Abstract
The corrosion of aluminum alloys (AA) caused by fungi has been considered as the typical reason causing the failure of aviation materials, but its corrosion mechanism is still unclear. In this work, AA 7075 corrosion induced by Fungus Aspergillus terreus with continued organic carbon starvation was investigated in artificial seawater. Results indicate that A. terreus can survive with organic carbon starvation and form a biofilm on AA surface, then accelerate AA corrosion. The pitting corrosion is more severe in biotic environments, and it is closely related to the initial spore concentration. The higher initial spore concentration, the more A. terreus survivors, leading to the more severe pitting corrosion. Acid corrosion caused by organic acids of A. terreus has no contribution to the corrosion acceleration of aluminum alloys. A. terreus can help to destroy the passive film of aluminum alloys, leading to more corrosion pits compared with the control.
Similar content being viewed by others
Introduction
Aluminum alloys (AA) with high mechanical strength, such as AA 7075, have been widely used in the aviation industry, vehicles, ships, and warships due to their special features, such as low density, satisfactory plasticity, and high corrosion resistance1,2,3. Al, Mg, Zn, and Cu are the main elements for the high-strength AA. A passive film with several nanometers will be formed on the AA surface, leading to a low corrosion rate. However, localized corrosion still can occur due to the presence of defects in the passive film and the harsh environment, which then leads to the service failure of engineering equipment4,5. So, corrosion of AA still is a big trouble for the safe running of engineering equipment.
In recent years, more and more researchers begin to focus on microbiologically influenced corrosion (MIC) and considered that MIC can accelerate the corrosion of metal especially in marine environments6,7,8. The microorganisms related to MIC include, but are not limited to, bacteria, archaea, fungi, lichens, and algae9,10,11. The adhesion of microorganisms and the subsequent biofilm formation on AA surface can surely accelerate the damage of surface passive film and then increase the localized corrosion rate. Effects of MIC on AA have also been studied in the literature. Guan et al.12 have found the localized corrosion of AA was enhanced by anaerobic sulfate-reducing bacteria (SRB), and element Al as a nutrient could improve the metabolic activity of SRB. Except for the bacteria, fungi such as Aspergillus niger, Penicillium frequentans, have been found on the corroded areas of aircraft13. Compared with the bacteria, fungi can grow well on the surface of AA in a humid environment, leading to more aggressive corrosion toward AA14. Dai et al.15 investigated AA 2024 corrosion induced by A. niger, and they found that the corrosion rate of AA with the assistance of A. niger increased by more than 4 times compared with the control. And A. niger also promoted the formation of severe pitting corrosion. Wang et al.16 also observed that the localized corrosion of AA 7075 was enhanced by A. niger, and the depth of corrosion pits could reach 247 μm after 18 days of testing.
In the aviation industry, it is necessary and urgent to investigate the mechanistic corrosion behavior and mechanism of the AA and confirm the main influence factors. For the AA MIC induced by fungi, acid corrosion has been confirmed as one of the key corrosion mechanisms accelerating pitting corrosion17,18. Because, amounts of organic acids with a high concentration, such as acetic acid, succinic acid, glutaric acid, glyoxylic acid, were secreted by fungi, leading to a low pH value of testing solution19. However, it is still unclear if there are some other possible corrosion mechanisms except for acid corrosion in the presence of fungi.
The theory of electron transfer, such as extracellular electron transfer (EET) and direct electron transfer (DET), have been applied to illustrate SRB corrosion behavior and mechanism by more and more researchers20,21,22. SRB cells even can produce some bio-nanowires with organic carbon starvation, and the SRB can acquire electrons from steel by these bio-nanowires23. Acquiring energy from metal is the main reason why microorganisms accelerate metal corrosion24. Similarly, fungi corrosion for AA can also aim to acquire energy or get some metal elements used to their metabolism. Some studies have also found that fungi, such as yeast and Cladosporium, can also have the EET process25,26. But, the studies about EET corrosion of fungi can not be found in the literature. Additionally, organic carbon starvation easily occurs in the environment for the fungi adhesion on the AA surface. It is significant to investigate fungi corrosion behavior and mechanism with continued organic carbon starvation in the marine condition. A. terreus, a mycelial fungus belonging to aspergillus, can secrete amounts of organic acids which can be easily found in the marine environment27,28. They can easily adhere to the metal surface. But, effects of A. terreus on AA corrosion is still unknown, and more works should be done to investigate AA corrosion mechanism caused by A. terreus.
In this work, the spores of the fungus A. terreus with a different concentration in artificial seawater were prepared without an organic carbon source. The mechanistic corrosion and mechanism of aluminum alloy 7075 caused by fungus A. terreus with continued organic carbon starvation were studied in artificial seawater using various electrochemical measurements and surface analysis. The effects of electron transfer mechanisms on AA corrosion was also discussed.
