DMSO or terazosin treatment. We observed that VTA TH+ fluorescence in all DMSO-treated mice had a strong and significant negative correlation with changes in switch time CV, such that greater VTA TH+ fluorescence values correlated with lower switch time CV (r = −0.59; p = 0.01). There was also a similar but non-significant relationship between switch time CV and VTA TH+ fluorescence values in dopamine-depleted mice treated with terazosin (r = −0.47; p = 0.15; Fig. 3e).
Representative histological images of TH fluorescence (red) from a VTA Vehicle mice treated with dimethyl sulfoxide (DMSO), b VTA 6-hydroxydopamine (6-OHDA) mice treated with DMSO, and c VTA 6-OHDA mice treated with terazosin (TZ). Scale bars represent 200 µm. d VTA TH fluorescence (in arbitrary units; AU) from 10 VTA Vehicle mice treated with DMSO (in black), 8 VTA 6-OHDA mice treated with DMSO (in red), and 11 VTA 6-OHDA mice treated with terazosin (in green). * p < 0.05. Each dot represents a single mouse, and the horizontal line represents the median value. e In VTA Vehicle and VTA 6-OHDA mice treated with DMSO, the percent change in coefficient of variability (CV) relative to presurgical baseline is strongly correlated with VTA TH fluorescence (red and black line). The relationship between VTA 6-OHDA and mice treated with terazosin is shown in green.
Acute terazosin and tamsulosin does not affect interval timing variability
We trained 8 mice to perform the same interval timing task described above. In these animals, we injected (intraperitoneally; IP) saline, terazosin (0.01 mg/kg or 0.4 mg/kg), or tamsulosin (0.01 mg/kg or 0.4 mg/kg) once per day in a pseudorandomized, counterbalanced design to investigate the effect of acute α1-andrenergic antagonism during the interval timing switch task. Acute terazosin did not change interval timing variability at low doses (saline: 32% (30–35%) vs 0.01 mg/kg terazosin: 30% (27–34%); Wilcoxon p = 0.25; Cohen’s d = 0.39) or high doses (saline: 28% (26–30%) vs 0.4 mg/kg terazosin: 33% (28–35%); Wilcoxon p = 0.20; Cohen’s d = 0.56; Fig. 4a). Similarly, acute tamsulosin did not change interval timing variability at low doses (saline: 29% (27–37%) vs 0.01 mg/kg tamsulosin: 38% (31–40%); Wilcoxon p = 0.64; Cohen’s d = 0.26) or high doses (saline: 30% (26–34%) vs 0.4 mg/kg tamsulosin: 28% (26–35%); Wilcoxon p = 0.95; Cohen’s d = 0.18; Fig. 4b). These data provide evidence that α1-adrenergic receptor antagonism alone does not affect interval timing variability.
Terazosin protects against cognitive symptoms of PD
An analysis of patient databases was used to determine whether glycolysis-enhancing terazosin, alfuzosin, or doxazosin (TZ/AZ/DZ) is associated with lower hazard of developing dementia compared to tamsulosin. We identified 14,184 men with PD who had not taken TZ/DZ/AZ or tamsulosin previously. After matching for demographics, comorbidities, and disease duration, 1508 men (754 matched pairs) remained. This matching successfully reduced imbalance between the groups (Supplementary Table 1) and resulted in a statistically significant 25% reduction (hazard ratio (HR) = 0.75; 95% CI: 0.57, 0.98) in the hazard of being diagnosed with dementia (Fig. 5).
Kaplan–Meier plot of 754 matched pairs of men aged 40 or older diagnosed with Parkinson’s disease (PD) and newly started on terazosin, alfuzosin, or doxazosin (TZ/DZ/AZ; green) or tamsulosin (red). The red and green lines denote cumulative incidence of PD patients remaining dementia free, and the shaded areas denote 95% CI.
Discussion
We tested the hypothesis that the glycolysis-enhancing drug terazosin is protective against cognitive dysfunction associated with PD. We found that VTA dopamine-depleted mice had increased interval timing variability, modeling cognitive deficits seen in PD patients8. Rodents with VTA dopamine depletion and terazosin had preserved interval timing variability. Finally, analysis of patient databases revealed that PD patients newly started on terazosin had less risk of developing dementia compared to PD patients taking tamsulosin, an α1-adrenergic receptor antagonist that does not impact glycolysis. Together these data provide evidence that terazosin is protective for cognitive, as well as motor, symptoms of PD. This is key as there are only a few minimally effective treatments for PD-related cognitive symptoms and no treatments that alter disease course.
