Abstract
Glass alteration in the presence of microorganisms has been a topic of research for over 150 years. Researchers from a variety of disciplines, including material science, biology, chemistry, geology, physics, and cultural heritage materials preservation have conducted experiments in this area to try and understand when, how, and why microorganism may interact and subsequently influence the alteration of glass. The breadth and depth of these studies are the topic of this review. This review presents a detailed history and a comprehensive overview of this field of research, while maintaining focus on the terrestrial alteration of anthropogenic silicate glasses. Within this manuscript is a schema for bio-interaction with silicate glasses and an outline of an evidence-based hypothesis on how these interactions may influence glass alteration processes. Topics discussed include microbial colonization of glass, development, and interactions of biofilms with glass surface, abiotic vs. biotic alteration processes, and signatures of bio-alteration. Future research needs and a discussion of practical drivers for this research are summarized.
Similar content being viewed by others
Introduction
Glass is included in many of our modern and daily activities. We wake up to glass-covered alarm clocks, eat breakfast in the company of glass containers full of coffee or juice, transport our children in cars with glass windows, communicate with our friends and family through glass-covered cellphones, and sit in glass clad buildings during our working hours. Every one of these interactions and the thousands of others between a glass and its surrounding environment may result in the colonization of its surface by microorganisms. If the organisms are not removed in a timely manner then their co-association with glass surfaces may last seconds, minutes, weeks, hours, and even millennia1. Some of these associations may be harmless or protective, others may be more damaging, decreasing the durability and service life of a glass. It is the latter interactions that have stimulated research on this topic for the past few decades.
At the time of writing this manuscript, a significantly larger number of studies have been conducted on abiotic alteration of glass than on the biotic alteration of glass. This has been in part due to the emphasis placed on abiotic alteration by certain industries (e.g., nuclear waste disposal)2,3. While abiotic-focused research has provided many important insights4, it has addressed only part of the cause of glass alteration in natural, non-sterile environments. The other part, one which is underpinned by biology, needs to be assessed as well. Understanding both abiotic and biotic alteration, and how they interact can aid in the development of a well-rounded model of glass alteration under natural conditions. Insight into the synergy of these two processes is especially important as international communities strive to place glass-including-devices and structures in outer space5, send them to other planets6, and dive them to the depths of our oceans7 for longer periods of time than previously attempted. Knowing a priori how and when these processes interact or override each other could mean the difference between a successful or failed mission.
Another driver for this review is the practice of vitrifying nuclear waste and the legal requirement that nuclear waste producers account for the long-term (thousands to tens-of-thousands of years) durability of their vitrified products4. At some disposal sites buried vitrified wastes may experience biotic contamination, as well as adverse abiotic conditions8. Therefore, it is important that biological parameters are considered in the disposal site performance assessment models9. As previously stated, this research community has accumulated vast knowledge of abiotic alteration processes, but not of biotic ones. Fulfilling this need is a focus of this review, which requires first a general understanding of the current state-of-the-field of glass bio-alteration.
To date, few literature reviews have covered this research topic10,11,12. The first detailed review was published in 1989 by Newton and Davidson, whose work showed a specific interest in the conservation of glass artifacts12. Their chapter outlined both the authors’ contributions to the field, as well as those by many others whose work will be mentioned below. In 1991, a few years after the release of Newton and Davidson’s publication, Koestler and Vedral released a comprehensive bibliography on the biodeterioration of cultural property materials that included many references to glass13. Several years later, Drewello and Weissmann published a more general overview that focused on the bio-alteration of silicate glasses10. More recently, in 2006 and 2016, literature reviews were included in the academic theses of Aouad14 and Shelley15, respectively, and, in 2017, a brief overview was published in a book by Pinna that focused on medieval stained glass windows from Europe16. Although no comprehensive review has been compiled in the two decades since Drewello and Weissmann (1997), many new manuscripts have been published and various advances have been made in analytical techniques for studying the phenomenon. In addition, and at the present, no schema (such as that for stone and mineral)17,18 has been proposed to hypothesize how and why terrestrial microbes interact with and alter silicate glasses. This review aims to address this gap.
This review opens with a brief historical timeline of research on the bio-alteration of glass and closes with a discussion of the general pathways by which bio-alteration may occur. A comprehensive list of glass colonizing organisms is not provided although a few key organisms are mentioned. The development of such a list is a topic of future work. The historical timeline presented here does not cite every paper published on the bio-alteration of glass, but focuses on manuscripts that identify biological agents and their possible mechanisms of action. An emphasis has been placed on the alteration of silicate-based anthropogenic glasses, but, and when appropriate, data on the bio-alteration of naturally formed silicate glasses, predominately volcanic in origin, and selected silicate minerals is also included. The alteration of glasses designed for biological and medical applications (i.e., bioglasses) is not discussed due to their vastly different alteration environment and glass chemistries.
Literature review and historical timeline
1830’s–1940’s
Microbial interactions with glass have been reported in the literature to at least 1831, when the influential Swedish mycologist Fries published his observations of lichen (fungi living in symbiotic association with photosynthetic algae or cyanobacteria) growth on church windows in Falsterbo, Sweden19. More than 40 years later (1879), the prolific British lichenologist Crombie noted, “Vitricole Lichens (which, in this country, we have only observed on broken pieces of bottles on garden wall tops, chiefly in Scotland)…”20. This statement was made in response to published observations made by Nylander, who had observed prolific lichen growth on glass exposed to nature for several years21. Similar findings of microorganisms growing on glass were communicated in 1881 by Egeling22, and reiterated in 1913 by Fink23.
In 1921, the English researcher Mellor published the first in a series of papers and letters on biologically induced alteration of anthropogenic glass24,25,26. A possible motivation for this undertaking may be found in her 1923 article where she states, “… technical and practical knowledge of the stained glass artist should be reinforced by the theoretical and laboratory studies of the scientific worker”26. Mellor’s papers were the culmination of time spent examining the surfaces of medieval church windows while abroad in France. In the papers, Mellor hypothesized a link between the presence of lichens and the alteration of glass surfaces24,26. This theory was summarized in her 1923 Nature paper26, where she listed over 20 lichen species growing on the glass panes and detailed the presence of pitting in the glass surfaces under the organisms. The pits measured up to 5 mm wide and almost 2 mm deep, and the number of pits varied both by the species of lichen and by the color and type (chemical composition) of glass. These and other results from Mellor’s research gave credence to the then controversial theories of organism-induced alteration of inorganic surfaces27,28, and she was one of the first to suggest a correlation between the composition of a glass and its vulnerability to alteration26.
Mellor’s work is foundational to this field of inquiry but has only been moderately cited over the years. Prior to the 1960’s, there are a few citations to Mellor’s articles in the lichenology literature29,30,31,32. Post the 1960’s many of the citations to her papers are found in publications by art restorers, conservators, and/or conservation scientists and few microbiologists11,33,34,35. This appears to follow the trend of an increasing interest in bio-alteration by the cultural heritage field during this time period. Since the late 1980’s, several geologists/geochemists have referenced her findings to emphasis the diversity of surfaces that lichens colonize and their roles as biological agents of geochemical processes (examples of these publications include36,37,38). A brief literature review reveals that Mellor’s work has become more popular within the last several decades with almost a third of citations to her 1923 paper occurring since 2000. In several of these modern papers, her findings are utilized to compare lichen species observed on glass with those “known” (as observed by Mellor) on naturally altered glasses. Given the nature of the works which have cited her papers an acknowledgment of Mellor’s role in establishing several of the fundamental areas of inquiry for this field seems qualified. Specifically, and as summarized in Fig. 1, Mellor’s work hypothesized that:
-
(i)
Different glass chemistries altered differently under observed conditions;
-
(ii)
Only certain kinds of organisms colonize glasses;
-
(iii)
These organisms left alteration “signatures” on the glasses; and
-
(iv)
Alteration could be abiotic, biotic, or driven by both.
1940’s–1960’s
Following Mellor’s publications, there appear to be relatively few publicly published articles on the bio-alteration of glass until the 1940’s when glass manufacturers and scientists began to document their frustrations around the alteration of optical glasses. Many clinical and research lab reports from this time detail biologically “fouled” (aka biofouling) and etched glass lenses within expensive microscopes and optical instruments39,40,41. Biofouling was particularly problematic for microscopes exposed to the hot and humid conditions of tropical or subtropic environments41,42,43. Under these conditions, the glass pieces were noted to deteriorate quickly. In some cases, the optics would become “hazed” or “pitted” within 4–8 weeks39,42. As noted, the deterioration of the optics occasionally resulted in financial hardship for researchers working in remote tropical outposts as the shipment of replacement pieces was often expensive, difficult to arrange, and could take many months to arrive. Researchers attributed the bio-colonization to the presence of organic debris on the surface of lenses and the presence of moisture; a problem which they believed could be mitigated by routine cleaning and good storage practices42,44. Recommended cleaning procedures have included sterilization and application of fungicides, such as meta-cresyl acetate (cresatin) or sodium ethylmercurithiosalicylate, to the glasses39,40,42. These treatments, while effective in the short term, did not prevent recolonization from occurring over time and had to be repeated regularly45. Some researchers also expressed concern that the cleaning agents themselves could damage the surfaces of the glass46. Given these limitations, scientists have since proposed alternative approaches to keep glass surfaces aseptic. Three of these developed methods that are still in use today include adding small amounts of biocidic elements (Pb, Ag, Cu, etc.) to glass matrices47, coating glass surfaces with a thin layer of a metal oxide or metal48, and reacting the glass surface with biologically active compounds49. Exposing glass surfaces to gamma irradiation also was suggested as a sterilization technique50.
From 1940 to 1960 research focused on describing visual signatures of bio-alteration, finding the source cause(s) of biological colonization, and the development of technologies and methods of mitigation. In contrast to the previous period, research appears to have been conducted by a mixture of industry and academia. Descriptive terms for the alteration textures, such as “etching”, “pitting”, and “hazing” appear within many publications from this time and echoes those previously utilized by Mellor. They have continued to be popular through time with many appearing in manuscripts from the 1990’s and 2000’s, by Koestler et al.51, Staudigel et al.52, Krumbien et al.11, and Furnes and Thorseth53,54, the latter describing bio-alteration of deep sea basaltic glasses.
It is curious to note that most of the classification of these signatures occurred near simultaneously with the introduction of more powerful microscopes and advanced imaging methods. That is, the very instruments that may have been damaged by microbial activity—a factor driving this research in the mid-20th century—made it possible to view these signatures of the deterioration and associate them with the presence of the organisms. Two significant advances in microscopic instruments during this period which enabled this work include the patenting of the electron microscope (EM) in 1940’s by the Ruskas55 (for which Ernst Ruska won a Nobel prize for in 1986 and that was seminal to the creation of the scanning electron microscope (SEM)) and the creation of confocal imaging by Minsky between 1955 and 1957 (refs. 56,57). In the 1960s, researchers would add onto these techniques green fluorescent protein (GFP) tagging58,59, which underwent further refinement in 1992 with the first reporting cloning of the GFP gene60, and the development of fluorescence imaging61. Pairing microscopes with GFP has enabled direct visualization and quantification of real-time, biological activity on relevant time length scales, and made measurement of alteration kinetics and mechanisms plausible in the second half of the 20th century.