Results
Identification of fungus A. terreus
The polymerase chain reaction (PCR) amplification of 16S rDNA is used to do the identification of fungus used in this work. According to the DNA sequence, the fungus used in this work is A. terreus.
Spore counts of A. terreus
Table 1 shows the spore counts of A. terreus initially and after 14 days of testing. The initial spore concentrations of Aspergillus terreus are 108 spores mL−1, 106 spores mL−1, 104 s spores mL−1, respectively. It aims to study the effects of spore concentration on AA corrosion. The amounts of both planktonic and sessile A. terreus can survive with continued organic carbon starvation after 14 days. But it is also seen that more than 90% A. terreus have died compared with the initial concentration. The presence of sessile spores suggests that A. terreus can grow on the surface of AA and form a biofilm. The initial pH value of artificial seawater is 8.2, but the pH values change to 8.2, 7.4, 6.9, and 6.1 corresponding to the control specimen and specimens with initial spore concentrations of 104 spores mL−1, 106 spores mL−1, and 108 spores mL−1, respectively.
SEM analysis
SEM images of surface films with different original spore concentrations after 14 days of testing are shown in Fig. 1. And the corresponding EDS analysis results of surface films located in various areas are shown in Table 2. For the control specimen in the absence of A. terreus, the scratch lines can be seen and only little corrosion products (Fig. 1a, a3), suggesting that the corrosion AA in the abiotic artificial seawater is slight. From the part enlarge image of Fig. 1a1, a2, some little corrosion product particles can be observed. And the EDS results of site A (Table 2) indicate that the key elements of corrosion products are O, Al, C, Mg, Cu, Cr. The biofilms of A. terreus covered on the specimen surface can be observed. When the initial spore concentration is 104 spores mL−1, there are amounts of corrosion products observed on the specimen surface, and a compact biofilm can also be seen (Fig. 1b, b1). The layered corrosion products agglomerate together (Fig. 1b2), and EDS results (Table 2) of site B indicate that the corrosion products are main the oxides of aluminum. Some hyphae with a big width can be found (Fig. 1d3), which is confirmed by the high C content of sit C (Table 2). And two spores in the hyphae can be observed. The growth and breed of A. terreus in biofilm are firstly found. With the increase of initial spore concentration, i.e., 106 spores mL−1, amounts of hyphae and spores of specimen surface can be found after 14 days of testing (Fig. 1c–c3). It is also seen that A. terreus like to grow together due to the presence of local agglomeration of hyphae and spores (Fig. 1c). The high contents of C, O, and Al at sites D and E (Table 2) confirm that the biofilms contain more organics because of the formation of biofilm. And some wide or thin hyphae can be found in Fig. 1c2, c3. The wide hyphae can be used to grow and breed. The initial pores concentration increases to 108 spores mL−1, also leading to compact surface biofilms, and some big agglomerates can be observed (Fig. 1d). From the part enlarge images of Fig. 1d1, d2, amounts of hyphae and spores accumulate together. EDS results of site F include a high content of C, O, and Al, similar to the results of site D, indicating the formation of a biofilm. Large amounts of spores can also be found from the other area of surface biofilm (Fig. 1d3), and EDS results of site G show the presence of C, O, and Al with high content.
a control, b 104 spores mL−1, c 106 spores mL−1, d 108 spores mL−1. Images (a1), (b1), (c1), and (d1) are the part enlarge of images (a), (b), (c), and (d) corresponding to the blue square area, respectively. Images (a2), (b2), (c2), and (d2) are the part enlarge of images (a1), (b1), (c1), and (d1), respectively. Images (a3), (b3), (c3), and (d3) are the different position of surface films corresponding to the control, 104 spores mL−1, 106 spores mL−1, and 108 spores mL−1, respectively.
Surface morphologies
Figure 2 shows the 2D and 3D surface morphologies of AA 7075 specimens without corrosion products caused by A. terreus corresponding to different initial spore concentrations after 14 days of testing. For the specimen in the absence of A. terreus, i.e., the control, the surface scratch of the specimen can be seen clearly (Fig. 2a, b), suggesting that the corrosion of the specimen in the absence of A. terreus is slight. But there are still some small corrosion pits that can be found from the part enlarged image of Fig. 2c. For the test solution with an initial spore concentration of 104 spores mL−1, the localized corrosion of the specimen is more severe compared with the control (Fig. 2d, e). There is a circular corrosion area in Fig. 2d, which can be the area of biofilm formation due to the agglomeration of A. terreus. Some big corrosion pits are apparently in Fig. 2f. Localized corrosion is apparently accelerated when the initial spore concentration increases to 106 spores mL−1 (Fig. 2g, h). The size of corrosion pits further increases, and some corrosion pits connect together forming a bigger corrosion pit (Fig. 2j). For the specimen with an initial spore concentration increases to 108 spores mL−1, the more severe localized corrosion can be easily recognized from Fig. 2k–m. The size of corrosion pits increases by several times compared with the other specimens. The depth of corrosion pits is more than 20 μm, which is also several times of the other specimens (Fig. 2m). The surface morphologies demonstrate that the uniform corrosion of all specimens is slight, but the localized corrosion is enhanced apparently with the gradual increase of the initial spore counts.
