Abstract
Neurodegenerative diseases are characterized by neuronal impairment and loss of function, and with the major shared histopathological hallmarks of misfolding and aggregation of specific proteins inside or outside cells. Some genetic and environmental factors contribute to the promotion of the development and progression of neurodegenerative diseases. Currently, there are no effective treatments for neurodegenerative diseases. It has been revealed that bidirectional communication exists between the brain and the gut. The gut microbiota is a changeable and experience-dependent ecosystem and can be modified by genetic and environmental factors. The gut microbiota provides potential therapeutic targets that can be regulated as new interventions for neurodegenerative diseases. In this review, we discuss genetic and environmental risk factors for neurodegenerative diseases, summarize the communication among the components of the microbiota-gut-brain axis, and discuss the treatment strategy of fecal microbiota transplantation (FMT). FMT is a promising treatment for neurodegenerative diseases, and restoration of the gut microbiota to a premorbid state is a novel goal for prevention and treatment strategies.
Similar content being viewed by others
Introduction
Neurodegenerative diseases are characterized by neuronal impairment and loss of function that lead to the progressive impairment of cognitive function1. The misfolding and aggregation of specific proteins inside or outside cells are the major shared histopathological hallmarks of neurodegenerative diseases2; examples include misfolded α-synuclein deposits in Parkinson’s disease (PD), amyloid-β (Aβ) aggregates, and neurofibrillary tangles are formed from hyperphosphorylated tau in Alzheimer’s disease (AD)3, mutated huntingtin (HTT) in Huntington disease4, and TAR DNA-binding protein 43 (TDP-43) in amyotrophic lateral sclerosis (ALS)5. AD and PD are the two most common neurodegenerative disorders.
The two most common neurodegenerative disorders: AD and PD
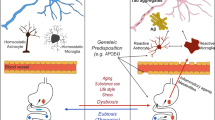
AD is characterized by cognitive dysfunction and progressive memory decline, and is caused by a complex interaction between genetic, lifestyle, environmental, and epigenetic factors3. The increased life expectancy worldwide has resulted in a significant increase in age-related diseases. Neurodegenerative disorders and dementia are increasing progressively with an incidence of 17.2 million people worldwide1. AD is one of the fastest-growing age-related diseases today6. Worldwide, 10% of people over the age of 65 years are affected by AD7, and after the age of 65, the risk of developing AD doubles every 5 years8. In the United States, 40% of people over 85 years old are cognitively impaired9; AD pathology probably contributes to 75–80% of these cases10, and more than 5 million individuals have AD11. The primary neuropathological criteria for AD diagnosis are the intracellular accumulation of hyperphosphorylated tau as neurofibrillary tangles and the extracellular deposition of Aβ as neuritic plaques12. Aβ is derived from Aβ precursor protein (APP) predominantly in endosomes by β-secretase and γ-secretase13. The autosomal dominant form of early-onset AD has been attributed to the overproduction of Aβ as the result of APP and presenilin 1 and 2 (PSEN1/2) mutations14. Synaptic activity both presynaptically and postsynaptically modulates neuronal Aβ release15. Aβ can aggregate into higher-order fibrils and oligomers, impair synaptic activity and cerebral capillary blood flow, and directly stimulate tau hyperphosphorylation12. Aβ accumulation may be a critical pathological process for the initiation of tau accumulation and neuroinflammation, which are the downstream events that may be the main drivers of neurodegeneration12. Tau protein is primarily expressed by brain neurons and is encoded by the MAPT gene12. The production of tau is related to the presence of amyloid proteins16, and the progression of tau pathology in AD requires Aβ deposition17. Tau spreads from cell to cell through neuronal connections, and this process can be facilitated by Aβ in animal models17. Tau pathology generally does not progress in the absence of amyloid pathology18. Elevating the Aβ level alone is sufficient to drive tau pathology in human neurons19. Recent research has suggested that tau spreads through neuronal communication pathways even in normal aging, and its spread is accelerated by the presence of Aβ in the human brain20. The rate of amyloid accumulation predicts the beginning of tau accumulation, whereas the rate of tau accumulation predicts the beginning of cognitive impairment21. Different individuals with “typical” AD may have distinct biochemical features of tau, including hyperphosphorylated soluble, oligomeric, seed-competent tau22. And microglial dysfunction contributes to the pathology of AD23 (Fig. 1). Microglia clear Aβ plaques and are involved in the development and progression of AD. When genes linked to AD risk (including SPI1, CR1, TREM2, MS4As, ABCA7, CD33, and INPP5D) are expressed in microglia, their phagocytic function is disrupted, and Aβ accumulates and activates the cascade that promotes subsequent neuronal degeneration23,24.
PD is the second most common neurodegenerative disease25. The incidence of PD increases progressively with age and affects 1 to 4% of individuals over the age of 60 years11,26 and over 5 million people worldwide27. AD and PD begin decades before the clinical manifestation of the first symptoms due to the formation of pathogenic protein aggregates.
Widespread aggregation of the α-synuclein protein in the form of Lewy bodies is a neuropathological hallmark of PD28 (Fig. 2). The coexistence of multiple pathological proteins in diseased brains is common in various neurodegenerative diseases, and one pathological protein could promote the spreading of another29. Approximately 50% of individuals with AD have α-synuclein, Aβ, and tau pathology in the brain30. Genetic and histopathological data suggest that Aβ plaques drive the spread of tau pathology31. Overexpression of α-synuclein has been observed to increase phosphorylated tau in mice32. Misfolded protein aggregates further activate the innate immune system in various neurodegenerative diseases. This implies that the inflammation induced by faulty protein clearance may be a common phenomenon in neurodegeneration. Astrocytes are activated by microglia via nuclear factor (NF)-κB signaling, thus further amplifying inflammation33, and exacerbating tissue damage and cellular dysfunction. High expression levels of tumor necrosis factor (TNF)-α and interleukin (IL)-1β have been linked to synaptic plasticity, learning, and memory34. Brain structure and function deteriorate via a feedforward loop that fuels further neurodegeneration, and no neuroprotective or neurorestorative therapies have been identified to date for treating neurodegenerative diseases. In this review, we discuss genetic and environmental risk factors for neurodegenerative diseases, summarize the communication among the components of the microbiota-gut-brain axis, and discuss the fecal microbiota transplantation (FMT) treatment strategy.