Increased interval timing variability models some aspects of cognitive dysfunction in PD and Alzheimer’s disease patients8,9,11,16,17. Importantly, the VTA is involved in PD and degenerates through the course of the disease18. Accordingly, we observed that VTA dopamine depletion increased interval timing variability, and terazosin reversed or prevented deficits. High doses of terazosin and tamsulosin did not affect interval timing variability, indicating that it was unlikely to be an α1-adrenergic receptor effect19,20.
We found that the level of VTA TH+ fluorescence correlates with interval timing variability21. However, this correlation does not hold for VTA dopamine-depleted mice treated with terazosin as this treatment likely changed the relationship between VTA TH+ fluorescence and interval timing variability. We note that there was a trend for more TH+ fluorescence in VTA dopamine-depleted mice treated with terazosin, and it is possible that more animals would provide reliable evidence of VTA neuroprotection with terazosin. Furthermore, terazosin might increase synaptic dopamine in the projection fields of VTA dopamine neurons, as was observed in nigrostriatal dopamine terminals4. Together, these data establish that VTA dopamine depletion models PD-like increased interval timing variability, which is protected by terazosin.
From analysis of patient databases, we previously showed that terazosin is associated with fewer PD diagnostic codes linked with cognition, relative to tamsulosin4. Here, we expand this finding and show that PD patients have less risk of developing dementia relative to patients taking tamsulosin. Because these data are derived from large administrative databases, it is difficult to discern whether these patients had interval timing deficits.
Our work has several limitations. First, there is no definitive rodent model of cognitive dysfunction in PD, in part because the underlying pathophysiology is complex and diverse. However, VTA dopamine depletion models at least one characteristic of cognitive dysfunction in PD. Second, the mechanistic link between enhanced glycolysis and protection of dopamine neurons, dopamine release, or TH levels and/or production is unclear. However, it is possible that terazosin is protecting against VTA 6-OHDA-induced cell death or enhancing TH or dopamine similar to that observed in the nigrostriatal pathway4. This is an important future direction to understand how glycolysis-enhancing therapeutics can modulate neurodegenerative disease. Third, our data from terazosin are retrospectively observational and depend on comparisons with tamsulosin6,7,22.
These data converge with our prior work on motor symptoms of PD and provide evidence that terazosin is protective not only for neurons in the substantia nigra4, but also in the ventral tegmental area. Future studies will rigorously test this idea with a prospective and placebo-controlled randomized trial, in which motor and cognitive aspects of PD are carefully measured, and with cellular, molecular, and preclinical studies to understand how enhancing glycolysis modulates brain circuits.
Methods
Mice
All experimental procedures were performed in accordance with the relevant guidelines of Protocol #0062039 and with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa. Wild-type male and female C57BL/6 mice were received from Jackson Labs (Bar Harbor, ME) at approximately 12–14 weeks of age and acclimated to the animal holding facility for 2 weeks. During acclimation, all mice were communally housed on a 12-hour light/dark cycle with ad lib access to laboratory rodent chow and water. To facilitate operant behavioral training (described below), mice were individually housed, weighed daily, and maintained on a restricted diet with ad lib access to water.
Interval timing switch task
The interval timing switch task is designed to capture an animal’s internal representation of time14,15,16,23, as in other interval timing tasks13,24. Mice are trained to switch from a short to a long nosepoke after approximately 6 s. This switch is an explicit time-based decision that requires working memory for temporal rules and attention to the passage of time, and models cognitive deficits in neurodegenerative disease8,9,16,17.