Figure 3 shows the SEM images of typical surface morphologies of specimens after 14 days of testing in the artificial seawater. The specimen with an initial spore concentration of 106 spores mL−1 is chosen to investigate effects of spores on pitting corrosion initiation of AA. The slight corrosion of the abiotic control specimen can be neglected, but some small pits can be found (Fig. 3a). For the biotic specimen, amounts of corrosion pits caused by A. terreus can be clearly found (Fig. 3b), and some residual inclusion particles due to corrosion can be found (Fig. 3c). This further verifies that A. terreus can enhance the corrosion of AA.
Electrochemical measurements
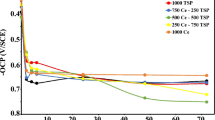
Figure 4 shows the time-dependent Nyquist and Bode plots of various specimens corresponding to different initial spore concentrations of A. terreus in the artificial seawater. For specimen in the absence of A. terreus, the diameters of Nyquist plots decrease gradually with time but with a big value (Fig. 4a), demonstrating that the control specimen’s corrosion is slight. The big width of phase angle (Fig. 4b) suggests that the surface passive film is good. For the specimen with an initial spore concentration of 104 spores mL−1, the diameters of Nyquist plots decrease from the 1st day to the 4th day, and change little from the 4th day to the 10th day (Fig. 4c). But there is an apparent increase after 10 days (Fig. 4c). For the specimen with an initial spore concentration of 106 spores mL−1, the diameters of Nyquist plots also decrease from 1th day to the 4th day, and then increase gradually with time. When the initial spore concentration increases to 108 spores mL−1, the diameters of Nyquist plots also decrease from 1th day to the 10th day, then have an increase (Fig. 4g). And it is also found that the diameters of Nyquist plots are small indicating a higher corrosion rate of the specimen (Fig. 4g). For the biotic specimen, the diameters of Nyquist plots have a fast decrease during the initial days, and also have an increase during the last days. These suggest that the presence of A. terreus can apparently accelerate AA corrosion initially, but has an inhibition effect in the later period.
All EIS data in this work are fitted using the equivalent circuit shown in Fig. 5a. In this equivalent circuit, Rs represents solution resistance. Qf and Rf are capacitance and resistance of the surface film, respectively. Qdl and Rct are assigned to a double-layer capacitance and a charge transfer resistance, respectively. In this work, using constant phase element (Q) to replace capacitance, because of the heterogeneity of specimen surface caused by corrosion. The changes of Rp values fitted from the EIS data in Fig. 4 with time using an electrochemical equivalent circuit are shown in Fig. 5b. And Rp represents the summation of fitted Rf and Rct, i.e., Rp = Rf + Rct. Generally, Rp values are inversely proportional to the corrosion rates, and the lower Rp values, the higher the corrosion rates. It is seen that the Rp values of abiotic control specimen decrease gradually with time, but they are bigger than these of biotic specimens, suggesting that the corrosion rate of the control specimen is small. For the biotic specimens, the Rp values are smaller than control, demonstrating that A. terreus can accelerate specimen corrosion. And it is also found that the Rp values of the biotic specimen have a fast decrease during the initial days, but also have an increase in the last days. The Rp values have a decline gradually with the increase of initial spore concentration. These indicate that the corrosion of specimen is related to the initial spore concentrations in the artificial seawater without organic carbon source. The more initial spore concentration, the higher the corrosion rate.
a electrochemical equivalent circuit, b changes of Rp, i.e., Rf + Rct, fitted from the EIS data in Fig. 5 with time.
Figure 6 shows the potentiodynamic polarization curves of AA specimens corresponding to different initial spore concentrations of A. terreus with continued organic carbon starvation after 14 days of testing in the artificial seawater. It is seen that both the cathodic and andic reactions are accelerated with the increase of initial spore concentration. And the higher initial spore concentration, the bigger the corrosion current density. These also suggest that the A. terreus with continued organic carbon starvation can promote AA corrosion, and the corrosion acceleration is related to the initial spore concentration. These results are corresponding to the EIS data (Figs. 4 and 5) and surface morphologies after removing corrosion products (Figs. 2 and 3).
WBE measurements
Figure 7 shows the changes of distributions of galvanic current density for the abiotic control WBE with time in the artificial seawater. There is a bigger cathodic site with a higher cathodic current density of more than 1 × 10−6 A cm−2 from 1st day to 14th day in the absence of A. terreus, while the anodic current densities are small. These suggest that the small cathode and big anode appear for the control specimen in the artificial seawater, thus leading to a low corrosion rate. It is also found that some local anodic sites still can be found. However, the anodic current densities are small in the initial 2 days. And the local anodic current density has an increase with time on the whole, and the biggest value can reach 6.87 × 10−7 A cm−2 at electrode 62 on the 10th day (Fig. 7e). These also demonstrate that even though the corrosion of control is slight, but there are still some anodic sites leading to tiny localized corrosion, corresponding to the surface morphologies in Fig. 2.