Genetic and environmental risk factors for neurodegenerative diseases
Some genetic risk factors that modulate the transmission of pathological proteins contribute to the promotion of neurodegenerative disease development and progression. Targeted deletions of CX3CR1 and TREM2 variants35 and altered complement expression have been associated with neurodegenerative phenotypes36. CX3CR1 is the receptor for the chemokine fractalkine (CX3CL1) and is part of a critical signaling pathway for microglia-neuron crosstalk. The CX3CL1/CX3CR1 axis is implicated in the regulation of cognitive functions and synaptic plasticity in the hippocampus. CX3CR1 deficiency exacerbates α-synuclein-A53T-induced neuroinflammation and neurodegeneration in PD37. TREM2 is a transmembrane glycoprotein. TREM2 regulates phagocytic pathways and suppresses inflammatory reactivity to regulate the reactive microglial phenotype38. TREM2 mutations decrease phagocytic activity, contribute to neurodegeneration by impairing the clearance of damaged neurons and aggregated proteins, and promote pro-inflammatory reactions35. It is estimated that the risk of AD attributable to genetic factors is 56–79%39. The role of epigenetic factors, DNA and histone modifications, and noncoding RNA in the development of neurodegenerative disease has been deeply investigated40. The APOE gene encoding apolipoprotein (Apo) E is the strongest genetic risk factor for developing AD. There are three predominant variants of APOE: APOE2, APOE3, and APOE4. People who carry the APOE4 gene variant are at higher-than-average risk of developing AD, and the variant is linked to defects in the blood–brain barrier (BBB) and subsequent cognitive decline41. APOE4 is associated with a fourfold increase in the risk of developing AD in people with one copy of this variant and a 15-fold increase in the risk of developing AD in people who have two copies42. Aβ and tau propagation are associated with ApoE43. The BIN1 gene encoding amphiphysin 2, which can inhibit tau propagation, is the second most prevalent risk locus for late-onset AD44. Rare coding variants in PLCG2, ABCA7, TREM2, and ABI3 have been identified in AD using genome-wide association studies (GWASs)45. The rare coding variants p.R62H (rs143332484) and p.R47H (rs75932628) in TREM2 and p.P522R in PLCG2 (rs72824905) are associated with the risk of AD45,46. Dysfunction in TREM2 increases amyloid plaque seeding47. CX3CR1+ mononuclear phagocytes express antifungal receptors and activate antifungal responses in a Syk-dependent manner and are essential for mediating interactions between intestinal mycobiota and host immunity at steady-state and during inflammatory disease48 (Fig. 1). The CX3CR1/CX3CL1 axis plays a key role in the phagocytosis of tau by microglia and is affected as AD progresses49. In hTau/CX3CR1−/− mouse models, microglial activation led to the secretion of IL-1, which promotes p38 MAPK-mediated tau hyperphosphorylation and aggregation50 (Fig. 1). A missense mutation in the gene encoding CX3CR1 led to changes in the gut fungal communities and to severe colitis, and impaired antifungal responses in Crohn’s disease patients51. G protein-coupled receptor 31 (GPR31) is highly and selectively expressed in intestinal CX3CR1+ cells. The bacterial metabolites lactic acid and pyruvic acid contribute to enhanced immune responses by inducing GPR31-mediated dendrite protrusion of intestinal CX3CR1+ cells48. Histone deacetylase 1 (HDAC1)-deficient mice display age-associated DNA damage accumulation and cognitive impairment. HDAC1 activation has the therapeutic potential for functional decline in brain aging and neurodegeneration52. Some studies have also shown that pathogens can act as triggers to induce the accumulation of Aβ1–42 monomers, reactive gliosis, and pro-inflammatory response, and are involved in the development of sporadic AD53.
Mutations in the gene encoding leucine-rich repeat kinase 2 (LRRK2) are the most common cause of hereditary PD54. LRRK2 activity promotes α-synuclein propagation via the phosphorylation of RAB3555. A genetic component for the apparently sporadic disease was not obvious in the early days of PD research. LRRK2 mutations can cause familial PD with age-dependent but variable penetrance; variants of the gene are also risk factors for sporadic PD56. Individuals with mutations in the genes PARKIN, PINK1, SNCA, GBA1, and LRRK2 show an increased risk of developing familial PD57 (Fig. 2). In addition, multiple mutations in genes such as C9orf72, TARDBP, and SOD1 are mainly expressed in a variety of nonneuronal cells which enhance immune dysregulation and neuroinflammation in the pathogenesis of ALS58.
Although considerable genetic research has highlighted the importance of copy number variations and de novo mutations in neurodegenerative disease etiology, environmental exposure has also been linked to the pathogenesis of these diseases59. Various environmental factors may modify and trigger psychiatric conditions. The burden of disease caused by environmental pollution is becoming a public health challenge worldwide, and 6.4 million deaths in 2015 were attributable to air pollution according to the Global Burden of Disease Study60. Experimental and epidemiologic evidence strongly supports the role of environmental exposure and gene–environment interactions in the incidence and progression of PD61. The toxicants of heavy metals, pesticides, detergents, solvents, and other industrial byproducts are highly relevant to neurologic disorders62. These toxicants can cross the BBB, potentially impacting the health and function of central nervous system (CNS) cells.