1960’s–1990’s
As stated above, Mellor’s work appears to become of significant interest to fields outside of lichenology in the 1960’s. It was in this decade that several papers, many of which utilized the aforementioned microscopes, provided direct evidence for Mellor’s hypothesis that organisms alter glass. As with Mellor’s work, the greater part of this research focused on the natural alteration of historical glasses62. Some of the most cited papers, and that provided additional credit to Mellor’s other findings, included those from the 1960’s from Prod’Homme63, Winter64, and Kerner-Gang65, from the 1970’s Collongues et al.66 and Bettembourg32, and one from 1981 by Tennent67. In the 80’s, Perez y Jorba et al. reported on the alteration of anthropogenic glass under natural conditions by more than one type of organism, providing some of the first evidence for the alteration of silicate glass by bacteria68. These authors noted circular pits associated with fungal filaments in the altered regions of medieval glasses; a finding that compliments those of Mellor’s68. Some of the altered regions observed by Perez y Jorba and coworkers had evenly spaced, concentric striations that occasionally were cracked and backfilled with S- and Mn-rich materials. The researchers suggested that these pits and striations were biotic in origin, primarily caused by ferrous iron (Fe2+)-oxidizing bacteria. Follow-up laboratory experiments by Koestler et al. provided empirical proof that Fe2+-oxidizing bacteria (specifically cyanobacteria) were able to cause alterations similar to those observed by Perez y Jorba et al.51,68. Koestler et al. also noted that potassium (aka potash) silicate glasses were more susceptible to pit formation than soda-lime silicate glasses, which exhibited more generalized surface etching without pitting. These results were in-line with the observation of Mellor that difference glass chemistries alter differently.
As previously mentioned, SEM played a key role in these research efforts. This may be due in part to the instrument being more accessible and affordable to the general scientific research community in the 1960’s and 1970’s69). SEMs of this time period allowed micron-scale visualization and measurement (length and depth) of the alteration textures on the glass surfaces, and their comparison to the position, shape, and size of microorganisms. From the early 1990’s onward it became near common practice for researchers in geomicrobiology to identify organisms on the glass surfaces by comparing SEM images with epifluorescence microscopy images, where the organism was stained by a fluorescing dye70,71,72. Other analytical methods used in conjunction with SEM imaging included stable isotope analysis (13C, 18O)70,73, energy dispersive spectroscopy or microprobe analysis51,53,71,72,74,75, transmission electron microscopy (TEM)75,76, and genomic analysis through polymerase chain reaction (PCR) amplification71. The samples analyzed in these studies came from natural environments70,71,72,73,74,75,76 and from substrates altered under controlled laboratory conditions51,52,53.
A major theme of this 30 years timespan was building upon past knowledge and techniques. More observational and experimental evidence was provided that dissimilar glass chemistries alter differently under similar biotically exposed conditions, a theory first posed by Mellor in the 1920’s. In addition, combining fluorescent tagging methods, stable isotope analysis, and elemental analysis with more mature imaging instrumentation permitted researchers to develop a more nuanced understanding of microbial colonization of glasses. Species growing on glasses could be more accurately documented, identified, and correlated with alteration phenomena with the use of micro-imaging51,68. As a result, researchers began looking beyond fungi as the singular source of glass alteration, leading to some of the first evidence for bacterial alteration of glass68. Descriptions of alteration expanded beyond physical symptoms of degradation to include possible chemical reactions between the organisms or their by-products with underlying glass surfaces11,68. The latter is foundational to modern studies of bio-alteration of glass as it indicated that it isn’t only physical processes, but also chemical processes that could have a significant impact on how glass alteration occurs. The significant method and theory development from this timeframe set the stage for the highly cited works from the geochemical community on the bio-alteration of basaltic glasses in the 1990’s.
1990’s to present
In the 1990’s, Thorseth et al.53,73,74, Torsvik et al.70, Giovannoni et al.71, Fisk et al.72 (the preceding works in collaboration and many in coauthorship with Harald Furnes), and Staudigel et al.52 provided a more geochemical-focused approach to bio-alteration studies. These researchers used microbial alteration textures (aka signatures) on basaltic glass as evidence of epilithic and endolithic microorganisms from ancient times. Although not focused on anthropogenically produced silicate glasses, these publications are important because they gave further weight to the idea that observed alteration textures could be biotically produced74.
Another important paper from this time written by Staudigel et al. broke with the decades long convention of describing bio-alteration as predominately causing degradation of a glass and, rather, discussed that it could also have the reverse affect—i.e., it could result in preservation77. Subsequent research by Aouad et al. in the 2000’s provided additional evidence to support this idea, although Aouad and coworkers did not agree with all of the prior works’ methodology77,78. This research will be discussed in greater detail in following sections.
In the early 2000’s Furnes et al. summarized their groups findings from the 90’s54. In this paper, the authors listed five features commonly found on volcanic glasses that have undergone biotic alteration54:
-
(i)
An alteration surface with “bio-generated textures” that are granular and/or tubular, and related in shapes and sizes with microorganisms;
-
(ii)
An association of filament texture with the shapes mentioned in i;
-
(iii)
A presence of DNA and RNA in or near these textures (young samples only);
-
(iv)
An association of C and N with the textures, particularly near the alteration front; and
-
(v)
A presence of C isotopes and in relevant bio-fractions of 12C and 13C isotopes in the alteration front.
Although these features were identified in natural glasses altered in marine settings, they can and have been applied to the analysis of naturally altered anthropogenic glasses (for an example see79). Like the past works of Krumbein et al.11, Perez y Jorba et al.68, and Koestler et al.51, this research highlighted that biogenic alteration signatures could be both physical and chemical in nature. Furnes et al. also made the important inclusion of using stable isotope analysis to try and separate biotic alteration from abiotic alteration. This analysis method can require mg to g sample sizes, and can be challenging on altered glass materials given the small (sometimes μm/μg or smaller sized) and localized formation of some alteration textures. At this length scale, the detection of biogenic C, N, or S or minerals may be more easily achieved, although source attribution may be difficult to ascribe.
As noted by the above researchers and in several other papers from the 80’s and 90’s, the hypothesized and experimentally tested processes by which organisms may cause localized alteration textures include microbially induced variations in solution pH and microbially produced ligand/glass surface interactions52,53,74,77,80. For the former process, the impact of organisms could be significant as local pH is known to effect the rate of glass alteration81,82,83. In addition, a 1996 study by Welch and Ullman reported that organic acids produced by microbes as part of their metabolic pathways were more effective at altering silicates than inorganic acids at the same pH (cited by Furnes et al. in the 2002 review)84. For the latter process several organic ligands that can be biologically secreted have been reported to interact with silicates15,85,86. Examples of these molecules include oxalic acid, citric acid, 2- keto-gluconic acid, tartaric acid, and salicylic 2-3 dihydoxybenzoic acids. Microbes that secrete Fe-binding ligands, such as multi-cation-chelating siderophores, have also been found to increase the dissolution rates of basaltic glasses if the preferred valence of Fe is present87,88. Some researchers have suggested that these chemicals (organic acids/bases and ligands) allow organisms attacking a glass surface to remove elements that are otherwise depleted in their local environment11,77. The commonality of this effect is a current topic of debate and is discussed in more detail in the biochemical alteration section of this review.
Another heavily deliberated topic for this period is whether such observed signatures are truly biotic in origin or if they occurred due to abiotic reactions that were subsequently mixed with biotic material. This sentiment had been previously and briefly discussed by Mellor24, reiterated by Koestler et al.17, Stuadigel et al.77 in the 1990’s along with others11, and appears to have come to the forefront of discussion in the 2000’s (for example, see refs. 14,89). Coming from the field of municipal waste glass durability, the mid-2000’s research of Aouad and Aouad et al. suggested that abiotic processes could have produced textures similar to those previously ascribed to biotic processes78,90. In his papers and his dissertation, Aouad suggested that bio-alteration of glass is relatively rare and that the bio-attributed alteration patterns found on most volcanic glasses may have occurred abiotically14. He also asserted that bio-alteration textures are not consistently observed on naturally altered samples; a statement which parallels early criticisms of Brill and Hood’s theories of dating using glass alteration layers91 by Newton92. In a response to Banerjee and Muehlenbach93 attributing tubular textures on volcanic glass to bio-alteration, Aouad noted that the textures could have formed through abiotic leaching in a less durable region of the glass14. To test this hypothesis, Aouad ran a series of experiments on silicate glasses under controlled biotic and abiotic conditions. For these glasses and the given experimental setup, a greater extent of alteration was found to occur under abiotic rather than biotic conditions14,90. Aouad et al. also showed through elemental release rates that biofilms (i.e., cells embedded in a matrix of extracellular polymeric substance (EPS)) can act as a protective layer, slowing the rate of glass alteration, and extend the lifetime of the glass. Biofilms and EPSs are discussed at a greater depth below.
The difficulty of making mechanism origin assignments has been further detailed in a 2007 paper by Benzerara et al.89 This study focused on colocalized identification of a glass derived element (e.g., Fe) with a biotic associated element (e.g., C) and extended the analyses to include elemental redox measurements. This was completed with focused ion beam milling, TEM, scanning transmission X-ray microscopy, near-edge X-ray absorption fine structure spectroscopy, and electron energy loss spectroscopy. The features investigated were <5 μm wide channels and ≈30 nm wide alteration layers in naturally altered basalt glasses. Although, the findings of this study were geologically interesting—showing direct evidence for oxidized Fe in the channels, a result contrary to prior, albeit lower resolution research on similar materials—they could not conclusively prove that the alteration was biotically produced89. Dimensional measurements of the channels indicated that the channels could be “compatible with a biogenic origin”89.
Research into the biological alteration of glass surfaces has continued to grow since the early 2000’s. Many studies, such as those by Schabereiter-Gurtne et al.94, Carmona et al.95, Marvasi et al.35, Piñar et al.96,97, Rodrigues et al.98, and Shelley15 have attributed textures on naturally altered, historical glasses to biologically induced alteration. More recently, this field of inquiry has expanded into the realm of nuclear waste glass durability research and the assessment of glass alteration analogs99,100,101,102. Glass alteration analogs are historical glasses (natural or anthropogenic) which have altered under natural conditions, and that are studied to help inform and provide context for glass alteration models103. These models are being utilized by the nuclear waste glass communities to predict the service life of synthesized glasses104.
In the 1990’s and 2000’s, glass bio-alteration research reached into the fields of geology53,54,70,71,72,73,74,89, municipal waste disposal14, and nuclear waste glass durability research90,99,101,102. With these shifts have come the revival of the discussion on how to identify biotic alteration from abiotic alteration on naturally altered glasses. Techniques such as stable isotope analysis and select, biologically relevant element detection were proposed and utilized to meet this challenge54,89. Research communities also began dialog on and providing greater experimental support for the alteration rate-slowing properties of organisms and biofilms77,90. Parallel to this, research into effects of biogenically produced organic acids on glass alteration rates grew as did interest in the altering effects of siderophore-producing organisms11,14,51,77. The interplay of physical and chemical alteration processes on glass alteration processes has arisen as an open question in the field11,14,54,88,90 along with a growing need to understand what kinds of organisms colonize and alter glass77,94,97.
Review of relevant literature as completed above suggests that there were four major theories proposed for this field from the early 1800’s onward. These theories are presented in no specific order below:
-
(i)
The alteration of glass by microorganisms can be identified by chemical and/or physical “signatures”;
-
(ii)
The alteration of glass can occur via a multitude of microorganisms (bacteria, fungi, and others);
-
(iii)
The alteration of glass by organisms can occur in multiple types of natural environments and be (partially) reproduced in the laboratory; and
-
(iv)
Abiotic environmental factors can play a synergistic role with microorganisms during alteration processes.