Figure 8 shows the changes of distributions of galvanic current density for the typical WBE with an initial spore concentration of 108 spores mL−1 with time in the artificial seawater. It is seen there are many anodic sites with a high anodic current density, which suggests that some more corrosion pits are formed in the presence of A. terreus corresponding to the surface morphologies (Fig. 2). Some anodic sites change their position with the increase of testing time, demonstrating that the MIC is a dynamic process. It is seen a bigger anodic current peak composed of several electrodes on the 14th day, and the biggest anodic current density at electrode 55 is 1.72 × 10−6 A cm−2 (Fig. 8f). These indicate that some anodic sites connect together, and then form a big corrosion pit, also corresponding to the surface morphology in Fig. 2k. There is a big cathode in the presence of A. terreus, which is contrary to the abiotic control specimen. Thus, the localized corrosion is accelerated in the presence of A. terreus even with continued organic carbon starvation.
Discussion
Fungi can be widely found, and they can grow well in a humid environment with proper organics. In this work, it is found that fungus A. terreus can survive with continued organic carbon starvation after 14 days of testing (Table 1). But more than 90% A. terreus have died. The A. terreus survivors can also adsorb and grow on AA surface, and then form a biofilm (Fig. 1). The A. terreus can produce spores through the hyphae (Fig. 1b3). So, one question is how the A. terreus grow without an organic carbon source. Some previous works have verified that some bacteria, such as SRB, could get electrons from Fe0 to obtain energy20,22,29. So, it can speculate that A. terreus also can get electrons from Al0. The direct electron transfer is difficult for the A. terreus initially due to the presence of a passive film of aluminum oxides. The electrical conductivity of aluminum oxide is worse. But, once the passive film is broken, the direct electron transfer can be responsible for the A. terreus. The study of Ramos et al.30 has demonstrated that the Escherichia coli survivors could get organic carbon from the release of dead cells without organic carbon, and they also found that 99% of Escherichia coli has died only after 2–3 days. Moroni et al.31 also found that fungi could survive for a long time after removing the source of pollutants, the organic nutrients of fungi. Fungi can subsist on almost any organic nutrients to maintain their biological activity. The survivors of fungus A. terreus can also get some amounts of organics from the dead cells. So, the higher the initial spore concentration of A. terreus, the more survivors in the absence of organic carbon source after 14 days (Table 1). Some researchers32,33 also found that some fungi, such as mycete, could get some metal elements, such as Fe, Cu, Zn and Mg, from AA to promote their growth, but leading to the broken of the passive film. Some hyphae with a big width are also firstly observed corresponding to a low initial spore concentration, which can be beneficial to get more nutrients. This means that the fungus A. terreus with a carbon starvation state will change their structure to adapt to the new environment. From the above analysis, it is seen that the survivors of fungus A. terreus can have a good ability to adapt to the new environment.
For AA corrosion, the anodic reaction is the dissolution of Al, and the cathodic reaction is the reduction of oxygen. Fungi can produce amounts of organic acids, leading to the cathodic reduction of H+ (refs. 17,24). The main reactions of AA corrosion include the oxidation of Al, the reduction of oxygen and H+.
Anodic reaction:
Cathodic reactions:
For the depolarization of oxygen, the supply of OH− will push Reaction (4) forward:
Acid corrosion has been the main factor that accelerates AA corrosion in the presence of Fungi17. In this work, the artificial seawater is alkaline, and the lowest pH value is 6.1 corresponding to the specimen with the initial spore concentration of 108 spores mL−1. These indicate that A. terreus can also produce some organic acids leading to the decrease of pH, but with a near-neutral value due to the limitation of organic carbon sources. So, acid corrosion can be neglected in this work.