Among all types of pollution, heavy metals are considered the greatest threat to human health because of their persistence and bioaccessibility in the environment. Increased industrialization has led to higher levels of heavy metals. Chronic exposure to transition metals such as manganese (Mn), iron (Fe), copper (Cu), and zinc (Zn) is linked to neurodegenerative disorders63. Conformational changes in disease-related proteins (Aβ, tau, and α-synuclein) are central to the pathogenesis of neurodegenerative diseases. The conformational changes of Aβ and its oligomerization are critical to the Aβ-induced neurodegeneration process. Aβ oligomers form insoluble aggregates termed amyloid fibrils. The pathogenesis of AD may be essentially altered by factors that accelerate oligomerization. Trace elements of Al3+, Zn2+, Cu2+, Mn2+, and Fe2+ are accelerating factors in protein conformational change; for example, they can enhance Aβ oligomerization64 (Fig. 3). Exposure to Mn2+ has been linked to an increased risk of neurodegenerative disorders according to our research and the research of others65,66,67. Mn2+ exposure promotes α-synuclein secretion and acts as a key amplifier of NLRP3 inflammasome signaling63,68. Mn2+ crosses the BBB as Mn2+ alone or in complex with transferrin or citrate67. Aluminum (Al) is a trivalent metal neurotoxin and is linked to the etiology of neurological disorders69. Al3+ enters the brain via a similar mechanism to Fe2+. Al3+ accumulation within the CNS induces pro-inflammatory signaling, irreversible brain cell damage, dysregulation of gene expression, and functional decline in cognition, memory, and behavior70. The mechanism of Al3+ toxicity is inflammatory neurodegeneration including amyloidogenesis, inflammasome activation, deficits in neurotrophic signaling and synaptogenesis, altered innate immune responses, reactive oxygen species (ROS), and α-synuclein production, and inability to remove self-aggregating waste from brain cells, cytoplasm, and parenchyma71,72. The imbalance of Zn2+ and Cu2+ plays a pivotal role in the mechanisms of AD and PD. Aβ aggregation and ROS production lead to excess intracellular Zn, release Zn from metallothioneins and may affect mitochondrial function and induce apoptosis. Excess Cu2+ is neurotoxic, and its neurotoxicity has traditionally been viewed as the result of its strong affinity for Aβ and its promotion of increased oxidative stress via the Fenton reaction73. Several studies have suggested that lead (Pb), arsenic (As), and methyl mercury (MeHg) are also neurotoxins and can disrupt brain function74, cause cognitive dysfunction75, and increase the risk of AD and PD by disrupting mRNA splicing, the ubiquitin-proteasome system, the electron transport chain, and oxidative stress76,77.
APP binds to metals (Al, Cu, Fe, Mn, or Zn). Under acceleratory conditions, Aβ self-aggregates and forms several types of oligomers (SDS-soluble oligomers, Aβ-derived diffusible ligands, globulomers, and protofibrils) finally forming insoluble aggregates (amyloid fibrils), and tight binding to the surface of neurons and form fibrillar deposits.
Pesticides are used to destroy, prevent, or control destructive pests. Food and Agriculture Organization (FAO) reported that ~3 million tons of pesticides are used globally every year. Exposure to pesticides has been identified as a risk factor for nervous system disorders, reproductive problems, and cancer linked to inflammasome activation78. Rotenone can easily cross the BBB and activate the NLRP3 inflammasome79, and rotenone exposure can cause nigrostriatal degeneration, α-synuclein accumulation, motor impairment, and neuroinflammation80. Paraquat is a widely used herbicide, and exposure to paraquat is also linked to an increased risk of PD and AD62. Paraquat induces ROS generation, cytotoxicity, and NLRP3 activation81. The organophosphate chlorpyrifos is widely used, and an estimated 3.2–4.1 million kilograms enter the environment annually in the United States alone82. Chlorpyrifos exposure can also increase the risk of PD by altering the expression of claudin5, ZO1, and TRPC4, which are important proteins for BBB integrity83.
The neurodegenerative process can be exacerbated by neuroinflammation84. Inflammasomes sense damage-associated molecular patterns and pathogen-associated molecular patterns. Growing evidence indicates that there is an association between inflammasome activation and neurodegenerative disease. Heavy metals and pesticides cause cellular damage by deregulating lysosomal function, impairing mitochondrial function, enhancing the spread of misfolded proteins, and potentially triggering an inflammatory response ranging from the induction of acute necrosis to more discrete cellular pathophysiologies, including protein misfolding, oxidative stress, and programmed cell death85. Inflammasomes may link environmental toxicant-driven cellular stress with neuroinflammation and ultimately cell death.
Genetic and environmental factors affect gut microbiota
The gut microbiota is a dynamic microbial system, and it can be modified by genetic and environmental factors. Previous studies have reported that the gut microbiota is constantly challenged by environmental factors such as exercise, diet, stress, altitude, temperature, toxicants/pollutants, and noise86,87. Environmental contaminants (heavy metals, pesticides, persistent organic pollutants, antibiotics, food additives, and nanomaterials) can affect the composition of the gut microbiota, leading to physiological disorders in the host and causing certain diseases88. The gut microbiota has become a new toxicological target for some environmental pollutants. A decreasing diversity of gut microbiota is often observed after exposure to heavy metals89. In our previous studies, Mn exposure led to decreased abundances of Prevotellaceae, Fusobacteriaceae, and Lactobacillaceae66,90. In addition, Nasuti et al.91 showed that changes in gut microbiota may be one of the reasons for the neurotoxicity of permethrin. Many studies have shown that antibiotic administration leads to disturbances in the microbial diversity and metabolism of the gut microbiome that might be linked to a multitude of diseases89.
The host’s genetic background can influence microbiota composition. The microbiomes of humans and mice are associated with host genetic variation, and several heritable bacterial taxa have been identified92,93. The gut microbiota, as an epigenetic factor influencing DNA methylation status in the SNCA promoter region, may affect α-synuclein expression and the risk of PD94. The APOE genotype, by influencing bile acid secretion, could affect the composition of the gut microbiota to favor the development of organisms triggering protein misfolding, increasing the risk for PD in synucleinopathies95,96. Moreover, TAS2R38 has been shown to be a genetic risk factor associated with the development of PD. Genetic variants of the TAS2R38 bitter taste receptor are associated with distinct gut microbiota traits in PD and are associated with a reduction in bacterial alpha diversity with a predominant reduction in the Clostridium genus97. The relative abundance of certain microbiota elements can be influenced by the genetic background of the subject, as demonstrated in a large study of monozygotic and dizygotic twins98. The APOE genotype is the strongest prevalent genetic risk factor for AD. Structural and specific gut microbiome profiles were strongly and significantly associated with APOE alleles99,100. Tran et al.99 reported that different APOE genotypes can influence the relative abundances of several bacterial taxa, such as Prevotellaceae and Ruminococcaceae and several butyrate-producing genera, in both humans and transgenic mice. Guardia-Escote et al.101 also showed that the composition of gut microbiota in early life can be modulated by the APOE genetic background. Environmental factors such as dietary habits, living conditions, and contamination of environmental matrices can also interact with genetic profiles to affect gut microbiota composition93,102.