Many of these theories were seeded in the 1920’s work of Mellor24,25,26, expanded upon or utilized by researchers in industry41, academia42, and in the field of cultural heritage preservation51,68 since at least the 1940’s, and had been carried forward in 1990’s by these groups along with the geochemical community53,70,71,72,73,74. Unlike most previous research efforts, the geochemical community focused on the bio-alteration of sea altered, natural glasses as opposed to man-made glasses. Although applied to different glass chemistries and environments, the geochemical approach used by these researchers helped establish analytical methods that aid in separating biotic alteration from abiotic alteration11,54. These avenues of investigation have since been built upon through carefully designed experiments and state-of-the-art imaging and bio-assessment instrumentation and techniques in the fields of cultural heritage preservation and vitrified waste disposal88,102. Findings from these research efforts are foundational to pursuing the current “big” questions of this field; some of which have been addressed more recently by those in fields of cultural heritage science87 and waste disposal78,88,90. These questions will be covered in more detail below and are outlined on a historical timeline in Fig. 1.
Topics of glass bio-alteration
With a review of relevant literature and introduction to the general concepts of the field complete, it is now possible to develop deeper discussions of the specialized topics in glass bio-alteration. The first topic to be addressed is the need for a general schema that outlines the steps of the bio-alteration of glass. The second is to discuss the effects and synergies of biotic and abiotic processes. Third is to examine the special case of biofilms and biofilm formation, followed by an outline of the process(es) of bio-alteration. Finally, chemical and physical signatures of alteration are to be addressed in greater detail than above and the role of time in bio-alteration processes will be discussed. These topics are addressed in the remainder of this manuscript.
General steps of bio-alteration
A general bio-alteration schema for silicate glass may be described in five general steps. These steps have been adapted from content presented in18,105 and are outlined in Fig. 2. This is a more detailed schema than that outlined in the review by Drewello and Weissmann10, and, as depicted in Fig. 2, is circular and can branch or terminate at any given step. The time lapse between one step and the next is variable and system dependent. Having a detailed scheme is important as it provides a general timeline with key development points for microbial interaction with glass; each of which could be studied in order to gain a better understanding of how biological glass alteration temporally progresses. The proposed steps of the schema are:
-
(i)
The substrate builds up organic material;
-
(ii)
The substrate is colonized by microbial (bacterial and/or fungal) spores and/or other propagules (i.e., filaments or fragments) with or without physical attachment;
-
(iii)
The colony or community grows and reproduces, and frequently develops a biofilm morphology(s);
-
(iv)
The organism and/or biofilm biomineralize; and
-
(v)
The organism(s) and/or biofilm detaches—if this occurs then these steps may be reinitiated51.
Beginning with step i, microbial growth is promoted by organic nutrient sources on the surface of an inorganic material105. However, some evidence suggests that microorganisms may be sustained (at least temporarily) on select glass types without the presence of traditional nutrients or other energy sources77. Generally, and most likely regardless of nutrient source, colonization will occur concurrently with or following the release of hydrolyzed ions from the glass surface14. Once a colony is established (step ii), the microbial community size and diversity may increase (step iii), with growth and reproduction rates dependent on the species, water availability, temperature, and nutrients85,105. An organism’s attachment and continued interaction with a glass surface may be dictated by biochemical interactions and biophysical interactions. Anchoring may occur through the microorganism’s EPSs. An established, growing colony may eventually form a biofilm of structured EPS (step iii) that may begin to mineralize (step iv). When the colony shrinks or dies, it can cause physical stress on the glass surface and/or possibly detach. This may occur synchronously with delamination of all or part of the glass alteration volume (step v). Some of these steps may occur very quickly, some within a few seconds, and some over months, years, or even decades.
Abiotic and biotic alteration
Given the outline above, bio-alteration may be integrated into the current, abiotic glass alteration schema. The abiotic process of glass alteration has been described by Vienna et al.4 and Gin106 and will not be extensively reiterated here. It begins with the attack of surface silanols and siloxanes by dissociated species of water, OH−, and H+, and follows with the release of glass-forming elements such as Si, alkali, and alkaline earth elements, and, if present, boron (B). Other elements may also be released, but at lower concentrations. As glass-forming elements move into the altering solution, more water-derived ions can penetrate the glass surface and interact with freshly exposed bulk glass. Surface attack by water species can be nearly instantaneous with glass solidification, i.e., just post quenching of the melt107, and requires only that silanols be present on the glass surface and accessible to water molecules in the environment. Almost as quickly, organic materials may contact the glass surface and begin building up, possibly, although not likely, allowing for immediate microbial colonization. It is important to note that for both biotic and abiotic alteration to occur water must be present. Within 20 h of first exposure, wild bacteria can establish and reproduce to form a colony on a surface108. During this timeframe and under abiotic and ambient conditions, most silicate glasses would still be undergoing initial (forward) glass alteration processes83. Significantly longer amounts of time (e.g., days) are needed for fungal reproduction109. Whether microbial colonization occurs or not, a glass can continue to alter in response to surrounding abiotic conditions (driven by pH, solution chemistry, etc.). If the glass is colonized, the likelihood of which can increase over time with continued exposure to organics and biological agents, then glass alteration may be affected by the organisms’ local interactions with the glass surface90. Interestingly, the presence of the glass substrate may also influence the growth rate of the colonizing organisms110. Although abiotic processes of alteration may override biotic ones, as alluded to in12, studies of bacterial growth on inorganic substrates indicate scenarios in which biotic processes can dominate11,90. Over long timescales (months, years, decades, and beyond), glass alteration rates are probably dictated by both, with biotic and abiotic processes waxing and waning in intensity with time, and in response to environmental stimuli.
With it established that organisms can inhabit glass surfaces, it is now necessary to discuss the likelihood of such colonization and subsequent substrate alteration. It is well known that organisms will colonize almost any earth mineral85, particularly those that contain elements that are of nutritional value105,111, and bio-colonization is likely to occur under almost any condition where organisms can live —on basaltic glass on the ocean floor to interior glass in space stations orbiting the earth. However, as is mentioned above and will be elucidated below, colonization does not always lead to bio-alteration. How often it does, and at what frequency has been and still is an open question for the field. A major challenge to answering this query stems from the issue of separating out biotic alteration process from abiotic alteration processes during alteration experiments. As previously stated, these processes can occur concurrently and, sometimes, result in similar alteration signatures78. Despite these challenges, the processes of bio-alteration can and have been studied, examples of which can be found in34,51,90. Success of such studies seems to depend on addressing a series of questions:
-
(i)
What type(s) of organism(s) have colonized?
-
(ii)
What is the type(s) of organisms driving alteration, or is it caused by abiotic factors?
-
(iii)
When did colonization take place?
-
(iv)
How did the organism(s) interact with the surface?
-
(v)
How long, and with what frequency did the organisms interact with the surface?
-
(vi)
How do the rates and mechanisms of alteration change with biotic and abiotic factors? and
-
(vii)
When is the biofilm/organism protective and when is it destructive?
The remainder of this manuscript focuses on developing responses to these questions and outlining areas needing more research with the aim of providing a foundation upon which this issue of the likelihood of bio-alteration can be more fully addressed.
Extracellular polymeric substances and biofilms
Understanding bio-alteration processes requires an introduction to EPSs and biofilms, as they are frequently identified and discussed in the glass bio-alteration literature as growing on bio-altered glass (see Fig. 3 for an example, other examples can be found in14,34,90). They are also a primary point of interaction between organisms and glass surfaces. EPS and biofilm characteristics associated with specific organisms and their potential effects on glass alteration will be addressed in future associated publications. As debated by Staudigel et al.77 in 1995 and mentioned in 1987 by Koestler et al.51, biofilms and EPSs on glass may either prevent or exacerbate alteration of glass surfaces. Older bio-alteration literature24,63 and several newer publications11,15,112 provided experimental evidence of the destructive effects of biofilms and EPSs on glass. However, within the past few decades Aouad78, Aouad et al.88, and Bachelet et al.113 presented significant physical evidence that biofilms can be protective. Therefore, a definition and general outline of EPS, and biofilm structure and function is needed to better understand their sometimes contradictory effects on glass surfaces.
Samples99 were analyzed with a FEI Helios NanoLab 660 (Hillsboro, OR) FIB-SEM with an energy dispersive spectrometer (EDS; EDAX Newark, NJ). An accelerating voltage of 5 kV–10 kV, and a working distance of ~4 mm was used in the analyses of these materials. Imaging was performed with an Everhart-Thornley secondary electron (SE) detector in field-free conditions, and through-the-lens detectors (TLD) for SE and back-scatter electron (BSE) imaging in immersion mode.
EPSs are hydrated biopolymers that occur with a singular or a grouping of embedded microorganisms114. Their primary components are exopolysaccharides and proteins115. Minor components are lipids, DNA, and humic substances. They are excreted by many microbes and either remain attached to the outside of the microbe’s cell wall or are secreted onto a surface. They form the building materials of bacterial colonies, making up ≈50%–90% of the organic carbon in a biofilm structure116. They give the functionality, structural integrity, and biophysical and biochemical properties to a biofilm51,117.
Biofilm constituents include EPSs, water, organic acids, lipids, enzymes, DNA, and organophosphates114. The concentration and types of these organics may not be consistent throughout the biofilm118. This is in part due to the biofilm’s heterogenous architecture that is the result of a variety of microcolonies within its organic mass119. Different kinds of microbes are often found at the bottom of a biofilm (i.e., inorganic substrate and solution/biofilm interface), as compared those in the middle of a biofilm. As microcolonies are also not spatial nor temporally static (see120 for an example), so neither are biofilms.
A biofilm can limit solute diffusion and restrict the movement of water and nutrients into or out its embedded microorganisms through the properties of its EPSs and proteins116. Biofilms can be strongly hydrating; holding on to and releasing water and nutrients (i.e., reactive solutes) to its microbial communities114. Solutes can move through or around a biofilm either by diffusion or convection. Diffusion occurs due to random molecular motion, is often slow, and dominates under low flow or dry conditions121. It is also the transport mechanism for solutes to a cell surface and cell clusters121. Convection is a faster process resulting from and dictated by bulk fluid flow under a pressure gradient122. When solution channels are present in a film, solute transport can be affected by both advection (a type of convection) within the channels and diffusion across the remaining biomass122. Limits to diffusion within a biofilm lead to solute and even physiological gradients that maintain biofilm heterogeneity 121.
Another important aspect of biofilms that influences glass alteration is their ability to adhere to surfaces. The extent of adhesion is determined by the chemical properties of their EPSs123. EPSs contain functional groups that are strongly polar and anionic, such as carboxylates (-COO−) and phosphates (-PO43−). These groups may react with Si species on a glass surface in a fashion similar to that of OH− in the formation of silanols86, but instead form silicate esters. The esters may react further with the glass network, resulting in dissolution. In addition, if amino acids are constituents in the EPS, they may undergo complexation reactions with the glass surface through their zwitterionic structures. In this scenario, a positively charged portion of an amino acid is electrostatically attracted to a negatively charged, deprotonated silanol site on the glass surface. The negatively charged section of the acid can then rotate to and connect with a nearby positively charged site. Research by Ehrlich et al. has suggested this is a viable reaction path for organosilanols on the surface of colloidal silica, although the pH of the solution had to be carefully constrained to achieve successful complexation86.