Briefly, mice were trained in standard operant chambers enclosed in sound-attenuated cabinets (MedAssociates, St. Albans, VT) that contained two light-equipped nosepoke response ports (left and right) on the front wall, a reward hopper located between the two nosepoke response ports, and another light-equipped nosepoke on the back wall, opposite the reward hopper. Operant training began by shaping the animal’s behavior. Trial initiation began with a response at the back nosepoke, at which point either the left or right front nosepoke was illuminated, and a response at the appropriate port resulted in a reward. Mice were then advanced to the interval timing switch protocol, in which each session was randomly organized into 50% short (6 s) and 50% long (18 s) trials. Either the left or right nosepoke was designated for short trials and the contralateral nosepoke for long trials (counterbalanced across experimental groups). A back nosepoke response initiated a trial, generating two identical light cues above the left and right nosepokes, along with an 8 kHz tone (72 dB) for both trial types. In short trials, mice received a reward for the first response at the designated nosepoke after 6 s. In long trials, mice would begin by responding at the short-trial-designated nosepoke. When there was no reinforcement after 6 s, the mouse would switch to the long-trial-designated nosepoke until a reward was delivered after 18 s. Once mice were performing optimally, they were taken off food restriction and underwent stereotaxic surgical procedures (outlined below). Behavioral changes were assessed by retraining the mice in the same interval timing switch task. Only switch trials were analyzed during both training periods.
Surgical procedures
On the day of surgery, 6-hydroxydopamine hydrobromide (6-OHDA; Millipore Sigma #162957, Darmstadt, Germany) and desipramine hydrochloride (Millipore Sigma #D3900) were freshly made and stored on ice away from light. 6-OHDA was prepared at 2 mg/ml in 0.03% ascorbic acid (AA) and desipramine at 4 mg/ml in 0.9% saline. Mice were anesthetized under 4.0% isoflurane at 400 ml/min, and surgical levels of isoflurane (1.5–3.0%) were maintained at approximately 120 ml/min (SomnoSuite, Kent Scientific, Torrington, CT, USA). Desipramine (25 mg/kg) was injected intraperitoneally (IP) to protect norepinephrine terminals against 6-OHDA25,26. An incision was made along midline and bilateral craniotomies drilled above the VTA (AP −3.3, ML +/−1.1). Mice were randomly assigned to four different groups: (1) midbrain vehicle injections treated with dimethyl sulfoxide (DMSO; Vehicle DMSO); (2) VTA dopamine depletion treated with DMSO (6-OHDA DMSO); (3) VTA dopamine depletion treated with terazosin (6-OHDA terazosin); and (4) midbrain vehicle injections treated with terazosin (Vehicle terazosin). The vehicle group received microinjections of 0.5 μl 0.03% AA bilaterally, and the dopamine depletion groups received equal volumes of 6-OHDA prepared in AA (AP −3.3, ML +/−1.1, DV −4.6 at 10° laterally). Vehicle or 6-OHDA was infused over 10 min (0.05 µl/min; Legato 130 Syringe Pump, kd Scientific, Holliston, MA, USA), with a 5-minute wait period before removing the needle. After the incision was closed, mice were moved to a clean cage with ad lib access to food and either DMSO- or terazosin-treated water (described below). All mice recovered for one week before transitioning back to the interval timing switch task for approximately 3–4 weeks of post-surgical behavioral training.
Mouse terazosin
Following surgery, terazosin (Tocris #1506, Minneapolis, MN) prepared in DMSO was delivered through ad lib access to treated water. Water for control mice was made with equivalent volumes of DMSO alone. Preparation of terazosin began with a 100 mM stock solution (42 mg/ml in DMSO) stored at −80 °C. The stock was diluted 1:100 to make a 1 mM (0.42 μg/μl in water) working solution. We added 106 μl of working solution, equating to 45 μg terazosin (0.42 μg/μl × 106 μl), to 300 ml water, to which mice had free access. The concentration of the final drinking water was 0.15 μg/ml terazosin (45 μg/300 ml). An average mouse drinks approximately 5 ml of water per day27, so each mouse consumed ~0.75 μg terazosin daily (0.15 μg/ml × 5 ml/day). Additionally, experimental mice weigh on average 0.025 kg27; thus, the terazosin dose received by our animals was ~0.03 mg/kg/day (0.75 μg/0.025 kg/day). All terazosin- and DMSO-treated water was replaced every 2 days for the duration of the experiment post-surgery4.