Diet is a principal environmental factor and an established modulator that influences gut microbiota composition103. Various dietary patterns, nutrients, and food components have the potential to substantially alter the gut microbiota composition. For example, the gut microbiota composition appears to be sensitive to caloric balance104. Cohousing mice harboring an obese twin’s microbiota with mice containing the lean co-twin’s microbiota fed low saturated fat, high fruit and vegetable diet can take on microbiota characteristics of lean mice105. High energy-dense diet rapidly altered the gut microbiota composition with increases in pro-inflammatory Proteobacteria proliferation and in Firmicutes/Bacteroidetes ratio in rats106. Moreover, a rapid shift in gut microbiota composition was observed in humans, with increased abundances of Alistipes, Bilophila, and Bacteroides, after consuming a high-fat/protein diet for 5 days103, and Bacteroides spp. are highly associated with animal proteins, but Prevotella spp. are highly associated with increased intakes of plant proteins107.
The microbiota-gut-brain axis
The gut-brain axis is a network comprising the gastrointestinal tract, the enteric nervous system (ENS), and the brain. Immunity, digestion, metabolism, satiety, and stress reactions can be regulated by bidirectional communication along the gut-brain axis108. Gut bacteria have been found to play crucial roles in neurodevelopment, neuroinflammation, and behavior109. A growing body of research has focused on the microbiota-gut-brain axis. The vagus nerve synapses on enteric neurons and enables gut-brain communication. The dysregulation of the microbiota-gut-brain axis has been increasingly implicated in psychiatric and neurological disorders, such as AD110, PD111, stroke109, and multiple sclerosis109. Gut microbial products can affect neuronal transcription and thus host behavior via gene–environment interactions112,113. For example, γ-aminobutyric acid (GABA), tryptophan, serotonin, histamine, and dopamine, which are neurotransmitters or precursors in the brain, can directly affect how neurons communicate with each other. The microbiota-gut-brain axis is a potential new therapeutic target for the effective treatment of CNS disorders via the immune system, direct ENS routes, and the vagus nerve by altering the recruitment of host neurochemical signaling and the production of bacterial metabolites108. Microbial metabolites are often most markedly altered in the disease state, and such metabolites [e.g., short-chain fatty acids (SCFAs), tryptophan, tyrosine derivatives, and trimethylamine N-oxide] have significant effects on physiological processes114.
There are many bidirectional communication pathways between the gut microbiota and the brain, including the autonomic nervous system (ANS), ENS, immune-modulatory responses, enteroendocrine signaling, neurotransmitters, and microbial metabolite signaling115. The ANS coordinates with the hypothalamic-pituitary-adrenal (HPA) axis to promote integrated communication between the brain and the gut, which is responsible for endocrine and physiological homeostasis and autonomic, motor, and behavioral functions. The ENS communicates with the CNS via intestinofugal neurons116. Enteroendocrine cells, such as enteroendocrine L cells and enterochromaffin cells, are essential for maintaining gut homeostasis; they can establish direct contact with luminal constituents via the apical surface117. Microbiota-derived neuromodulatory metabolites include catecholamines, histamine, 5-hydroxytryptamine (5-HT), GABA, and tryptophan precursors and metabolites, which are involved in host mood, behavior, and cognition118. Branched-chain amino acids (BCAAs) participate in a variety of biochemical functions in the peripheral and CNS119,120. BCAAs enhance protein synthesis through the mTOR signaling pathway, reduce protein oxidation, and have positive effects on mitochondrial biogenesis and ROS scavenging120. SCFAs are key players in microbiota-gut-brain axis communication that influence intestinal mucosal immunity, barrier integrity, and function, as well as BBB integrity and neuroinflammation121. Acetate, propionate, and butyrate are the most abundant SCFAs in the human body, and SCFAs might influence the microbiota-gut-brain axis via interaction with free fatty acid receptors (FFARs) and/or inhibition of histone deacetylases (HDACs)122. SCFAs have been implicated in gastrointestinal function123, immune function124, autism spectrum disorder (ASD)122, PD125, and AD126. SCFAs interact with gut mucosal enteroendocrine cells and can migrate into the CNS127. Neuromodulators, SCFAs, bile acids, bacteriocins, and choline are immunomodulatory and activate the innate immune system. Pro-inflammatory cytokines within the brain are released when the innate immune response is activated128. In turn, astrocytes are activated by microglia via NF-κB signaling due to the upregulation of these pro-inflammatory cytokines30, leading to further amplification of inflammation and the immune response129, deterioration of brain structure and function, and disease pathology130 (Fig. 4). A recent study found that Aβ deposits were observed in the gastrointestinal tract of AD patients and transgenic mice overexpressing APP131,132. Enteric Αβ directly induces cerebral amyloidosis and AD-like dementia may be by retrograde axonal transportation via the vagus132.
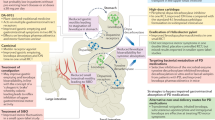
Fecal microbiota transplantation in neurodegenerative diseases
Research on the role of the gut microbiome in regulating brain function is growing rapidly. The microbiome may be a key susceptibility factor for neurodegenerative diseases. Perturbations of the gut microbiota are associated with multiple diseases. The microbiota in the gastrointestinal tract impacts the development and function of the nervous, metabolic, and immune systems133. Microbiome reconfiguration can alter its function and may modify disease symptoms134. Transplantation of microbiota from patients with ASD135, schizophrenia (SCZ)136, and irritable bowel syndrome (IBS) into wild-type mice promoted indication-specific behavioral symptoms137, such as hallmark autistic behaviors for ASD; locomotor hyperactivity decreased anxiety and depressive-like behaviors, and increased startle responses for SCZ; and faster gastrointestinal transit, intestinal barrier dysfunction, innate immune activation, and anxiety-like behavior for IBS. FMT and antibiotic and probiotic interventions have shown promise for the treatment of neurodegenerative diseases in limited human trials. FMT is a procedure in which stool from a healthy donor is placed into another patient’s intestine138. FMT from a healthy donor resolved recurrent Clostridioides difficile infections and was suggested to prevent multiple sclerosis disease progression for over 10 years139. FMT temporarily improved leg tremors and other PD symptoms in a PD patient140. Xue et al.141 reported that FMT via colonoscopy can relieve the motor and non-motor symptoms with acceptable safety in PD patients based on a small-scale trial. FMT cured epilepsy in a case with Crohn’s disease142. Many animal studies have suggested a positive effect of FMT on neurodegenerative diseases.