Surface morphologies (Fig. 2), EIS data (Figs. 4 and 5), polarization curves (Fig. 6), and the WBE results (Figs. 7 and 8) demonstrate that A. terreus can accelerate AA corrosion and the corrosion is more severe with the increase of initial spore concentrations in artificial seawater. The passive film of AA in the abiotic solution can have a good protective effect, leading to a slight corrosion of AA (Fig. 2a). But the corrosion is more severe in the presence of A. terreus, suggesting the broken of passive film is enhanced by the A. terreus. In this work, A. terreus is difficult to get an organic caron source due to their absence, causing more than 90% A. terreus has died (Table 1). So, the A. terreus survivors can get electrons from Al to get energy for their metabolism. The A. terreus survivors can have enough power to destroy the passive film due to the energy starvation, then promote the electron transfer from Al to A. terreus. Some areas beneath biofilm can cause the accumulation of acid, which may accelerate the dissolution of the passive film. The bode plots show that the width of phase angle decreases gradually with the initial spore concentration on the whole compared with the control specimen (Fig. 5), which also demonstrates that the passive films are destroyed by A. terreus. And the protective effect of the passive film will have an abrupt decline due to the broken, then causing the increase of corrosion rate. The broken of the passive film can promote the electron transfer from Al to A. terreus. SEM images of surface films indicate that the biofilms are not homogeneous and there are apparent agglomerations of biofilm on the part location (Fig. 1). For the scattered A. terreus, they can directly get electrons from electrons Al, and leading to small corrosion pits (Figs. 2 and 3). The measure results of WBE also demonstrate that there are more anodic sites compared with the control (Figs. 7 and 8). The previous studies22,34,35 have indicated that the bacteria with organic carbon starvation could accelerate the localized corrosion of steel. Similarly, fungi can also do it according to the results of this work. In addition, the higher the initial spore concentration of A. terreus, the more A. terreus survivors (Table 1). The more A. terreus survivors can lead to more serious damage of passive film, then results in a higher corrosion rate. So, the AA corrosion is accelerated gradually with the increase of initial spore concentration (Figs. 4–6).
It is also found that localized corrosion is enhanced gradually with the increase of initial spore concentration. Localized corrosion, such as pitting corrosion, is the chief reason causing the failure of engineering equipment36,37. A pitting corrosion mechanism as shown in Fig. 9 was put forward to illustrate the localized corrosion induced by A. terreus survivors with continued organic carbon starvation. As observed from the SEM images of biofilms, the agglomeration growth of A. terreus tends to grow together, and form a large biofilm cluster (Fig. 1). The agglomeration of A. terreus can be beneficial to their metabolism due to organic carbon starvation. Fungus A. terreus is aerobic, so that amounts of dissolved oxygen (DO) beneath the biofilm cluster will be consumed by A. terreus fast. The transportation of DO from the solution to the area beneath the biofilm cluster will be difficult due to the hindering effect of the biofilm cluster. And then this will lead to an anaerobic environment beneath the biofilm cluster. So, an oxygen concentration cell will generate, while the area without oxygen beneath the biofilm cluster is as the anode and the other area containing DO is as the cathode. Pitting corrosion will be apparently accelerated due to the formation of oxygen concentration cell38. The electrons released by the anodic area will transfer to the cathodic area promoting the reduction of oxygen. A. terreus in the cathodic area can get electrons in different ways. Some A. terreus on the AA surface can directly get electrons through the direct contact between A. terreus and AA. But the direct electron transfer between A. terreus and AA still lacks some key shreds of evidence, and these will need a greater degree of study in the future. Some A. terreus can indirectly get electrons by some electron mediators, such as riboflavin39 and flavin adenine dinucleotide (FAD)40. There are amounts of filamentous nano hyphae produced by A. terreus, and some called vegetative hyphae similar to the nanowire produced by bacteria41,42, can be used to deliver electrons. Electron mediators and filamentous nano hyphae can be the main ways used to deliver electrons. Because most A. terreus cannot grow in an anoxic environment. A. terreus survivors in the biofilm only get electrons from the indirect ways from Al0 due to the organic carbon starvation. And the higher the initial spore concentration, the higher A. terreus survivors, which means more A. terreus can get electrons from Al. The cathodic reaction will be promoted. The acceleration of the cathodic oxygen reduction reaction can also enhance the anodic reaction, i.e., the dissolution of Al. The polarization curves also demonstrate that the presence of spores enhances both the anodic and cathodic reactions (Fig. 6). So, the localized corrosion is more severe for the specimen when the initial spore concentration is higher. It also should be noted that some areas covered with the corrosion products, such as Al(OH)3, can also limit the diffusion of DO, then form an oxygen concentration cell promoting pitting corrosion43.
Methods
Aluminum alloy specimens
Aluminum alloy (AA) 7075 as experimental specimens with a different size was used to do corrosion study. It has a chemical composition (wt.%) of 6.00 Zn, 2.50 Cu, 2.40 Mg, 0.40 Si, 0.50 Fe, 0.30 Mn, 0.18 Cr, 0.20 Ti, and Al balance. For the electrochemical measurements, the working area of the cylindrical AA electrode sealed by epoxy resin was 0.785 cm2. Specimens with a size of 10 mm × 10 mm × 5 mm were prepared to do surface analysis. All AA specimens were mechanically polished with 400, 600, 800, and 1200 grit silicon carbide papers in series. And then the polished specimens were degreased with acetone, and washed with anhydrous ethanol, respectively. The specimens were sterilized by an ultraviolet (UV) lamp at least 30 mins before use.