Histology
Mice were anesthetized with ketamine (100 mg/kg IP) and xylazine (10 mg/kg IP) and transcardially perfused with cold phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA). Brains were removed and post-fixed in 4% PFA overnight, followed by immersion in 30% sucrose for approximately 48 h. The fixed brains were sliced at 40 μm coronal sections of the midbrain, using a cryostat (Leica Biosystems, Deer Park, IL). Sections were then blocked for one hour in 5% normal goat serum (NGS) in PBST (0.3% Triton X-100 in 1× PBS). After blocking, sections were incubated overnight at 4 °C in anti-tyrosine hydroxylase antibody (Millipore #AB152, Burlington, MA) diluted to 1:1000 in 5% NGS. The sections were washed with PBST three times over 30 min before a 2 h incubation in goat anti-rabbit IgG (H+L) Alexa Fluor 568 secondary antibody (Invitrogen #A11036, Waltham, MA) diluted to 1:1000 in 5% NGS. After three more PBST washes, the sections were mounted with ProLong Diamond Antifade Mountant with DAPI (Invitrogen #P36962) on Superfrost microscope slides (Fisher Scientific, Waltham, MA).
Brain sections were imaged using VS-ASW-S6 imaging software (Olympus, Center Valley, PA). Histological targeting of the VTA was confirmed by two independent authors, one blinded to treatment conditions. Eight mice, in which the injection missed the VTA, were excluded. For the remaining mice, the Count and Measure analysis tool in cellSens Dimension Desktop (Olympus, Shinjuku, Tokyo, Japan) was used to quantify tyrosine hydroxylase positive (TH+) fluorescence levels in the VTA. Fluorescence levels in each animal were determined by averaging the mean pixel intensities of three VTA sections. Anterior-posterior coordinates of the VTA were determined following prior literature and ranged from −3.1 to −3.5, respectively. Outlines of the regions of interest were referenced from Franklin & Paxinos, 200828.
Acute terazosin and tamsulosin
In a separate group of mice (n = 8) trained in the interval timing switch task, we investigated the acute effects of terazosin and tamsulosin. Tamsulosin is an α1-adrenergic antagonist used for similar clinical interventions as terazosin but does not enhance glycolysis4. Mice were injected (IP) once per day with either saline, terazosin (0.01 mg/kg or 0.4 mg/kg), or tamsulosin (0.01 mg/kg or 0.4 mg/kg) in a pseudorandomized, counterbalanced design. Injections were administered approximately 20 min prior to starting the interval timing switch task.
Statistics
For experiments with VTA dopamine depletion and chronic terazosin administration, we analyzed effects of dopamine depletion and terazosin using two-sided non-parametric Wilcoxon tests. Four-to-five sessions of interval timing behavior were collected and analyzed per mouse, both before surgery and approximately 16 days post-surgery. Post-surgery switch time coefficients of variability (CV) and mean switch times were normalized to each mouse’s pre-surgical baseline to account for animal-specific variability in timing behavior. For acute pharmacological administration of terazosin and tamsulosin, each drug treatment was compared to injections of saline one day prior using two-sided non-parametric Wilcoxon tests. All data was analyzed with custom routines written in MATLAB and R, and all statistics was reviewed by the Biomedical Epidemiology Research and Design core in the Institute for Clinical and Translational Sciences at the University of Iowa.
Administrative database search
Using the IBM MarketScan Research Databases, we identified men aged 40 or older taking terazosin, alfuzosin, or doxazosin (collectively, TZ/DZ/AZ) or tamsulosin, not in conjunction with finasteride or dutasteride. We restricted our analysis only to men as the most common use of TZ/DZ/AZ or tamsulosin is to treat benign prostatic hyperplasia, a condition that only affects men. Men who switched between the TZ/DZ/AZ and tamsulosin classes were excluded. To ensure that we identified men with PD who were newly started on TZ/DZ/AZ or tamsulosin, we required: (1) at least 12 months of enrollment prior to the observed first dispensing date with prescription drug coverage; (2) at least two dispensing events to occur in the first year following the first dispensing date; 3) the PD diagnosis date must have occurred before the TZ/DZ/AZ or tamsulosin start date; and 4) the men must have been free of a dementia diagnosis at the start of medication. Dementia was defined as ICD-9-CM: 289.9, 290.0, 290.1, 290.2, 290.3, 290.4, 290.43, 294.1, 294.8, 331.0, 331.1, 348.3 or ICD-10-CM: F01.51, F03.90, F05, F06.0, F06.8, F29. To ensure that we identified new cases of PD, the first observed diagnosis of PD or dispensing of levodopa must have occurred within at least 12 months after the insurance enrollment date; health insurance claims data do not include detailed measures of PD severity, e.g., Unified Parkinson’s Disease Rating Scale scores or cognitive function. The administrative database search and subsequent analysis described below was performed on a fully deidentified secondary database and was not considered human subject research, per the US Department of Health and Human Services29. Therefore, this study was exempt from institutional review board approval.