Germ-free wild-type mice and their offspring had ASD-like symptoms and displayed alternative splicing of ASD-relevant genes when FMT was performed with stool from children with ASD. ASD symptoms decreased when GABA receptor agonists were administered to the ASD model135. Decreased cerebral oxidative stress was observed in another study after FMT from a normal hamster in an ASD hamster model143.
Gut bacteria control the differentiation and function of immune cells144,145. Braak’s hypothesis posits that PD may start in the gut, triggered by a pathogen, and spread to the brain146. Gut microbiota dysbiosis is linked to PD134. Sampson et al.134 reported that gut microbiota are required for motor deficits, microglial activation, and α-synuclein pathology in an α-synuclein-overexpressing mouse model, revealing that alterations in the human microbiome represent a risk factor for PD. Elevated levels of probiotics and depletion of anti-inflammatory SCFA-producing bacteria have been confirmed in PD patients147. α-Synuclein-overexpressing germ-free mice that underwent FMT with stool from PD-affected patients exhibited enhanced physical impairments134. The PD mice model showed improved motor function in the pole descent test and traction test and inhibited the TLR4/TBK1/NF-κB/TNF-α signaling pathway-mediated gut inflammation and neuroinflammation after receiving feces from healthy mice in another study148. Conversely, wild-type mice administered fecal matter from PD mice displayed impaired motor function and decreased striatal dopamine and serotonin levels, while FMT had no side effects on behavioral functions and neurotransmitters in normal mice148.
Another study found that the seizure threshold increased after transplantation with Parabacteroides merdae, Akkermansia muciniphila, and Parabacteroides distasonis in mice149. There are numerous publications about the relationship between AD and gut microbiota. The composition of the gut microbiota of AD patients differed from that of healthy controls at the taxonomic level, such as Bacteroides, Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales150. Gut microbial alterations have been associated with cognitive impairment151 and Aβ load110 in older adults. Gut microbiota alterations may stimulate inflammatory pathways that trigger neuroinflammation152. The pro-inflammatory cytokines IL-6, CXCL2, NLRP3, and IL-1β and the anti-inflammatory cytokine IL-10 are released by TLRs, and they can cross the BBB via both diffusion and cytokine transporters110. Patients with cognitive impairment and brain amyloidosis have more pro-inflammatory gut bacteria in their feces110. Furthermore, germ-free wild-type mice that received AD feces showed lower levels of neuro-related fecal metabolites and poorer cognitive function153. Microbial-mediated intestinal and systemic immune dysfunction is an important component of the pathogenesis of AD, and FMT from healthy wild-type mice into transgenic AD model mice with AD-like pathology, amyloid deposits, and neurofibrillary tangles alleviated the formation of Aβ plaques and neurofibrillary tangles, glial reactivity, and cognitive impairment154.
FMT may reverse the decrease in cognitive function induced by antibiotics. Wild-type mice showed a cognitive decline after broad-spectrum antibiotic therapy. However, memory and spatial learning were improved after receiving anti-aging mouse feces155. Human microbiome transplantation protected germ-free mice from death caused by acute arsenic toxicity156. According to our research, Mn exposure increased Aβ and inflammatory factor production in the brain and caused hippocampal degeneration and necrosis66,90. FMT from normal rats alleviated the neurotoxic effects of Mn exposure by altering the gut microbiota66.
The literature suggests a potential beneficial effect of healthy donor FMT. FMT may be a promising treatment option for neurodegenerative diseases, and restoration of the gut microbiota to a premorbid state is a novel goal for prevention and treatment strategies157. However, for microbiome-linked diseases, the gut microbiota required for successful treatment remains unknown. When preparing FMT, careful measures should be taken to maintain the viability of the diverse bacterial population. Meanwhile, inherent risks of FMT include the possibility of aspiration with bowel perforation after a colonoscopy and upper gastrointestinal delivery158. Some mild gastrointestinal symptoms have been reported after FMT, including constipation, diarrhea, fever, abdominal discomfort, flatulence, bloating, belching, vomiting, nausea, and borborygmus159. FMT also has the risk of infection transmission160, such as bacterial translocation, and bacterial infections caused by multidrug-resistant organisms. With the COVID-19 pandemic, FMT could potentially transmit SARS-CoV-2. SARS-CoV-2 genetic material, including live virus, can be detected in feces even after the resolution of respiratory symptoms161,162. Autoimmune diseases and metabolic syndrome are associated with disturbances in the gut microbiome and should also be assessed as potential long-term risks related to FMT158.
Conclusions
Despite significant advances in our understanding of the pathobiology of neurodegenerative diseases, pathobiology is complex, and we have not yet identified an effective treatment for neurodegenerative diseases in humans. It has been revealed that bidirectional communication exists between the brain and the gut. The microbiota in the gastrointestinal tract impacts the development and functions of the immune, metabolic, and nervous systems and is associated with multiple diseases. The latest findings reviewed here improve our understanding of the genetic and environmental risk factors of neurodegenerative disease. Moreover, the discovery of the communication among the components of the microbiota-gut-brain axis has led to the idea of ameliorating neurodegenerative diseases by FMT. FMT may be a promising treatment option with great potential to treat neurodegenerative diseases in the future. However, we should also be aware that FMT could increase the risk of bacterial translocation, sepsis, and bacterial infections caused by multidrug-resistant organisms.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Bianchi, V. E., Herrera, P. F. & Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. https://doi.org/10.1080/1028415X.2019.1681088 (2019).
Vaquer-Alicea, J. & Diamond, M. I. Propagation of protein aggregation in neurodegenerative diseases. Annu. Rev. Biochem. 88, 785–810 (2019).
Scheiblich, H., Trombly, M., Ramirez, A. & Heneka, M. T. Neuroimmune connections in aging and neurodegenerative diseases. Trends Immunol. 41, 300–312 (2020).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Power, R., Prado-Cabrero, A., Mulcahy, R., Howard, A. & Nolan, J. M. The role of nutrition for the aging population, implications for cognition and Alzheimer’s disease. Ann. Rev. Food Sci. Technol. 10, 619–639 (2019).
Maiese, K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann. Med. 46, 587–596 (2014).