Culturing and inoculation of Aspergillus terreus
Aspergillus terreus isolated from the Xisha Sea area of China was used in this work, and the species of fungus was identified using polymerase chain reaction (PCR) amplification of 16S rDNA. The DNA sequence of A. terreus is as blow:
GCCAACCTCCCACCCGTGACTATTGTACCTTGTTGCTTCGGCGGGCCCGCCAGCGTTGCTGGCCGCCGGGGGGCGACTCGCCCCCGGGCCCGTGCCCGCCGGAGACCCCAACATGAACCCTGTTCTGAAAGCTTGCAGTCTGAGTGTGATTCTTTGCAATCAGTTAAAACTTTCAACAATGGATCTCTTGGTTCCGGCATCGATGAAGAACGCAGCGAAATGCGATAACTAATGTGAATTGCAGAATTCAGTGAATCATCGAGTCTTTGAACGCACATTGCGCCCCCTGGTATTCCGGGGGGCATGCCTGTCCGAGCGTCATTGCTGCCCTCAAGCCCGGCTTGTGTGTTGGGCCCTCGTCCCCCGGCTCCCGGGGGACGGGCCCGAAAGGCAGCGGCGGCACCGCGTCCGGTCCTCGAGCGTATGGGGCTTCGTCTTCCGCTCCGTAGGCCCGGCCGGCGCCCGCCGACGCATTTATTTGCAACTTGTTTTTTTCCAGGTTGACCTCGGATCAGGTAGGGATACCCGCTGAACTTAAGCATATCTA
A potato dextrose liquid medium with a composition (g L−1) of dextrose 20, NaCl 30, potato infusion 200 was used to culture A. terreus at 37 °C. The culture medium of A. terreus was also sterilized at 121 °C for more than 20 mins. Both the planktonic and sessile spores of A. terreus were enumerated using a plate count method.
Spore suspension preparation of A. terreus
A. terreus were incubated at 37 °C for ~10 d, and the hyphae were removed using sterilized gauze. Then A. terreus culture solution containing amounts of spores were filtered using a filter membrane with the pore size of 0.22 μm. The spores were bigger than the pore size of filter membranes so that the spores adhere to the surface of the filter membrane. More and more spores were enriched after several cycles and then transferred to artificial seawater. The spores were dispersed in artificial seawater by ultrasound for several mins. The enriched spore solution with a high concentration was diluted to prepare the various test solution. The spore concentration is tested using spread plate method. The spore concentrations of test solution were 108 spores mL−1, 106 spores mL−1, 104 spores mL−1, respectively. The total volume of the test solution was 250 mL. The details and steps of the separation and enrichment of A. terreus spores are shown in Fig. 10a. The abiotic seawater was used as the control. The artificial seawater used in this work was prepared according to the standard of ASTMD 1141-9837. The chemical composition of artificial seawater can be found in Table 3. The artificial seawater was also autoclaved at 121 °C for more than 20 min.
Characterizations of surface morphologies
Scanning electron microscope (SEM) and EDS (JSM-IT200, JEOL, Japan) were applied to investigate and analyze the surface morphologies of biofilms or corrosion product films. After 14 days of testing, the biofilm specimens were immersed for 8 h in a phosphate buffer solution containing 2.5% (w/w) glutaraldehyde to kill A. terreus. Then the specimens were dehydrated using ethanol solution with different concentrations (50%, 60%, 70%, 80%, 90%, and 100%) in a series, and each for 10 mins. Afterward, the high-purity N2 was used to dry the biofilm-covered specimens. The biofilm-covered specimens were coated with a thin gold film due to the low electrical conductivity of the biofilm.
The corrosion products were removed using a pickling solution containing an inhibitor. The surface morphologies without corrosion products and biofilm were analyzed by a Leica 3D stereoscopic microscope (DVM6, Germany) and SEM. The localized corrosion in the biotic and abiotic test solution was also recognized.
Electrochemical measurements
An electrochemical workstation (CS350H, Corrtest, China) with a three-electrode cell was used to do the electrochemical measurement, and the schematic diagrams of the setup are shown in Fig. 10b. The saturated calomel electrode (SCE), AA 7075 specimen, and a platinum plate were used as the reference electrode, working electrode, and counter electrode, respectively. Prior to electrochemical impedance spectroscopy (EIS) measurements, open-circuit potential (OCP) was monitored firstly. When it became stable, EIS was scanned at the OCP using a 10 mV sinusoidal wave from 105 to 10−2 Hz. Potentiodynamic polarization curves were conducted on the last day of testing after EIS measurements, and the potential scanning range was from −0.25 V to +0.5 V vs. corrosion potential (Ecorr) at a scanning rate of 0.5 mV s−1. EIS data are fitted well using Zview2 software. More than three independent specimens are used to do each test.
Wire beam electrode
A wire beam electrode (WBE) including 100 pieces of AA 7075 disks was used in this work, and the diameter of a single microelectrode was 1 mm. WBE measurements were performed using an electrochemical instrument (CST520, Corrtest, China), including the potential and current density scans, and the reference electrode was also SCE. Each microelectrode was regarded as the working electrode one at a time in series, and the other electrodes were as counter electrode. All microelectrodes were connected once finishing WBE measurements. The schematic diagram of the setup for the galvanic current distribution of WBE is shown in Fig. 10c.