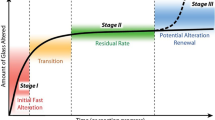
Biomineralization is the process by which minerals (mostly carbonates) are formed in biofilms. This mineralization is relevant to microbial interaction with glass as mineralized biofilms are often found on glasses altered at or in contact with a soil layer (see Fig. 3). Although relatively few studies have examined biomineralization as part of glass alteration (for example, see124,125), research by Jantzen and coworkers has shown that inorganic crystallization processes associated with a resumption of alteration during abiotic alteration may significantly increase the overall amount of dissolved glass126. Resumption of alteration is defined as an increase in the glass alteration rate following a leveling off period (referred to in the literature as the residual rate regime). It is possible that biomineralization of a biofilm could have a similar effect. The biogenic minerals could incorporate elements released from the glass matrix; a reaction which then might result in unsaturated conditions at the glass alteration front and drive the release of those elements to a higher rate. The biomineralization process may either enhance a biofilm’s structure or destabilize it, with the effect being dependent largely on the mineralization mechanism. The two fundamental mechanisms of biomineralization are biologically induced and organic matrix-mediated mineralization127. This second category was redefined by Mann in 1983 as biologically controlled mineralization128.
Biologically induced mineralization is the result of indirect interactions between an organism and its surrounding environment, and vary in type and amount by the nature of the interaction127. The organism(s) may influence the growth of such minerals through pH, pCO2, and the type of chemicals it secrets into the precipitation volume. The resulting biogenic minerals tend to be morphologically heterogeneous and variable in the amounts of bound water and trace elements incorporated into their crystalline structures129. According to Weiner and Dove, this variability is not always distinct from that of minerals produced abiotically, making it difficult to predict a mineral’s source (biotic or abiotic)130. External morphologies or additional identification of organic binders between crystals may have to been assessed to determine origin.
Biologically controlled mineralization occurs when an organism directly controls mineral nucleation, morphology, growth, and deposition through cellular activities128. In a recent book on this topic, Mann relates these processes to those which produce bones, shells, and teeth131. Weiner and Dove state that this process may occur extra-, inter-, or intracellularly relative to the cells actively controlling the process130. Extracellular mineralization occurs outside the cell, intercellular mineralization occurs between cells of the same or different organisms, and intracellular mineralization occurs inside the cell within specialized vesicles or vacuoles. Minerals produced intracellularly may be exported outside the cell or released during cell lysis130. The morphology of the minerals may be organism(s) or biofilm specific116,127,128,130, and may vary during biofilm/organism growth and development (see132 for the case of gypsum and calcite formation by a cyanobacterium). Several examples of biogenic minerals produced from the bio-alteration of glass are listed in Table 1.
Processes of bio-alteration
With establishment of the nature and general functionality of a biofilm, it is possible to discuss how biofilms and their embedded organisms may cause alteration of glass surfaces. Both Perez y Jorba68 and Krumbien11 allude to two general processes of bio-alteration:
-
(i)
Biophysical, resulting from physical forces; and
-
(ii)
Biochemical, resulting from chemical interactions.
In separating the biophysical and the biochemical contributions to alteration processes, the authors highlighted the distinct functions a glass may provide for an organism and/or biofilm. That is, it may be a physical support, a nutrient source, or both. To better comprehend how and when glass–organism(s) interactions occur it is necessary to understand an organism’s attachment processes and its metabolic needs. This topic is the subject of future work. Here, the processes are described more generally and historical insight into how they were developed is provided. A summary outline of the processes is provided in Fig. 4 to accompany this content. It is important to note that although discussed separately in this and other manuscripts (for example, see11,68), in nature biophysical and biochemical processes are most likely synergistic.
Biophysically induced alteration occurs when an organism’s/biofilm’s interaction with a surface results in mechanical stress. For individual organisms, these effects can be localized, but for biofilms they may be more global. These biophysical interactions do not directly cause the chemical leaching of elements from a glass substrate. Rather, the attachment of a microorganism(s) to a glass can causes its surface to mechanically alter. Non-biotic examples of biophysical alteration include fissuring or spalling of a glass surfaces due to humidity cycling and as illustrated in refs. 51,34. Similar biophysical forces have been observed on marble surfaces, where fungi are proposed to cause preexisting fissures to increase in size133.
Microorganism may induce mechanical stress when they adhere, expand or shrink, and/or detach from an anchoring substrate85. Two mechanisms for microorganisms attaching to surfaces have been discussed by van Loosdrecht et al.134: general physiochemical forces or cell-specific structures (appendages, fimbria, pili, etc.). Both mechanisms can produce mechanical stress, albeit on different length scales. Adhesion by physiochemical forces usually precedes interaction by cell-specific structures134. The adhesion process begins with long distance attraction between the organism and the surface (van der Waals forces), and, if they become sufficiently close, may be followed by short distance attraction (steric repulsion and H-bonding). Once the organism contacts the surface it may anchor through the production of EPSs that work as a bridging material between the organism and the surface135. If there are no cell-specific structures, or if they are unavailable, then adhesion may be driven by the physiochemical forces and attachment by EPSs, as described above134.
Biophysical interactions may be modeled, in part, by physiochemical parameters. This is because many microorganisms (e.g., bacteria) may be approximated as hydrated colloids134. As reviewed by Bos et al.136 and discussed by van Loosdrecht et al.134, physiochemical attachment may be influenced by surface (substrate and organism) hydrophobicity, charge distribution, solution chemistry (i.e., pH and ionic strength), contact angle, and roughness. Most cell surfaces are negatively charged, and their hydrophobicity varies over the cell’s life cycle. In contrast, silicate glass surfaces are usually hydrophilic134,137, with the degree of the glass’s surface hydrophilicity being related to its composition138, atomic topology139, and exposure conditions140, among other factors. The contact angle between water and a surface is sometimes considered a proxy for its hydrophobicity134,137. Generally, bacteria adhere more strongly to hydrophobic surfaces (e.g., metals oxides) than hydrophilic surfaces (e.g., salts)134,137. In addition, the extent to which bacteria will adhere to glass surfaces has been found to be negatively correlated with a bacterium’s zeta potential134,137. The zeta potential is the electric charge of the bacteria at the shear plane. It is widely used as a proxy for bacterial cell surface charge. For Gram-negative bacteria, increased adhesion to a glass surface may occur if the bacteria have long chain lipopolysaccharides and if they produce a significant amount of EPSs137. Increased surface roughness141 and a higher ionic strength in the altering solution (1 mM–100 mM)137 have been determined to increase bacterial adhesion. A list of the parameters that affect bacterial adhesion to silicate glass, compiled mainly from the work of Li and Logan137, are summarized in Table 1.
As helpful as these models are for understanding microbial attachment to glass, they are limited. In 2004, Li and Logan suggested that better, or at least additional, models are needed to characterize how bacteria attach to inorganic surfaces137. Currently, there is a thermodynamic model142 and a “classical” DLVO (Derjaguin, Landau, Verwey, Overbeek) theory (partially based on the work of van Oss et al. starting in the 1980’s, e.g.,143). Possible new models (including variations upon the previous two) have been reviewed thoroughly by Bos et al.136 and by Sharma and Rao144, and will not be discussed in greater detail here. However, Vadillo-Rodríguez and Logan brought these reviewed models into question because they treat surfaces as having a relatively homogeneous distribution of active adhesion sites145. Experimental results reported in the afore-listed citation suggest that the active sites are more heterogeneously distributed than previously thought, and that the models need to be updated. The extent of such heterogeneity could be estimated from the ratio of active to inactive surface sites (-Si-OH, -Si-O-Si-, and Si-O-X) for bacteria adhesion. Measurement of these ratios could be achieved by analytical methods (by NMR, FT-IR, etc.), or possibly by topological constraint modeling146.
Attachment, expansion, shrinkage, and detachment of a microorganism from a glass surface as described above can be the result of variations in an organism’s EPS gene expression—which can be induced by surface contact itself147, the hydration/dehydration of the biofilm135, and film’s stiffness148. In regards to gene expression, first bacteria sense a surface before attaching to it, a process that triggers the expression of several essential genes149. Similar processes of regulated gene expression have been found during swarming, growth, and migration of a microbial community on a surface147. The details of these processes are organism specific (e.g., is different for Gram-negative bacteria vs. Gram-positive bacteria vs. fungi)147, and will be discussed in relation to each organism type in a future publication.
The effects of hydration/dehydration of biofilms on glass surfaces can be just as complicated, and often are conflated with the viscoelasticity (i.e., stiffness) of the biofilm135. However, and in general, when a biofilm hydrates it expands, which may cause tension stress to an underlying, inorganic surface150. When a biofilm dehydrated it contracts, which may cause compressive stress to a surface. If the mechanical strength of the inorganic surface varies from one region to the next, hydration/dehydration may cause shear stresses to develop. Repeated hydration/dehydration cycles can result in fatigue in a glass alteration layer and may cause mechanical failure. Comparing the viscoelasticity of the biofilm to the strength of the surface may predict how quickly and to what extent this physical alteration may occur151.
Biochemical alteration occurs when glass-forming elements are removed from a surface due to a chemical environment that is produced and/or mediated by an organism. Biochemical alteration may be divided into the two categories: passive alteration and active alteration. The naming convention used here is consistent, albeit contextually different (i.e., applied to glass alteration rather than mineral formation), with that proposed by Ehrlich in his 1998 review152. In passive alteration, an organism excretes chemicals that are part of its normal life cycle (e.g., metabolites such as carbon dioxide, organic acids, or the EPSs of biofilms), which may or may not induce dissolution of the contacted glass surface. Alternatively, in active alteration an organism excretes chemicals directly (e.g., siderophores) to remove elements from the glass. Extracted elements such as Fe, Mn, Mg, and P may be of nutritional benefit of the organism. It is generally presumed that passive alteration is more common than active alteration14; however, there have been examples of possible active alteration presented in the literature where experiments were conducted for long timeframes (>100 days) without the “amendment” of nutrients for the altering microorganisms77. These processes need not be mutually exclusive and may even be synergetic.
The chemical environment of the biofilm (pH, ionic strength, chemical composition, etc.) is a factor for whether chemical alteration will occur passively, actively, or by a mixture of both pathways, and should also be discussed. Studies on biofouled glass electrodes suggests that species within a biofilm can produce buffering substances that can shift the pH of an interfacial solution153. These shifts are most likely localized as biofilms are chemically heterogenous; a feature due to metabolic activities of inhabiting species and solute diffusion154. They can also be transient as biofilm chemical gradients can vary with time and environmental conditions.
Under laboratory conditions, the concentration of biogenic buffers produced have also been related to the amount of buffering chemicals added at the beginning of an experiment155. In nature, such ex situ buffer agents could be introduced by other organisms or changes in the macro-environment (e.g., rainfall, flooding, fires, etc.). In research on Arthrobacter-inhabited biofilms, Fe-rich and Fe-poor silicate glass and mineral samples aged under buffered conditions were measured to have no significant pH change (<0.04) within the biofilms grown on silicate substrates, but a significant pH change (0.27–1.08) was measured for the same samples aged in unbuffered conditions155. Depending on the starting pH of the alteration solution, the activity of the microorganisms in this environment, and the chemistry of the glass, a pH shift of 1 unit, as suggested by Liermann et al.155, could have a significant effect on glass alteration rates, particularly during the first stage of glass alteration83. As cautioned by the authors of the study155, the measured differences in the unbuffered scenarios may not be as large in a natural setting, but a change could be expected under unsaturated conditions with acid producing microbes. The organic acids detected in the buffered solutions includes acetic, formic, oxalic, and citric acids155, with more formic and acetic acids being detected in the post-alteration solutions of the low-Fe silicate glass.