Hirtz, D. et al. How common are the “common” neurologic disorders? Neurology 68, 326–337 (2007).
Yaffe, K. et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch. Neurol. 68, 631–636 (2011).
Gandy, S. & DeKosky, S. T. Toward the treatment and prevention of Alzheimer’s disease, rational strategies and recent progress. Annu. Rev. Med. 64, 367–383 (2013).
Maiese, K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br. J. Clin. Pharmacol. 82, 1245–1266 (2016).
Long, J. M. & Holtzman, D. M. Alzheimer disease, an update on pathobiology and treatment strategies. Cell 179, 312–339 (2019).
Haass, C., Kaether, C., Thinakaran, G. & Sisodia, S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2, a006270 (2012).
Naj, A. C. & Schellenberg, G. D. Alzheimer’s Disease Genetics Consortium (ADGC), Genomic variants, genes, and pathways of Alzheimer’s disease, An overview. Am. J. Med. Genet. B Neuropsychiatr. Genet. 174, 5–26 (2017).
Cirrito, J. R. et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58, 42–51 (2008).
Sato, C. et al. Tau kinetics in neurons and the human central nervous system. Neuron 97, 1284–1298 (2018).
He, Z. et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 24, 29–38 (2018).
Wang, L. et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. JAMA Neurol. 73, 1070–1077 (2016).
Choi, S. H. et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515, 274–278 (2014).
Vogel, J. W. et al. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat. Commun. 11, 2612 (2020).
Hanseeuw, B. J. et al. Association of amyloid and Tau with cognition in preclinical Alzheimer disease, a longitudinal study. JAMA Neurol. 76, 915–924 (2019).
Dujardin, S. et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 26, 1256–1263 (2020).
Efhymiou, A. G. & Goate, A. M. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 12, 43 (2017).
Murphy, M. P. & LeVine, H. III Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 19, 311–323 (2010).
Mishra, A. K. et al. Aberrant autophagy and Parkinsonism, does correction rescue from disease progression? Mol. Neurobiol. 51, 893–908 (2015).
Braczynski, A. K., Schulz, J. B. & Bach, J. P. Vaccination strategies in tauopathies and synucleinopathies. J. Neurochem 143, 467–488 (2017).
Lashuel, H. A., Overk, C. R., Oueslati, A. & Masliah, E. The many faces of alpha-synuclein, from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 14, 38–48 (2013).
Dehay, B. et al. Targeting α-synuclein for treatment of Parkinson’s disease, mechanistic and therapeutic considerations. Lancet Neurol. 14, 855–866 (2015).
Peng, C., Trojanowski, J. Q. & Lee, V. M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurosci. 16, 199–212 (2020).
Irwin, D. J. et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 72, 587–598 (2012).
Sepulcre, J. et al. In vivo tau, amyloid, and gray matter profiles in the aging brain. J. Neurosci. 36, 7364–7374 (2016).
Haggerty, T. et al. Hyperphosphorylated Tau in an α-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur. J. Neurosci. 33, 1598–1610 (2011).
Kirkley, K. S., Popichak, K. A., Afzali, M. F., Legare, M. E. & Tjalkens, R. B. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflammation 14, 99 (2017).
Tsarouchas, T. M. et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 9, 4670 (2018).
Walter, J. The triggering receptor expressed on myeloid cells 2, a molecular link of neuroinflammation and neurodegenerative diseases. J. Biol. Chem. 291, 4334–4341 (2016).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Castro-Sanchez, S., Garcia-Yague, A. J., Lopez-Royo, T., Casarejos, M., Lanciego, J. L. & Lastres-Becker, I. Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of Parkinson’s disease. Glia 66, 1752–1762 (2018).
Guerreiro, R. & Hardy, J. Genetics of Alzheimer’s disease. Neurotherapeutics 11, 732–737 (2014).
Ridge, P. G. et al. Assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol. Aging 41, 200.e13–200.e20 (2016).
Lardenoije, R. et al. Age-related epigenetic changes in hippocampal subregions of four animal models of Alzheimer’s disease. Mol. Cell. Neurosci. 86, 1–15 (2018).
Ishii, M. & Iadecola, C. Risk factor for Alzheimer’s disease breaks the blood-brain barrier. Nature 581, 31–32 (2020).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease, pathobiology and targeting strategies. Nat. Rev. Neurosci. 15, 501–518 (2019).
Sepulcre, J. et al. Neurogenetic contributions to amyloid beta and tau spreading in the human cortex. Nat. Med. 24, 1910–1918 (2018).
Calafate, S., Flavin, W., Verstreken, P. & Moechars, D. Loss of Bin1 promotes the propagation of tau pathology. Cell Rep. 17, 931–940 (2016).
Sims, R. et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384 (2017).
van der Lee, S. J. et al. A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer’s disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol. 138, 237–250 (2019).
Parhizkar, S. et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 22, 191–204 (2019).
Morita, N. et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature 566, 110–114 (2019).
Bolós, M. et al. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol. Neurodegener. 12, 59 (2017).
Bhaskar, K., Konerth, M., Kokiko-Cochran, O. N., Cardona, A., Ransohoff, R. M. & Lamb, B. T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31 (2010).
Leonardi, I. et al. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science 359, 232–236 (2018).
Pao, P. C. et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 11, 2484 (2020).
Itzhaki, R. F. et al. Whittum-Hudson, microbes and Alzheimer’s disease. J. Alzheimers Dis. 51, 979–984 (2016).
Steger, M. et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5, e12813 (2016).
Bae, E. J. et al. LRRK2 kinase regulates alphasynuclein propagation via RAB35 phosphorylation. Nat. Commun. 9, 3465 (2018).
Kluss, J. H., Mamais, A. & Cookson, M. R. LRRK2 links genetic and sporadic Parkinson’s disease. Biochem. Soc. Trans. 47, 651–661 (2019).
Abeliovich, A. & Gitler, A. D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539, 207–216 (2016).
Beers, D. R. & Appel, S. H. Immune dysregulation in amyotrophic lateral sclerosis, mechanisms and emerging therapies. Lancet Neurol. 18, 211–220 (2019).
Goldman, S. M. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 54, 141–164 (2014).
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015, a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
Fleming, S. M. Mechanisms of gene-environment interactions in Parkinson’s disease. Curr. Environ. Health Rep. 4, 192–199 (2017).