Data availability
The data presented in this article is available upon request to the authors.
References
Jiang, L. et al. Discovery of aluminum alloys with ultra-strength and high-toughness via a property-oriented design strategy. J. Mater. Sci. Technol. 98, 33–43 (2022).
Wang, L., Liang, J., Li, H., Cheng, L. & Cui, Z. Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl. Corros. Sci. 178, 109076 (2021).
Zhou, B. et al. Microstructure evolution of recycled 7075 aluminum alloy and its mechanical and corrosion properties. J. Alloys Compd. 879, 160407 (2021).
Pandey, V., Singh, J., Chattopadhyay, K., Srinivas, N. S. & Singh, V. Influence of ultrasonic shot peening on corrosion behavior of 7075 aluminum alloy. J. Alloys Compd. 723, 826–840 (2017).
Stannard, T. J. et al. 3D time-resolved observations of corrosion and corrosion-fatigue crack initiation and growth in peak-aged Al 7075 using synchrotron X-ray tomography. Corros. Sci. 138, 340–352 (2018).
Cai, H., Wang, P., Zhang, D., Wang, Y. & Li, E. An intelligent self-defensive coating based on sulfide ion responsive nanocontainers for suppression of microbiologically influenced corrosion induced by sulfate reducing bacteria. Corros. Sci. 188, 109543 (2021).
Jogdeo, P. et al. Onset of microbial influenced corrosion (MIC) in stainless steel exposed to mixed species biofilms from equatorial seawater. J. Electrochem. Soc. 164, C532 (2017).
Ma, Y. et al. Microbiologically influenced corrosion of marine steels within the interaction between steel and biofilms: a brief view. Appl. Microbiol. Biotechnol. 104, 515–525 (2020).
Qian, H. et al. Laboratory investigation of microbiologically influenced corrosion of Q235 carbon steel by halophilic archaea Natronorubrum tibetense. Corros. Sci. 145, 151–161 (2018).
Stamps, B. W. et al. In situ linkage of fungal and bacterial proliferation to microbiologically influenced corrosion in B20 biodiesel storage tanks. Front. Microbiol. 11, 167 (2020).
Liu, H., Sharma, M., Wang, J., Cheng, Y. F. & Liu, H. Microbiologically influenced corrosion of 316L stainless steel in the presence of Chlorella vulgaris. Int. Biodeterior. Biodegrad. 129, 209–216 (2018).
Guan, F. et al. Interaction between sulfate-reducing bacteria and aluminum alloys—Corrosion mechanisms of 5052 and Al-Zn-In-Cd aluminum alloys. J. Mater. Sci. Technol. 36, 55–64 (2020).
Smirnov, V., Belov, D., Sokolova, T., Kuzina, O. & Kartashov, V. Microbiological corrosion of aluminum alloys. Appl. Biochem. Microbiol. 44, 192–196 (2008).
Lee, J. S., Ray, R. I. & Little, B. J. An assessment of alternative diesel fuels: microbiological contamination and corrosion under storage conditions. Biofouling 26, 623–635 (2010).
Dai, X. et al. Corrosion of aluminum alloy 2024 caused by Aspergillus niger. Int. Biodeterior. Biodegrad. 115, 1–10 (2016).
Wang, J. et al. Study of the corrosion behavior of Aspergillus niger on 7075-T6 aluminum alloy in a high salinity environment. Bioelectrochemistry 129, 10–17 (2019).
Zhang, T., Wang, J., Zhang, G. & Liu, H. The corrosion promoting mechanism of Aspergillus niger on 5083 aluminum alloy and inhibition performance of miconazole nitrate. Corros. Sci. 176, 108930 (2020).
Jirón-Lazos, U. et al. Localized corrosion of aluminum alloy 6061 in the presence of Aspergillus niger. Int. Biodeterior. Biodegrad. 133, 17–25 (2018).
Xiao, C., Zhang, H., Fang, Y. & Chi, R. Evaluation for rock phosphate solubilization in fermentation and soil–plant system using a stress-tolerant phosphate-solubilizing Aspergillus niger WHAK1. Appl. Biochem. Biotechnol. 169, 123–133 (2013).
Dou, W. et al. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros. Sci. 150, 258–267 (2019).
Beese-Vasbender, P. F., Nayak, S., Erbe, A., Stratmann, M. & Mayrhofer, K. J. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim. Acta 167, 321–329 (2015).
Venzlaff, H. et al. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 66, 88–96 (2013).
Sherar, B. et al. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 53, 955–960 (2011).
Li, Y. et al. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J. Mater. Sci. Technol. 34, 1713–1718 (2018).