Beyond organic acids, other biogenic chemicals can be present in biofilms and their surrounding chemical environment. For example, and in the previously described study, rates of dissolution can also be affected by the presence of siderophores155. Siderophores are organic compounds that bind (chelate) and transport Fe to microorganisms. Some siderophores can also bind and transport other essential elements such as Mo, V, Zn, and Mn156. Siderophores can be produced by fungi, bacteria, and lichen under a variety of conditions as Fe, and these other elements are necessary nutrients for most organisms. As suggested by Callot et al. and Perez et al., siderophores may play an important role in the biogenic etching, and subsequent release of Fe from both crystalline and amorphous Fe bearing solids under extreme conditions80,87. Siderophore production, as described here, is an example of active biochemical alteration.
Whether biochemical alteration happens actively or passively, one defining indicator of it occurring is the depletion of certain elements from a glass surface. Depleted elements may include Si, Al, alkali and alkaline earth metals, and transition metals (Fe, Mn, Ti, etc.). As already stated, it can be very difficult to determine if elemental depletion under natural conditions is the result of biotic or abiotic processes14,54,66,157, although it is likely that both play a role52. Several recent studies suggest that there may be differences between the two processes in the types and concentrations of elements released from a glass14,15,53, with increased Fe and Mg release rates being noted by Aouad for glasses (basaltic and industrial chemistries) altered by isolated and lab grown Pseudomonas aeruginosa. Aouad also found (for experiments run >100 days) that the concentration of most elements were lower in the altering solution for the biotically altered vs. abiotically altered test glasses (see Table 2)14. For select glass chemistries tested, the rate of (most) elemental release under sterile conditions was found to be 26%–79% greater than those measured in the biotic experiments14. These dissolved elements were predominately found in the altering solution, glass alteration layer, and, if alteration was completed under biotic conditions, the biofilm layer. The concentrations of Si, Mg, Ca, Fe, and K were higher in the solid alteration layers for the biologically exposed glasses as compared those altered under abiotic conditions14. In addition, and for biotically altered glasses, the biofilms were enriched in Ni, Co, Pb, Cd, Cs, Th, Ba, and Zn, with the exception of one altered glass (SON68, the inactive French reference nuclear waste glass)14. A summary of elemental concentration in altering solutions vs. alteration layers vs. the biofilms is provided in the Table 2.
Other glass alteration experiments that have followed elemental release rate were conducted on basaltic glasses using bacteria from a natural deposit (Surtsey tuff) by Thorseth et al. and using microorganisms native to seawater by Staudigel et al.52,53. Thorseth et al. observed bacterial and biofilm attachment to the glass substrates within the first 46 days of exposure and indicators of glass alteration after 181 days53. The newly formed biofilms accumulated both Al and Si, most likely from the glass, along with many other elements (e.g., Na, Fe, and Ca) that could have been sourced from either the glass or growth medium53. Thorseth et al. noted that concentrations of these elements varied over the course of and between sets of experiments, attributing the observation to variations in the microbial community itself. After 394 days, the bacteria had taken up very little Si (an element which only could have come from the glass) inside their cells; a finding similar to Aouad14. Both Thorseth’s and Aouad’s studies underline the important experimental practice for glass bio-alteration studies of conducting elemental release inventories with not only altering solution samples, but also with produced solids, biofilms, and (possibly) the microorganisms in the study.
The need for this practice has been briefly discussed in the 1998 paper by Stuadigel et al.52. In this publication, the authors reported that the presence of microorganisms accelerated basaltic glass alteration. This statement was supported by their finding of increased Sr in produced sediments. Surprisingly, they did not observe an increase in Si in the alteration solution from the biotic experiments as compared to the abiotic experiments, a common indicator of glass alteration. Rather, a significant decrease was measured for Si release for both biotic experiments. The authors’ attributed this result to either to the glass having a low release rate in the provided conditions or its immediate precipitation or (possibly biotic) utilization. A completely inventory of Si in these locations, which appears to not have been possible at the writing of their publication, may have provided more clarity on observed alteration processes.
These reports on occasionally contradictory elemental release rates naturally leads to a discussion of the true significance of such findings for biotic experiments. Generally, the leaching of an element from a glass under biotic conditions does not conclusively prove that the element was leached biotically. In a similar respect, just because an element that could be metabolized by an organism is leached does not mean that it will be metabolized. Aouad attributes a similar sentiment to Crovisier (as quoted in14):
“Ce n’est pas parce que je vois des moutons dans une grotte que je dois conclure que les moutons creusent la pierre.”
(It’s not because I see sheep in a cave that I must conclude that the sheep dig the stone)
As outlined by Furnes in54, determination of glass alteration by bioactivity requires identification of specific indicators of an organisms’ interaction with a glass surface and its removed elements. Changes in elemental released rates can be one such indicator, but should be assessed in the context of others for determination of source cause (i.e., biotic or abiotic). Examples of other indicators (or signatures) could include the presence of biogenic minerals54,68,158 (see Table 3), or the enrichment within an organism of an element with isotopic ratios of the glass and/or altering solution.
Signatures of bio-alteration
Visual signatures of bio-alteration on glass surfaces has been extensively discussed in the literature and will only be briefly mentioned here. These signatures include pitting, tubes, and surface etching, along with half-circle alteration patterns visible only in cross section11,25,51,54,89,93,101,157. The shapes and sizes of these features may be related to the type of organism(s) observed interacting with the glass surface26,53. An example of pitting and half-circle alteration patterns is shown in Fig. 5A, B, and was found in a cross section of a naturally altered vitrified hillfort glass99,101. The association of these textures with a biologically relevant materials (biofilms, fimbria, etc.) and biologically produced chemical residuals can aid in their assignment to biological sources.
Samples102 were analyzed with a FEI Helios NanoLab 660 (Hillsboro, OR) FIB-SEM with an energy dispersive spectrometer (EDS; EDAX Newark, NJ, example shown in image B). An accelerating voltage of 5 kV–10 kV, and a working distance of ~4 mm were used in the analyses. Image A was collected on a FIB sample with a JEOL ARM200F (JEOL, Peabody, MA) in scanning transmission electron microscope (STEM) mode operated at 200 keV. Image B was collected with an Everhart-Thornley secondary electron (SE) detector in field-free conditions, and through-the-lens detectors (TLD) for SE and back-scatter electron (BSE) imaging in immersion mode using the above listed conditions. Images reproduced from Weaver et al.99. Left image was reproduced with permission from102, copyright (John Wiley and Sons, 2018).
Another indicator is the enrichment or depletion of biologically relevant elements in or near the glass alteration layer, and the formation of biologically produced minerals10,158. This section will predominately focus on the latter topic as the release of individual elements has been presented in detail above. Examples of biologically relevant elements that are part of microbial life cycles and associated with glass alteration can include H, C, O, N, S, P, K, Ca, Mg, and Si and select transition metals (i.e., Mn and Fe)159. The biogenic minerals incorporating these elements can be divided into five main groups: carbonates, sulfates, silicates (including clays), phosphates, and metals, metal oxides and sulfides. Their formation has been previously outlined above. For each group example, minerals that have been identified on altered glasses are listed in Table 3.
Some of biogenic-like minerals are formed on glasses altered under abiotic conditions, thus care should be taken in ascribing their source as abiotic or biotic when they have been produced under unknown or uncontrolled altering conditions130. For example, calcite minerals were found on the surface of medieval glass windows in Spain125. Although the surface was exposed to microorganisms, calcite was determined to be abiotic in origin because there were no calcium oxalates present near the deposits. If both calcite and calcium oxalates had been present, then they would had been most likely biotic in origin. This context of the surrounding environment including associated minerals is important when predicting mineral sources.
Clays, a common product found on abiotically altered glasses, also may be produced biotically. Cuadros et al.124 studied microbially induced clays formed from Fe- and Mg-rich rhyolitic glasses under a variety of solution conditions. They reported the importance of glass chemistry in determining the type of alteration products that formed, but also that clays formed in the biofilms covering the glass samples. The authors suggested that the clay was formed as Fe absorption onto the microorganisms’ cell walls and EPS structures, and the reaction of the Fe with dissolved Si and Al in the glass-altering solution or held within the biofilms124. The extent and rate of clay formation was suggested (although not measured) to be related to the porosity of the biofilm and temporal changes in its architecture.
Bio-alteration and time
Such temporal changes in biofilms and organism activity is of significance to glass bio-alteration research and is very dependent on both the life cycles of the organisms and rates of biotic exposure. As stated above, exposure of glass surfaces by ambient organic material and microorganisms can occur as soon as the glass begins to cool from its molten state. Based on exposure rates and other environmental factors, microbes could begin to colonize a surface within minutes, but also hours, years, or never occur at all. Indeed, others have noted detailed examples in which Roman glass samples excavated from a naturally dry environment showed little to no indication of alteration even after more than a thousand years of exposure160. If colonization does occur, laboratory evidence suggests that bio-alteration processes can be detected within days88. For example, within three days, and with only bacteria present, Aouad et al. noted that there was a significant increase in the amount of Fe released into solution as compared to an abiotic control sample88. The authors attributed this release to the production of a chelating agent (pyoverdine) by the bacteria. This same chelating agent was detected after six days in alteration experiments on synthetic basaltic glasses during a different study by Perez et al.87. Three to six days is significantly less than the months Staudgiel et al77. reported for the formation of biofilms in their studies of the alteration of man-made glasses using naturally sourced (i.e., seawater) solutions inoculated with bacteria.
Physical manifestation of changes in glass alteration rates due to the presence of a biofilm can take considerably longer. In many studies, the timescale of the formation of physical signatures of alteration (pitting and etching) corresponds with the weeks, months, or years needed for biofilm formation14,77. Once established biofilms can persist on surfaces anytime from a few days to decades, a feature that is in part due to their ability to dynamically respond to environment changes161,162,163. This fact suggests that bio-alteration rates for systems, including biofilms should be studied on several timescales—hours, days, months, and years—so as to make an accurate assessment of the extent and impact of bio-alteration on glass over time.
Summary and future research needs
Synergistics of abiotic and biotic alteration
As outlined in Fig. 1, there are several overarching concepts which have been developed in this field since the early 1800’s. One is that natural alteration of silicate glass is a synergism between abiotic and biotic sources of alteration, and their chemical and physical processes. However, and as pointed out by researchers from Mellor26 to Aouad78, these sources of alteration appear to occur in parallel, with one possibly dominating during one time period and then the other dominating during another time period. As argued above and elsewhere, “signatures” of these sources may be similar if not indistinguishable, thus making identification of a primary driver difficult. Questions to be addressed on this topic are:
-
(i)
Under what conditions does biotic alteration outperform abiotic alteration and vice versa?
-
(ii)
What are the synergistic effects of one process on the other? and
-
(iii)
How do the timescales of these alteration processes change overall glass alteration rates?