Tanner, C. M. et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872 (2011).
Sarkar, S. et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal. 12, eaat9900 (2019).
Kawahara, M., Kato-Negishi, M. & Tanaka, K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics 9, 619–633 (2017).
Wang, H. et al. iTRAQ-based proteomic technology revealed protein perturbations in intestinal mucosa from manganese exposure in rat models. RSC Adv. 7, 31745–31758 (2017).
Wang, H. et al. The gut microbiota confers protection in the CNS against neurodegeneration induced by manganism. Biomed. Pharmacother. 127, 110150 (2020).
Jenkitkasemwong, S. et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl Acad. Sci. USA 115, E1769–E1778 (2018).
Harischandra, D. S. et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci. Signal. 12, eaau4543 (2019).
Mirza, A., King, A., Troakes, C. & Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 40, 30–36 (2017).
Garza–Lombó, C., Posadas, Y., Quintanar, L., Gonsebatt, M. E. & Franco, R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure, Redox signaling and oxidative stress. Antioxid. Redox Signal. 28, 1669–1703 (2018).
Mold, M., Linhart, C., Gómez-Ramírez, J., Villegas-Lanau, A. & Exley, C. Aluminum and amyloid-β in familial Alzheimer’s disease. J. Alzheimers Dis. 73, 1627–1635 (2020).
Lukiw, W. J. et al. Aluminum in neurological disease - a 36 year multicenter study. J. Alzheimers Dis. Parkinsonism 8, 457 (2019).
Mezzaroba, L., Alfieri, D. F., Colado Simão, A. N. & Vissoci Reiche, E. M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 74, 230–241 (2019).
Farina, M., Avila, D. S., Da Rocha, J. B. & Aschner, M. Metals, oxidative stress and neurodegeneration, A focus on iron, manganese and mercury. Neurochem. Int. 62, 575–594 (2013).
Rodrigues, E. G. et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ. Health 15, 1–9 (2016).
Cholanians, A. B. et al. Arsenic induces accumulation of α-synuclein, Implications for synucleinopathies and neurodegeneration. Toxicol. Sci. 153, 271–281 (2016).
Karri, V. et al. Differential protein expression of hippocampal cells associated with heavy metals (Pb, As, and MeHg) neurotoxicity: deepening into the molecular mechanism of neurodegenerative diseases. J. Proteom. 187, 106–125 (2018).
Kim, K. H., Kabir, E. & Jahan, S. A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 575, 525–535 (2017).
Won, J. H., Park, S., Hong, S., Son, S. & Yu, J. W. Rotenone-induced impairment of mitochondrial electron transport chain confers a selective priming signal for NLRP3 inflammasome activation. J. Biol. Chem. 290, 27425–27437 (2015).
Martinez, E. M. et al. Editor’s highlight, Nlrp3 is required for inflammatory changes and nigral cell loss resulting from chronic intragastric rotenone exposure in mice. Toxicol. Sci. 159, 64–75 (2017).
Liu, Z. et al. Silymarin attenuated paraquat-induced cytotoxicity in macrophage by regulating Trx/TXNIP complex, inhibiting NLRP3 inflammasome activation and apoptosis. Toxicol. Vitr. 46, 265–272 (2018).
Chen, L., Na, R., Boldt, E. & Ran, Q. NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol. Aging 36, 2533–2543 (2015).
Li, W. & Ehrich, M. Transient alterations of the bloodbrain barrier tight junction and receptor potential channel gene expression by chlorpyrifos. J. Appl. Toxicol. 33, 1187–1191 (2013).
Ransohoff, R. M. How neuroinflammation contributes to neurodegeneration. Science 353, 777–783 (2016).
Cannon, J. R. & Greenamyre, J. T. Gene-environment interactions in Parkinson’s disease: specific evidence in humans and mammalian models. Neurobiol. Dis. 57, 38–46 (2013).
Karl, J. P. et al. Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 9, 2013 (2018).
Gubert, C., Kong, G., Renoir, T. & Hannan, A. J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 134, 104621 (2020).
Xia, J. Z. et al. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci. Total Environ. 631–632, 439–448 (2018).
Feng, P. et al. A Review on gut remediation of selected environmental contaminants: possible roles of probiotics and gut microbiota. Nutrients 11, 22 (2019).
Wang, H. et al. The gut microbiota attenuate neuroinflammation in manganese exposure by inhibiting cerebral NLRP3 inflammasome. Biomed. Pharmacother. 129, 110449 (2020).
Nasuti, C. et al. Neonatal exposure to permethrin pesticide causes lifelong fear and spatial learning deficits and alters hippocampal morphology of synapses. J. Neurodev. Disord. 6, 1–11 (2014).
Beaumont, M. et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 17, 189 (2016).
Turpin, W. et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat. Genet. 48, 1413–1417 (2016).
Matsumoto, L. et al. CpG demethylation enhances alpha-synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS ONE 5, e15522 (2010).
Friedland, R. P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimers Dis. 45, 349–362 (2015).
Tsuang, D. et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 70, 223–228 (2013).
Vascellari, S. et al. Genetic variants of TAS2R38 bitter taste receptor associate with distinct gut microbiota traits in Parkinson’s disease: a pilot study. Int. J. Biol. Macromol. 165, 665–674 (2020).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Tran, T. T. T. et al. APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J. 33, 8221–8231 (2019).
Parikh, I. J. et al. Murine gut microbiome association with APOE alleles. Front Immunol. 11, 200 (2020).
Guardia-Escote, L. et al. APOE genotype and postnatal chlorpyrifos exposure modulate gut microbiota and cerebral short-chain fatty acids in preweaning mice. Food Chem. Toxicol. 135, 110872 (2020).
Di Ciaula, A., Stella, A., Bonfrate, L., Wang, D. Q. H. & Portincasa, P. Gut microbiota between environment and genetic background in familial Mediterranean fever (FMF). Genes 11, 1041 (2020).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Vaughn, A. C. et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gutbrain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. 77, 18–30 (2017).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Long-Smith, C. et al. Microbiota-gut-brain axis, new therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 60, 477–502 (2020).
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V. & Dinan, T. G. The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194 (2020).
Cattaneo, A. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68 (2017).
Hilton, D. et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 127, 235–241 (2014).