Liu, Y. et al. Carbonaceous nanomaterials stimulate extracellular enzyme release by the fungus Cladosporium sp. and enhance extracellular electron transfer to facilitate lignin biodegradation. Sci. Total Environ. 696, 134072 (2019).
Wang, R. et al. Nanomaterials facilitating microbial extracellular electron transfer at interfaces. Adv. Mater. 33, 2004051 (2021).
Parvatkar, R. R., D’Souza, C., Tripathi, A. & Naik, C. G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 70, 128–132 (2009).
Yang, Z. et al. Antifungal fatty acid derivatives against Penicillium italicum from the deep-sea fungus Aspergillus terreus SCSIO 41202. Nat. Prod. Res. 35, 4394–4401 (2021).
Xu, D. & Gu, T. Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int. Biodeterior. Biodegrad. 91, 74–81 (2014).
Ramos, J. L. et al. Responses of Gram-negative bacteria to certain environmental stressors. Curr. Opin. Microbiol. 4, 166–171 (2001).
Moroni, B. & Pitzurra, L. Biodegradation of atmospheric pollutants by fungi: a crucial point in the corrosion of carbonate building stone. Int. Biodeterior. Biodegrad. 62, 391–396 (2008).
Miečinskas, P., Leinartas, K., Uksienė, V. & Juzeliūnas, E. QCM study of microbiological activity during long-term exposure to atmosphere—aluminium colonisation by Aspergillus Niger. J. Solid State Electrochem. 11, 909–913 (2007).
De Leo, F., Campanella, G., Proverbio, E. & Urzì, C. Laboratory tests of fungal biocorrosion of unbonded lubricated post-tensioned tendons. Const. Build. Mater. 49, 821–827 (2013).
Fan, Y. et al. Early corrosion behavior of X80 pipeline steel in a simulated soil solution containing Desulfovibrio desulfuricans. Bioelectrochemistry 141, 107880 (2021).
Eduok, U., Ohaeri, E. & Szpunar, J. Accelerated corrosion of pipeline steel in the presence of Desulfovibrio desulfuricans biofilm due to carbon source deprivation in CO2 saturated medium. Mater. Sci. Eng. C 105, 110095 (2019).
Zelmati, D., Bouledroua, O., Hafsi, Z. & Djukic, M. B. Probabilistic analysis of corroded pipeline under localized corrosion defects based on the intelligent inspection tool. Eng. Fail. Anal. 115, 104683 (2020).
Alamri, A. H. Localized corrosion and mitigation approach of steel materials used in oil and gas pipelines-an overview. Eng. Fail. Anal. 116, 104735 (2020).
Schafer, G., Gabriel, J. & Foster, P. On the role of the oxygen concentration cell in crevice corrosion and pitting. J. Electrochem. Soc. 107, 1002–1004 (1960).
Wang, D. et al. Aggressive corrosion of carbon steel by Desulfovibrio ferrophilus IS5 biofilm was further accelerated by riboflavin. Bioelectrochemistry 142, 107920 (2021).
Jia, R., Yang, D., Xu, D. & Gu, T. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry 118, 38–46 (2017).
Yang, Y. et al. Long-distance electron transfer in a filamentous Gram-positive bacterium. Nat. Commun. 12, 1–9 (2021).
Meysman, F. J. et al. A highly conductive fibre network enables centimetre-scale electron transport in multicellular cable bacteria. Nat. Commun. 10, 1–8 (2019).
Yin, L. et al. Numerical simulation of micro-galvanic corrosion of Al alloys: Effect of density of Al(OH)3 precipitate. Electrochim. Acta 324, 134847 (2019).
Acknowledgements
This paper was supported by the Guangdong Basic and Applied Basic Research Foundation (2019A1515011135), Fundamental Research Funds for the Central Universities (19lgzd18), National Natural Science Foundation of China (51901253), and the Open Project Program of Hubei Key Laboratory of Materials Chemistry and Service Failure (2020MCF02).
Author information
Authors and Affiliations
Contributions
Y.Z.: investigation, methodology, and writing—original draft. J.H.: investigation and methodology. L.Z.: investigation. Z.J.: data collection. H.L.: data collection. L.L.: conceptualization and supervision. Z.G.: conceptualization, formal analysis, and supervision. G.M.: conceptualization and supervision. H.L.: conceptualization, supervision, and writing—review. H.L.: supervision, conceptualization, writing—review and editing, and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., He, J., Zheng, L. et al. Corrosion of aluminum alloy 7075 induced by marine Aspergillus terreus with continued organic carbon starvation. npj Mater Degrad 6, 27 (2022). https://doi.org/10.1038/s41529-022-00236-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-022-00236-2
This article is cited by
-
Research on accelerating the corrosion of 7B04 aluminium alloy in the Lacticaseibacillus paracasei environment
Journal of Applied Electrochemistry (2024)
-
Biodegradation of materials: building bridges between scientific disciplines
npj Materials Degradation (2023)