An evolution in current glass alteration sample monitoring methods and analyses is needed to answer these questions as most current methods do no follow both organic and inorganic elemental release rates in real time or in situ. Such needed methodology has been already outlined for ex situ samples (see Furnes et al.54 and Benzerara et al.89), but these techniques need to be extended for use during active experiments. One way in which this could be achieved is by coupling micro-level isotopic analysis methods, such as sensitive high-resolution ion microprobe with reverse geometry (SHRIMP-RG)164 with microscopy to colocate on glass surfaces organisms and alteration textures, and to test their alteration layers for biologically relevant N isotopic enrichment in real time.
Time
Additional work is needed to develop a deeper comprehension of the temporal component of glass bio-alteration. This should be completed on timescales relative to the lifetimes of organisms (minutes to years) and biofilms (months to years). Questions to be addressed on this topic include:
-
(i)
How long does an organism need to interact with a glass to cause significant alteration?
-
(ii)
How does an organism’s life cycle and life cycle length affect glass alteration processes, and, by extension, rates of degradation?
To date only a handful of studies on select silicate glass chemistries have addressed the timing of alteration (for examples, see51,52,78), and more work needs to be completed on a greater variety of glass chemistries and alteration relevant microorganisms. Having this information can assist in development of a more global and better time-resolved schema for bio-alteration of glass. This would be greatly aided by tests done at finer timescales (hours and days) and for longer periods of time (>six months), using data analyzed from organisms, bioproducts, surfaces and solutions, and coupled with microorganism abundance and frequencies in mixed colonies and biofilms, using DNA and RNA sequencing. The analysis of surfaces and solutions should include not only inorganic components (Si, Al, Fe, etc.), but also organic ones (N, C, S, P, organic molecules, etc.). In addition, transition metal redox reactions may have a temporal differentiation in systems that involve bacteria, playing a greater role in early dissolution and sorption processes than later88. Therefore, tests like those proposed above, but with the inclusion of redox analysis (preferably on the micrometer or lower scale and colocated to areas of biological activity), coupled with the detection of organic molecules should be conducted.
Colony diversity
As mentioned upon above, another topic to address is that of alteration processes in mixed-species colonies. In nature, many types of microorganisms are embedded in a single biofilm and the frequency and abundance of individual types may influence how glasses alter77. From this knowledge arises this question: what is the effect of colony composition on glass alteration processes and rates? This question can be tricky to address as it will have an interplay with the above discussed issue of microbial residence time and can be affected by changes in the external environment (addressed below). Successful execution of this research will require pinpointing the effects of keystone microbial species and mixed colonies.
Water in the biofilm
Most of the above questions have been stated regarding singular organisms or colonies; however, similar questions should be asked for biofilms. The dynamics of microbial populations and the development of EPSs and other substances to the establishment of a biofilm are still not well understood. This is a question larger than the focus of this review, and, therefore, needs to be reduced to sub-questions that are specific to the current topic. On such subtopic is water—the substance through which glass alteration is mediated. An example question in this area is: how do EPSs and the resulting biofilm take-up, hold, and release water? As EPSs and biofilms are characterized by water retention and desiccation tolerance, understanding their water uptake is important to gaining insight into their interactions with glass surfaces.
Just as important as knowing how water is regulated in biofilms is knowing what it contains. Water within a biofilm can include a vast inventory of organic molecules, organisms, and inorganic materials165. Some of these substances can affect the pH and chemical activity of water. Several abiotic glass alteration studies have shown that shifts in pH and composition can affect glass alteration rates or/and processes83,166,167. Thus, the chemistry of water within a biofilm should also be considered when discussing the physical containment and transport of water within a biofilm.
Biofilm architecture and alteration rates
Another query for research that can dictate the presence of water in biofilms is: how do individual biofilms become heterogeneous in terms of hydrophilicity and hydrophobicity? The degree of heterogeneity and the mechanisms that produce it are not well understood115, although it is hypothesized to be related to diffusion gradients of reactive solutes (i.e., nutrients) within a film121. Biofilms are inherently porous and may act as molecular/elemental sieves, selectively taking up, moving, and exporting elements and molecules, thus forming the chemical gradients. This may be a positive feature of the film as it may act as a protective interface to the glass14, but this has only been shown for a handful of systems and needs further study with a larger variety of microorganisms.
In answering the above two questions, it may then be possible to then address the bigger issue of: how do biofilms influence glass alteration processes? As previously stated and cited, it has been experimentally demonstrated that as biofilms mature and generate biogenic minerals they may protect a glass from the outside environment90. This may be observed as a reduction in the extraction of elements from the glass surface14, or an inhibition of the “pumping mechanism” (i.e., the movement of elements out of the glass and into the unsaturated, alteration solution) that has been identified as a significant factor in glass alteration126. Alternatively, it may accelerate glass alteration as is known to occur with select glass chemistries under abiotic conditions126,168, and is suggested to occur under biotic conditions52. More tests which involve judiciously controlled biofilm growth on glass with careful monitoring of biological, biogenic, and inorganic components, such as those conducted by Aouad14 are needed to answer these questions. These tests also need to be completed with relevant microorganisms and as multiple replicates. Replication will be particularly important in order to gain insight into the true variance of bio-alteration rates; a necessary and key parameter for incorporating results from these alteration studies into long-term glass alteration models.
Impact of external environment
One more important question to ask is: what effect do changes in an external environment have on glass bio-alteration rates? It is an open question whether organisms exposed to very dry climates alter glass in the same manner as those exposed to very wet climates. As described above, most colonies undergo hydration/dehydration cycles at least on a seasonal basis. How much does this change in available water affect the rate of glass alteration and are the changes predominately physical or chemical in nature? Knowing the frequency and impact of these wetting and drying events can provide a more insight into how silicate glasses alter under natural, seasonal cycles.
As expressed above, there is a significant number of questions to be answered on this topic. It is at this point many researchers may ask why should such work be undertaken? The drivers for completing this work appear to be motivated by a combination of scientific curiosity and economic necessity. Beyond the relevance to the issue of nuclear waste glass disposal, as herein described in detail, and in regards to the latter, microbial alteration is recognized as a key to reclamation of precious transition and rare earth metals from manufactured materials, including glasses18,169. If the mechanisms of alteration were better understood, microorganisms might be more efficiently harnessed for the bioremediation or abatement of toxic metals (e.g., Pb, Hg, Ar) from the large quantity of glass that is produced for electronic devices170. At the same time, the need for antimicrobial glass is increasing, with a projected market growth of over 6% by 2023, in the areas of medicine, energy production (solar cells), food production, and military hardware (i.e., hand-held technology and motor vehicles)171. These areas of economic growth suggest that research on biological alteration will continue to expand in the coming years172, and, therefore, provide a practical basis for why these questions should be addressed.
Conclusion
This review presents a historical timeline of publications on the topic of bio-induced glass alteration. A general outline of how organisms interact with silicate glass surfaces either through biophysical and/or biochemical processes, and developed from these and other relevant publications is provided. In this review, biophysically alteration is defined as a process where mechanical stresses cause alteration to a glass surface. Biochemical alteration is defined as a process where chemicals produced by microorganisms’ cause leaching of elements from a glass surface. The leaching of these elements can either be passive, occurring as a result of an organism’s normal metabolic activity, or active, occurring as a result of an organism producing enzymes or chemicals to mediate the removal of select elements from glass. One overarching topic of discussion in this literature has been the chemical and physical signatures of these different alteration processes. Possible signatures of biological alteration may include the leaching of certain biologically relevant elements, the biogenic formation of minerals, and the presence of pitting or etching textures on the surface of glasses in association with enriched organic materials. However, research suggests that these features are not always clear indicators of biological alteration, and there are questions about when and under what conditions biotic alteration dominates abiotic alteration of glass surfaces. These questions can be addressed, in part, by investigating the types of organisms that colonize glass, the topic of a future publication.
Data availability
All data used to compile this manuscript can be made upon reasonable request to the corresponding author.
References
Koestler, et al. in The Science, Responsibility, and Cost of Sustaining Cultural Heritage (ed W. E. Krumbein, Brimblecombe, P., Cosgrove, D. E. & Staniforth, S) 149–163 (Wiley, 1994).
Cailleteau, C. et al. Insight into silicate-glass corrosion mechanisms. Nat. Mater. 7, 978–983 (2008).
Gin, S., Ryan, J. V., Kerisit, S. & Du, J. Simplifying a solution to a complex puzzle. npj Mater. Degrad. 2, 35–36 (2018).
Vienna, J. D., Ryan, J. V., Gin, S. & Inagaki, Y. Current understanding and remaining challenges in modeling long‐term degradation of borosilicate nuclear waste glasses. Int. J. Appl. Glass Sci. 4, 283–294 (2013).
David, L. Window Wars in Space: Quest for the ‘Big View’ High Above Earth. https://www.space.com/39691-window-sizes-spacecraft-blue-origin-virgin-galactic.html (2018).
Osborne, C. A city of glass: Elon Musk reveals plans for life on Mars. https://www.zdnet.com/article/a-city-of-glass-elon-musk-reveals-plans-for-life-on-mars/ (2016).
Robson, D. A glass sub to probe the ocean depths. https://www.bbc.com/future/article/20141205-glass-sub-for-the-deepest-depths (2014).
Spor, H., Trescinski, M. & Libert, M. F. In Materials Research Society Symposium Proceedings (eds C. G. Interrante & R. T. Pabalan) 771 (Cambridge University Press, 1992).
Kim, J.-S., Kwon, S.-K., Sanchez, M. & Cho, G.-C. Geological storage of high level nuclear waste. KSCE J. Civil Eng. 15, 721–737 (2011).
Drewello, R. & Weissmann, R. Microbially influenced corrosion of glass. Appl. Microbiol. Biotechnol. 47, 337–346 (1997).
Krumbein, W. E., Urzì, C. E. & Gehrmann, C. Biocorrosion and biodeterioration of antique and medieval glass. Geomicrobiol. J. 9, 139–160 (1991).
Newton, R. G. & Davison, S. Conservation of Glass, 154 (Butterworth & Co. Ltd., 1989).
Koestler, R. & Vedral, J. Biodeterioration of cultural property: a bibliography. Int. Biodeterior. 28, 229–340 (1991).
Aouad, G. Influence de Pseudomonas aeruginosa sur la Dégradation de Silicates: Incidence sur la Stabilité de Matrices de Confinement de Déchets et d’un Mâchefer Industriel. PhD thesis, University of Strasbourg (2006).
Shelley, W. L. Biocorrosion of Archaeological Glass (University of California, 2016).
Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives (Apple Academic Press; CRC Press, 2017).
Koestler, R., Warscheid, T. & Nieto, F. Biodeterioration: Risk factors and their management. Saving Our Architectural Heritage: The Conservation of Historic Stone Structures. Dahlem Workshop Report ES20 25-36 (1997).
Gadd, G. M. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156, 609–643 (2010).
Fries, E. Lichenographia Europaea Reformata. Praemittuntur Lichenologiae Fundamenta. Caput X. Sedes Lichenum s. solum & stationes, LXXXIV (Typis Berlingianis, 1831).
Crombie, J. M. Vitricole lichens and the Schwendenerian Hypothesis. J. R. Microsc. Soc. 2, 929 (1879).
Nylander, W. Circa lichenes vitricolas notula. Flora (Regensburg) 62, 303–304 (1879).
Egeling, G. Ein Beitrag zur lösung der frage bezüglich der ernährung der flechten. Plant Syst. Evol. 31, 323–324 (1881).