Administrative database analysis
Men taking TZ/DZ/AZ were matched to men taking tamsulosin in a two-stage process. We used the “gold standard” design in pharmacoepidemiology studies known as the active comparator, new user design30 and extreme restriction to limit the cohort to the same condition31. First, we estimated a propensity score model incorporating the following criteria: age; health care utilization during the lookback period (rate of hospitalization, rate of outpatient encounters); baseline health status (mean number of diagnoses per outpatient encounter, rate of unique outpatient diagnoses, the 29 Elixhauser comorbidities); factors for prescribing decision (diagnosis of benign prostatic hyperplasia, diagnosis of slow urinary stream, diagnosis of abnormal prostate-specific antigen (PSA), diagnosis of orthostatic hypotension, diagnosis of other hypotension, procedural claim for testing PSA, procedural claim for a uroflow study, procedural claim for a cystometrogram); and medication start date. The propensity score model was estimated using a boosted tree with the depth and number of rounds selected by testing performance on a 25% held-out validation set. Once the depth and number of rounds were selected, we used the entire data set to generate and estimate the propensity score model. Second, we required the time between the diagnosis of PD and the medication start date to be +/−180 days between possible matches. This was done to ensure a similar duration of disease and to potentially reduce unobserved heterogeneity due to differing PD severity. Within the set of possible matches based on the time between PD diagnosis and the medication start date, we selected the nearest possible match based on the log odds estimated by our propensity score model. We imposed a 20% pooled standard deviation caliper to exclude poor matches. After matching, we followed the medical records of men for 8 years to track the rates of developing dementia. We compared the hazard of developing dementia with Kaplan-Meier survival curves and Cox proportional hazards regression. Standard errors were clustered to account for the propensity score matching.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
IBM MarketScan Research Databases for Commercial Claims and Medicare Supplemental is used under license which prohibits redistribution. All other raw data are available at https://narayanan.lab.uiowa.edu/article/datasets.
Code availability
All code is available at https://narayanan.lab.uiowa.edu/article/datasets.
References
Dauer, W. & Przedborski, S. Parkinson’s disease. Neuron 39, 889–909 (2003).
Saxena, U. Bioenergetics failure in neurodegenerative diseases: back to the future. Expert Opin. Ther. Targets 16, 351–354 (2012).
Wellstead, P. & Cloutier, M. An energy systems approach to Parkinson’s disease. WIREs Syst. Biol. Med. 3, 1–6 (2011).
Cai, R. et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J. Clin. Invest. 129, 4539–4549 (2019).
Schultz, J. L. et al. A pilot to assess target engagement of terazosin in Parkinson’s disease. Parkinsonism Relat. Disord. 94, 79–83 (2022).
Sasane, R. et al. Parkinson disease among patients treated for benign prostatic hyperplasia with α1 adrenergic receptor antagonists. J. Clin. Invest. 131, e145112 (2021).
Simmering, J. E., Welsh, M. J., Liu, L., Narayanan, N. S. & Pottegård, A. Association of glycolysis-enhancing α-1 blockers with risk of developing Parkinson disease. JAMA Neurol. 78, 407 (2021).
Singh, A. et al. Timing variability and midfrontal ~4 Hz rhythms correlate with cognition in Parkinson’s disease. Npj Park. Dis. 7, 14 (2021).
Parker, K. L., Chen, K.-H., Kingyon, J. R., Cavanagh, J. F. & Narayanan, N. S. Medial frontal ∼ 4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J. Neurophysiol. 114, 1310–1320 (2015).
Buhusi, C. V. & Meck, W. H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765 (2005).
Kim, Y.-C. et al. Optogenetic stimulation of frontal D1 neurons compensates for impaired temporal control of action in dopamine-depleted mice. Curr. Biol. 27, 39–47 (2017).
Kim, Y.-C. & Narayanan, N. S. Prefrontal D1 dopamine-receptor neurons and delta resonance in interval timing. Cereb. Cortex 29, 2051–2060 (2019).
Narayanan, N. S., Land, B. B., Solder, J. E., Deisseroth, K. & DiLeone, R. J. Prefrontal D1 dopamine signaling is required for temporal control. Proc. Natl Acad. Sci. USA 109, 20726–20731 (2012).