Stilling, R. M., Bordenstein, S. R., Dinan, T. G. & Cryan, J. F. Friends with social benefits, hostmicrobe interactions as a driver of brain evolution and development? Front. Cellular Infect. Microbiol 4, 147 (2014).
Stilling, R. M., Dinan, T. G. & Cryan, J. F. Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis. Genes Brain Behav. 13, 69–86 (2014).
Zhang, L. S. & Davies, S. S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 8, 46 (2016).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198 (2017).
Sperringer, J. E., Addington, A. & Hutson, S. M. Branched-chain amino acids and brain metabolism. Neurochem. Res. 42, 1697–1709 (2017).
Zhang, S., Zeng, X., Ren, M., Mao, X. & Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: a review. J. Anim. Sci. Biotechnol. 8, 10 (2017).
Neinast, M., Murashige, D. & Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 81, 139–164 (2019).
Silva, Y. P., Bernardi, A. & Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11, 25 (2020).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Gill, P. A., van Zelm, M. C., Muir, J. G. & Gibson, P. R. Review article, short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 48, 15–34 (2018).
Erny, D., Hrabě de Angelis, A. L. & Prinz, M. Communicating systems in the body, how microbiota and microglia cooperate. Immunology 150, 7–15 (2017).
Unger, M. M. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72 (2016).
Zhang, L. et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 60, 1241–1257 (2017).
Overduin, J., Schoterman, M. H., Calame, W., Schonewille, A. J. & Ten Bruggencate, S. J. Dietary galacto-oligosaccharides and calcium, effects on energy intake, fat-pad weight and satiety-related, gastrointestinal hormones in rats. Br. J. Nutr. 109, 1338–1348 (2013).
Taipa, R. et al. Proinflammatory and anti–inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 76, 125–132 (2019).
Morales, I., Farías, G. & Maccioni, R. B. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation 17, 202–204 (2010).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Eisele, Y. S. & Duyckaerts, C. Propagation of Aβ pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 131, 5–25 (2016).
Sun, Y. et al. Intra-gastrointestinal amyloid-β1-42 oligomers perturb enteric function and induce Alzheimer’s disease pathology. J. Physiol. 598, 4209–4223 (2020).
Cho, I. & Blaser, M. J. The human microbiome, at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Sharon, G. et al. Human gut microbiota from Autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618 (2019).
Zheng, P. et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5, eaau8317 (2019).
De Palma, G. et al. Transplantation of fecalmicrobiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med 9, eaaf6397 (2017).
Gupta, A. & Khanna, S. Fecal microbiota transplantation. JAMA 318, 102 (2017).
Makkawi, S., Camara-Lemarroy, C. & Metz, L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 5, e459 (2018).
Huang, H. et al. Fecal microbiota transplantation to treat Parkinson’s disease with constipation, a case report. Medicine 98, e16163 (2019).
Xue, L. J. et al. Fecal microbiota transplantation therapy for Parkinson’s disease: a preliminary study. Medicine 99, e22035 (2020).
He, Z. et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease, the first report. World J. Gastroenterol. 23, 3565–3568 (2017).
Aabed, K. et al. Ameliorative effect of probiotics [Lactobacillus paracaseii and Protexin(R)] and prebiotics (propolis and bee pollen) on clindamycin and propionic acid-induced oxidative stress and altered gut microbiota in a rodent model of autism.Cell. Mol. Biol. 65,1–7 (2019).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Del Tredici, K. & Braak, H. A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol. 115, 379–384 (2008).
Wallen, Z. D. et al. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 6, 11 (2020).
Sun, M. F. et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice, gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 70, 48–60 (2018).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741 (2018).
Zhuang, Z. Q. et al. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimers Dis. 63, 1337–1346 (2018).
Manderino, L. et al. Preliminary evidence for an association between the composition of the gut microbiome and cognitive function in neurologically healthy older adults. J. Int. Neuropsychol. Soc. 23, 700–705 (2017).
Lin, C. et al. Microbiota-gut-brain axis and toll-like receptors in Alzheimer’s disease. Comput. Struct. Biotechnol. J. 17, 1309–1317 (2019).
Fujii, Y. et al. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosc. Biotechnol. Biochem. 83, 2144–2152 (2019).
Kim, M. S. et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69, 283–294 (2020).
Zhan, G. et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 10, 1257–1267 (2018).
Coryell, M., McAlpine, M., Pinkham, N. V., McDermott, T. R. & Walk, S. T. The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat. Commun. 9, 5424 (2018).
Allegretti, J. R., Mullish, B. H., Kelly, C. & Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 394, 420–431 (2019).
Gupta, S., Mullish, B. H. & Allegretti, J. R. Fecal microbiota transplantation: the evolving risk landscape. Am. J. Gastroenterol. 116, 647–656 (2021).
Baxter, M. & Colville, A. Adverse events in faecal microbiota transplant: a review of the literature. J. Hosp. Infect. 92, 117–127 (2016).
Cheng, Y. W. et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: a multicenter experience. Am. J. Transpl. 19, 501–511 (2019).
Wang, W. et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323, 1843–1844 (2020).
Xiao, F. et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158, 1831–1833.e3 (2020).
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 31802256) and Gansu Province Science Fund for Distinguished Young Scholars (No. 20JR5RA579) that was granted to Hui Wang.
Author information
Authors and Affiliations
Contributions
H.W. and Y.S. were responsible for the review. H.W., F.Y., S.Z., and R.X. performed the literature search. H.W. wrote the original draft of the manuscript with review and editing by Y.S. All authors have approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Yang, F., Zhang, S. et al. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. npj Parkinsons Dis. 7, 70 (2021). https://doi.org/10.1038/s41531-021-00213-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-021-00213-7
This article is cited by
-
Converged avenues: depression and Alzheimer’s disease– shared pathophysiology and novel therapeutics
Molecular Biology Reports (2024)
-
Gene–environment interactions in Alzheimer disease: the emerging role of epigenetics
Nature Reviews Neurology (2022)
-
Gut–Brain Axis, Neurodegeneration and Mental Health: A Personalized Medicine Perspective
Indian Journal of Microbiology (2022)
-
Pharmacophore based virtual screening of cholinesterase inhibitors: search of new potential drug candidates as antialzheimer agents
In Silico Pharmacology (2022)