Fink, B. The nature and classification of lichens II: the lichen and its algal host. Mycologia 5, 97–166 (1913).
Mellor, E. The decay of window glass from the point of view of lichenous growths. J. Glass Technol. 8, 182–186 (1924).
Mellor, E. Les lichens vitricoles et leur action mecanique sur les vitraux d’eglise. C. R. Seances Acad. Sci. 173, 1106–1108 (1921).
Mellor, E. Lichens and their action on the glass and leadings of church windows. Nature 112, 299–300 (1923).
Martin, G. Action of lichens on Glass. Bot. Gaz. 73, 423–423 (1922).
Fry, E. J. A suggested explanation of the mechanical action of lithophytic lichens on rocks (shale). Ann. Bot. 38, 175–196 (1924).
Perez-Llano, G. A. Lichens their biological and economic significance. Bot. Rev. 10, 1 (1944).
Llano, G. A. Economic uses of lichens. Econ. Bot. 2, 43 (1948). 15.
Llano, G. A. Economic Uses of Lichens (Smithsonian Institution, 1951).
Bettembourg, J.-M. Composition et altération des verres de vitraux anciens. Verres Réfract 30, 36–42 (1976).
Caneva, G., Nugari, M. P., Nugari, M. & Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation, 407 (Getty Conservation Institute, 2008).
Gorbushina, A. A. & Palinska, K. A. Biodeteriorative processes on glass: experimental proof of the role of fungi and cyanobacteria. Aerobiologia (Bologna) 15, 183–192 (1999).
Marvasi, M. et al. Bacterial community analysis on the Mediaeval stained glass window “Natività” in the Florence Cathedral. J. Cult. Herit. 10, 124–133 (2009).
Sterflinger, K. Fungi as geologic agents. Geomicrobiol. J. 17, 97–124 (2000).
Adamo, P. & Violante, P. Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl. Clay Sci. 16, 229–256 (2000).
Jones, D., Wilson, M. & McHardy, W. in Biodeterioration 7, 129–134 (Springer, 1988).
Hutchinson, W. The fouling of optical glass by micro-organisms. J. Bacteriol. 54, 45–46 (1947).
Nagamuttu, S. Moulds on optical glass and control measures. Int. Biodeterior. Bull. 3, 25–27 (1967).
Richards, O. W. Some fungous contaminants of optical instruments. J. Bacteriol. 58, 453 (1949).
Turner, J. S., McLennan, E. I., Rogers, J. S. & Matthaei, E. Tropic-proofing of optical instruments by a fungicide. Nature 158, 469–472 (1946).
Jones, F. L. Deterioration of glass in tropical use. J. Am. Ceram. Soc. 28, 32–32 (1945).
Jones, F. New treatments for optical glass. Chem. Eng. News 19, 390 (1941).
Baker, P. An evaluation of some fungicides for optical instruments. Int. Biodeterior. Bull. 3, 59–64 (1967).
Baker, P. Possible adverse effects of ethyl-mercury chloride and meta-cresyl-acetate if used as fungicides for optical/electronic equipment. Int. Biodeterior. Bull. 4, 59–5 (1968).
Jin, Y., Milia, C. D. & Wolcott, C. C. Antimicrobial glass articles and methods of making and using same. USA patent US20140370303A1 (2014).
Page, K. et al. Titania and silver–titania composite films on glass—potent antimicrobial coatings. J. Mater. Chem. 17, 95–104 (2007).
Baigozhin, A. The attaching of biologically active compounds to the surfaces of optical materials. Russ. Chem. Rev. 49, 1112–1118 (1980).
Berk, S. & Teitell, L. Radioactive materials in prevention of mold growth in optical instruments. Ind. Eng. Chem. 46, 778–784 (1954).
Koestler, R. J., Santoro, E. D., Ransick, L., Brill, R. H. & Lynn, M. in Biodeterioration Research 1, 295–307 (Springer, 1987).
Staudigel, H. et al. Biologically mediated dissolution of volcanic glass in seawater. Earth Planet. Sci. Lett. 164, 233–244 (1998).
Thorseth, I., Furnes, H. & Tumyr, O. Textural and chemical effects of bacterial activity on basaltic glass: an experimental approach. Chem. Geol. 119, 139–160 (1995).
Furnes, H. et al. Identifying bio-interaction with basaltic glass in oceanic crust and implications for estimating the depth of the oceanic biosphere: a review. Geol. Soc. Spec. Publ. 202, 407–421 (2002).
Ruska, E. & Ruska, H. Electronic microscope. USA patent US2266082A (1941).
Minsky, M. Confocal scanning microscope. Rapport technique, Patent 3 (1955).
Minsky, M. Memoir on inventing the confocal scanning microscope. Scanning 10, 128–138 (1988).
Tsien, R. Y. The green fluorescent protein. Ann. Rev. Biochem. 67, 509–544 (1998).
Shimomura, O., Johnson, F. H. & Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Physiol. 59, 223–239 (1962).
Prasher, D. C., Eckenrode, V. K., Ward, W. W., Prendergast, F. G. & Cormier, M. J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111, 229–233 (1992).
Patterson, G. H., Knobel, S. M., Sharif, W. D., Kain, S. R., & Piston, D. W. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 73, 2782–2790 (1997).
Sackler, A. M. in Arthur M. Sackler Colloquia of the National Academy of Sciences (ed J. Winter) 72–87 (The National Academies Press, 2005).
Prod’homme, M. Actions des microorganisms sur les surfaces vitreuses. in Proc. VIII nt. Cong. Glass, Brussels, Belgium. Gordon & Breach, New York, 17 (1965).
Winter, A. Alteration des surfaces des verres anciens. In Proc. VIII nt. Cong. Glass, 229 (Gordon & Breach, New York, 1965).
Kerner-Gang, W. Zur frage der entstehung von schimmelpilzspuren auf optischen gläsern. Mater. Organismen 3, 17 (1968).
Collongues, R., Perez y Jorba, M., Tilloca, G. & Dallas, J. Nouveaux aspects du phenomene de corrosion des vitraux anciens des eglises francaises. Verres Réfract. 30, 43–55 (1976).
Tennent, N. Fungal growth on medieval glass. J. Br. Soc. Master Glass Painters 17, 64–68 (1981).
Perez Y Jorba, M., Dallas, J., Bauer, C., Bahezre, C. & Martin, J. Deterioration of stained glass by atmospheric corrosion and micro-organisms. J. Mater. Sci. 15, 1640–1647 (1980).
Wells, O. C. & Joy, D. C. The early history and future of the SEM. Surf. Interface Anal. 38, 1738–1742 (2006).
Torsvik, T., Furnes, H., Muehlenbachs, K., Thorseth, I. H. & Tumyr, O. Evidence for microbial activity at the glass-alteration interface in oceanic basalts. Earth Planet. Sci. Lett. 162, 165–176 (1998).
Giovannoni, S. J., Fisk, M. R., Mullins, T. D. & Furnes, H. Genetic evidence for endolithic microbial life colonizing basaltic glass/sea water interfaces. Proc. ODP, Sci. Results 148, 207–214 (1996).
Furnes, H., Thorseth, I. H., Tumyr, O., Torsvik, T. & Fisk, M. R. in Ocean Drilling Program (eds Alt, J. C., Kinoshita, H., Stokking, L. B. & Michael, P. J.) 483–487 (Texas A&M University, 1996).
Thorseth, I. H., Torsvik, T., Furnes, H. & Muehlenbachs, K. Microbes play an important role in the alteration of oceanic crust. Chem. Geol. 126, 137–146 (1995).
Thorseth, I., Furnes, H. & Heldal, M. The importance of microbiological activity in the alteration of natural basaltic glass. Geochim. Cosmochim. Acta. 56, 845–850 (1992).
Alt, J. C. & Mata, P. On the role of microbes in the alteration of submarine basaltic glass: a TEM study. Earth Planet. Sci. Lett. 181, 301–313 (2000).
Knowles, E., Wirth, R. & Templeton, A. A comparative analysis of potential biosignatures in basalt glass by FIB-TEM. Chem. Geol. 330, 165–175 (2012).
Staudigel, H., Chastain, R., Yayanos, A. & Bourcier, W. Biologically mediated dissolution of glass. Chem. Geol. 126, 147–154 (1995).
Aouad, G. et al. Mise au point d’un milieu de culture pour l’étude de l’altération de silicates en présence de Pseudomonas aeruginosa. C. R. Geosci. 337, 1340–1347 (2005).
Rölleke, S. et al. Analysis of bacterial communities on historical glass by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. J. Microbiol. Methods 36, 107–114 (1999).
Callot, G., Maurette, M., Pottier, L. & Dubois, A. Biogenic etching of microfractures in amorphous and crystalline silicates. Nature 328, 147 (1987).
Mazer, J. J. Kinetics of Glass Dissolution as a Function of Temperature, Glass Composition and Solution. PhD thesis, Northwestern University, (1987).
Wang, Y., Jove-Colon, C. F., Lenting, C., Icenhower, J. & Kuhlman, K. L. Morphological instability of aqueous dissolution of silicate glasses and minerals. NPJ Mater. Degrad. 2, 27 (2018).
Vienna, J. D., Neeway, J. J., Ryan, J. V. & Kerisit, S. N. Impacts of glass composition, pH, and temperature on glass forward dissolution rate. NPJ Mater. Degrad. 2, 1–12 (2018).
Ullman, W. J., Kirchman, D. L., Welch, S. A. & Vandevivere, P. Laboratory evidence for microbially mediated silicate mineral dissolution in nature. Chem. Geol. 132, 11–17 (1996).
Krumbein, W. E. Microbial geochemistry. 336 (Blackwell Scientific Publications, 1983).
Ehrlich, H., Demadis, K. D., Pokrovsky, O. S. & Koutsoukos, P. G. Modern views on desilicification: biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 110, 4656–4689 (2010).
Perez, A. et al. Bioalteration of synthetic Fe(III)-, Fe(II)-bearing basaltic glasses and Fe-free glass in the presence of the heterotrophic bacteria strain Pseudomonas aeruginosa: Impact of siderophores. Geochim. Cosmochim. Acta. 188, 147–162 (2016).
Aouad, G., Crovisier, J.-L., Geoffroy, V., Meyer, J.-M. & Stille, P. Microbially-mediated glass dissolution and sorption of metals by Pseudomonas aeruginosa cells and biofilm. J. Hazard. Mater. 136, 889–895 (2006).
Benzerara, K. et al. Alteration of submarine basaltic glass from the Ontong Java Plateau: a STXM and TEM study. Earth Planet. Sci. Lett. Letters 260, 187–200 (2007).
Aouad, G. et al. The role of biofilm on the alteration of glasses: example of basaltic and nuclear glasses. in Am. GPU Fall Mtg. Abs. (2006).
Brill, R. H. & Hood, H. P. A new method for dating ancient glass. Nature 189, 12 (1961).
Newton, R. G. The enigma of the layered crusts on some weathered glasses, a chronological account of the investigations. Archaeometry 13, 1–9 (1971).
Banerjee, N. R. & Muehlenbachs, K. Tuff life: bioalteration in volcaniclastic rocks from the Ontong Java Plateau. Geochem. Geophys. Geosyst. 4, 1–22 (2003).
Schabereiter-Gurtner, C., Pinar, G., Lubitz, W. & Rölleke, S. Analysis of fungal communities on historical church window glass by denaturing gradient gel electrophoresis and phylogenetic 18S rDNA sequence analysis. J. Microbiol. Methods 47, 345–354 (2001).