Balci, F. et al. Interval timing in genetically modified mice: a simple paradigm. Genes Brain Behav. 7, 373–384 (2008).
Bruce, R. A. et al. Experience-related enhancements in striatal temporal encoding. Eur. J. Neurosci. 54, 5063–5074 (2021).
Larson, T. et al. Mice expressing P301S mutant human tau have deficits in interval timing. Behav. Brain Res. 432, 113967 (2022).
Gür, E. et al. Interval timing is disrupted in female 5xFAD mice: an indication of altered memory processes. J. Neurosci. Res. 97, 817–827 (2019).
Alberico, S. L., Cassell, M. D. & Narayanan, N. S. The vulnerable ventral tegmental area in Parkinson’s disease. Basal Ganglia 5, 51–55 (2015).
Buhusi, C. V., Matthews, A. R. & Buhusi, M. mPFC catecholamines modulate attentional capture by appetitive distracters and attention to time in a peak-interval procedure in rats. Behav. Neurosci. 136, 418–429 (2022).
Matthews, A. R. Dissociation of the role of the prelimbic cortex in interval timing and resource allocation: beneficial effect of norepinephrine and dopamine reuptake inhibitor nomifensine on anxiety-inducing distraction. Front. Integr. Neurosci. 6, 111 (2012).
Gür, E., Duyan, Y. A., Arkan, S., Karson, A. & Balcı, F. Interval timing deficits and their neurobiological correlates in aging mice. Neurobiol. Aging 90, 33–42 (2020).
Gros, P. et al. Exposure to phosphoglycerate kinase 1 activators and incidence of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 36, 2419–2425 (2021).
Tosun, T., Gür, E. & Balcı, F. Mice plan decision strategies based on previously learned time intervals, locations, and probabilities. Proc. Natl Acad. Sci. USA 113, 787–792 (2016).
Emmons, E. et al. Temporal learning among prefrontal and striatal ensembles. Cereb. Cortex Commun. 1, tgaa058 (2020).
Thiele, S. L., Warre, R. & Nash, J. E. Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J. Vis. Exp. JoVE https://doi.org/10.3791/3234 (2012).
Torres, E. M. & Dunnett, S. B. Animal Models of Movement Disorders: Volume I (eds Lane, E. L. & Dunnett, S. B.) 267–279 (Humana Press, 2012).
Nicolaus, M. L., Bergdall, V. K., Davis, I. C. & Hickman-Davis, J. M. Effect of ventilated caging on water intake and loss in 4 strains of laboratory mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 55, 525–533 (2016).
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates. (Elsevier Academic Press, 2008).
Protections (OHRP), O. for H. R. Human Subject Regulations Decision Charts: 2018 Requirements. HHS.gov https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts-2018/index.html (2010).
Lund, J. L., Richardson, D. B. & Stürmer, T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr. Epidemiol. Rep. 2, 221–228 (2015).
Secrest, M. H. et al. Extreme restriction design as a method for reducing confounding by indication in pharmacoepidemiologic research. Pharmacoepidemiol. Drug Saf. 29, 26–34 (2020).
Acknowledgements
The IBM MarketScan Research Databases for Commercial Claims and Medicare Supplemental was provided by the University of Iowa. This work was supported by a faculty fellowship through the Iowa Neuroscience Institute to JES and NIH R01s MH116043, NS120987 to NSN.
Author information
Authors and Affiliations
Contributions
M.A.W. and K.S. contributed equally to this manuscript as co-first authors. M.A.W., K.S., and N.S.N. designed the animal experiments. J.E.S. and N.S.N. designed the human PD database analysis. M.A.W., K.S., and M.O. performed all animal experiments. M.A.W. and K.S. independently verified histological targeting. E.E.T., K.S., and G.M.A. performed histological immunofluorescent analysis. M.A.W., K.S., and Q.Z. maintained and delivered terazosin. M.A.W., K.S., J.E.S., and N.S.N. performed all statistical analyses. M.A.W., K.S., J.E.S., and N.S.N. wrote the manuscript, and all authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weber, M.A., Sivakumar, K., Tabakovic, E.E. et al. Glycolysis-enhancing α1-adrenergic antagonists modify cognitive symptoms related to Parkinson’s disease. npj Parkinsons Dis. 9, 32 (2023). https://doi.org/10.1038/s41531-023-00477-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00477-1