Carmona, N. et al. Biodeterioration of historic stained glasses from the Cartuja de Miraflores (Spain). Int. Biodeterior. Biodegradation 58, 155–161 (2006).
Piñar, G. et al. Microscopic, chemical, and molecular-biological investigation of the decayed medieval stained window glasses of two Catalonian churches. Int. Biodeterior. Biodegradation 84, 388–400 (2013).
Piñar, G., Ripka, K., Weber, J. & Sterflinger, K. The micro-biota of a sub-surface monument the medieval chapel of St. Virgil (Vienna, Austria). Int. Biodeterior. Biodegradation 63, 851–859 (2009).
Rodrigues, A. et al. Fungal biodeterioration of stained-glass windows. Int. Biodeterior. Biodegradation 90, 152–160 (2014).
Weaver, J. L., Pearce, C. I., Sjoblom, R. & McCloy, J. S. Pre-viking Swedish hillfort glass: a prospective long-term alteration analogue for vitrified nuclear waste. Int. J. Appl. Glass Sci. 9, 540–554 (2018).
Matthews, B., Arey, B., Pearce, C., Kruger, A. & Tri-beam, F. I. B. Sample preparation to study alterations in ancient glass from Broborg, a Vitrified Swedish Hillfort. Microsc. Microanal. 25, 872–873 (2019).
Weaver, J. L. et al. Microscopic identification of micro-organisms on pre-viking Swedish Hillfort Glass. Microsc. Microanal. 24, 2136–2137 (2018).
Plymale, A. et al. Niche partitioning of microbial communities at an ancient vitrified hillfort: implications for vitrified radioactive waste disposal. Geomicrobiol. J 38, 1–21 (2020).
Weaver, J. L., McCloy, J. S., Ryan, J. V. & Kruger, A. A. Ensuring longevity: ancient glasses help predict durability of vitrified nuclear waste. Am. Ceram. Soc. Bull. 95, 18–23 (2016).
Verney-Carron, A., Gin, S. & Libourel, G. Archaeological analogs and the future of nuclear waste glass. J. Nucl. Mater. 406, 365–370 (2010).
Warscheid, T. & Braams, J. Biodeterioration of stone: a review. Int. Biodeterior. Biodegradation 46, 343–368 (2000).
Gin, S. Open scientific questions about nuclear glass corrosion. Proc. Mater. Sci. 7, 163–171 (2014).
Ménez, B., Pasini, V. & Brunelli, D. Life in the hydrated suboceanic mantle. Nat. Geosci. 5, 133 (2012).
Gibson, B., Wilson, D. J., Feil, E. & Eyre-Walker, A. The distribution of bacterial doubling times in the wild. Proc. Royal Soc. B. 285, 20180789 (2018).
Rousk, J. & Bååth, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 78, 17–30 (2011).
Brehm, U., Gorbushina, A. & Mottershead, D. in Geobiology: Objectives, Concepts, Perspectives, 117–129 (Elsevier, 2005).
Jackson, T. A. & Keller, W. D. A comparative study of the role of lichens and” inorganic” processes in the chemical weathering of recent Hawaiian lava flows. Am. J. Sci. 269, 446–466 (1970).
Jorba, P. Y., Mazerolles, L., Michel, D., Rommeluere, M. & Bahezre, J. in 1er Colloque du Programme Franco-Allemand de Recherche sur la Conservation des Monuments Historiques., 213–219 (Programme franco-allemand de recherche pour la conservation des monuments, 1993).
Bachelet, M. et al. Biodegradation of the french reference nuclear glass SON 68 by Acidithiobacillus thiooxidans: protective effect of the biofilm, U and REE retention (American Geophysical Union, 2008).
Flemming, H. C., Neu, T. R. & Wozniak, D. J. J. Bacteriol 189, 7945–7947 (2007).
Flemming, H.-C., Neu, T. R. & Wingender, J. The Perfect Slime: Microbial Extracellular Polymeric Substances (EPS) 140, 162 (IWA Publishing, 2016).
Donlan, R. M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8, 881 (2002).
Flemming, H.-C., Wingender, J., Griebe, T. & Mayer, C. in Biofilms: Recent Advances in Their Study and Control (ed Evans, L. V.) 19–34 (Harwood Academic Publishers, 2000).
Lewandowski, Z. in Biofilms: Recent Advances in Their Study and Control (ed Evans, L. V.) 1–17 (Harwood Academic Publishers, 2000).
Stoodley, P., Boyle, J. D., Dodds, I. & Lappin-Scott, H. M. Consensus Model of Biofilm Structure. Report No. 0952043254, 8 (University of Exeter, 1997).
Tolker-Nielsen, T. et al. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182, 6482–6489 (2000).
Stewart, P. S. Diffusion in biofilms. J. Bacteriol. 185, 1485–1491 (2003).
Stewart, P. S. Mini-review: convection around biofilms. Biofouling 28, 187–198 (2012).
Harimawan, A. & Ting, Y.-P. Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion. Colloids Surf. B Biointerfaces 146, 459–467 (2016).
Cuadros, J. et al. Microbial and inorganic control on the composition of clay from volcanic glass alteration experiments. Am. Min. 98, 319–334 (2013).
Aulinas, M. et al. Weathering patinas on the medieval (S. XIV) stained glass windows of the Pedralbes Monastery (Barcelona, Spain). Environ. Sci. Pollut. Res. 16, 443 (2009).
Jantzen, C. M., Trivelpiece, C. L., Crawford, C. L., Pareizs, J. M. & Pickett, J. B. Accelerated Leach Testing of Glass (ALTGLASS): II. Mineralization of hydrogels by leachate strong bases. Int. J. Appl. Glass Sci. 8, 84–96 (2017).
Lowenstam, H. A. Minerals formed by organisms. Science 211, 1126–1131 (1981).
Mann, S. in Inorganic Elements in Biochemistry, 125–174 (Springer, 1983).
Frankel, R. B. & Bazylinski, D. A. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 54, 95–114 (2003).
Weiner, S. & Dove, P. M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1–29 (2003).
Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Vol. 5, 26 (Oxford University Press on Demand, 2001).
Beveridge, T. J. Ability of bacteria to promote the formation of fine-grained minerals on their surfaces. In Instruments, Methods, and Missions for the Investigation of Extraterrestrial Microorganisms 3111, 378–387 (1997). International Society for Optics and Photonics.
Gorbushina, A. et al. Role of black fungi in color change and biodeterioration of antique marbles. Geomicrobiol. J. 11, 205–221 (1993).
van Loosdrecht, M. C., Lyklema, J., Norde, W. & Zehnder, A. J. Bacterial adhesion: a physicochemical approach. Microb. Ecol. 17, 1–15 (1989).
Garrett, T. R., Bhakoo, M. & Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 18, 1049–1056 (2008).
Bos, R., Van der Mei, H. C. & Busscher, H. J. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol. Rev. 23, 179–230 (1999).
Li, B. & Logan, B. E. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf. B Biointerfaces 36, 81–90 (2004).
Angeli, F., Gaillard, M., Jollivet, P. & Charpentier, T. Influence of glass composition and alteration solution on leached silicate glass structure: a solid-state NMR investigation. Geochim. Cosmochim. Acta. 70, 2577–2590 (2006).
Yu, Y., Krishnan, N. A., Smedskjaer, M. M., Sant, G. & Bauchy, M. The hydrophilic-to-hydrophobic transition in glassy silica is driven by the atomic topology of its surface. J. Chem. Phys. 148, 074503 (2018).
Hirama, Y., Takahashi, T., Hino, M. & Sato, T. Studies of water adsorbed in porous vycor glass. J. Colloid Interface Sci. 184, 349–359 (1996).
Shellenberger, K. & Logan, B. E. Effect of Molecular scale roughness of glass beads on colloidal and bacterial deposition. Environ. Sci. Technol. 36, 184–189 (2002).
Absolom, D. R. et al. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 46, 90–97 (1983).
Van Oss, C., Good, R. & Chaudhury, M. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 111, 378–390 (1986).
Sharma, P. K. & Hanumantha Rao, K. Analysis of different approaches for evaluation of surface energy of microbial cells by contact angle goniometry. Adv. Colloid Interface Sci. 98, 341–463 (2002).
Vadillo-Rodríguez, V. & Logan, B. E. Localized attraction correlates with bacterial adhesion to glass and metal oxide substrata. Environ. Sci. Technol. 40, 2983–2988 (2006).
Pignatelli, I., Kumar, A., Bauchy, M. & Sant, G. Topological control on silicates’ dissolution kinetics. Langmuir 32, 4434–4439 (2016).
O’Toole, G., Kaplan, H. B. & Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54, 49–79 (2000).
Gloag, E. S., Fabbri, S., Wozniak, D. J. & Stoodley, P. Biofilm mechanics: implications in infection and survival. Biofilm 2, 100017 (2020).
Tuson, H. H. & Weibel, D. B. Bacteria–surface interactions. Soft Matter 9, 4368–4380 (2013).
Yan, J., Nadell, C. D., Stone, H. A., Wingreen, N. S. & Bassler, B. L. Extracellular-matrix-mediated osmotic pressure drives Vibrio cholerae biofilm expansion and cheater exclusion. Nat. Commun. 8, 327–327 (2017).
Peterson, B. W. et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 39, 234–245 (2015).
Ehrlich, H. L. Geomicrobiology: its significance for geology. Earth Sci. Rev. 45, 45–60 (1998).
Munro, W. A., Thomas, C. L. P., Simpson, I., Shaw, J. & Dodgson, J. Deterioration of pH electrode response due to biofilm formation on the glass membrane. Sens. Actuators B Chem. 37, 187–194 (1996).
Stewart, P. S. & Franklin, M. J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6, 199–210 (2008).
Liermann, L. J., Barnes, A. S., Kalinowski, B. E., Zhou, X. & Brantley, S. L. Microenvironments of pH in biofilms grown on dissolving silicate surfaces. Chem. Geol. 171, 1–16 (2000).
Johnstone, T. C. & Nolan, E. M. Beyond iron: non-classical biological functions of bacterial siderophores. Dalton Trans. 44, 6320–6339 (2015).
Perez y Jorba, M., Dallas, J., Collongues, R., Bahezre, C. & Martin, J. Étude del’alteration des vitraux anciens par microscopie electronique a balayage et microsonde. Silic. Ind. 43, 89–99 (1978).
Garcia-Valles, M., Gimeno-Torrente, D., Martínez-Manent, S. & Fernández-Turiel, J. Medieval stained glass in a Mediterranean climate: Typology, weathering and glass decay, and associated biomineralization processes and products. Am. Min. 88, 1996–2006 (2003).
Drewello, U. et al. Biogenic surface layers on historical window glass and the effect of excimer laser cleaning. J. Cult. Herit. 1, S161–S171 (2000).
Bellendorf, P. et al. Archaeological Glass: the Surface and Beyond, 137–144 (Corning, 2010).
Helmstetter, C., Cooper, S., Pierucci, O. & Revelas, E. On the bacterial life sequence. Cold Spring Harb. Symp. Quant. Biol. 33, 809–822 (1968).
Stamets, P. Mycelium Running: How Mushrooms Can Help Save The World, 19 (Ten Speed Press, 2005).
Gaylarde, C., Ogawa, A., Beech, I., Kowalski, M. & Baptista-Neto, J. A. Analysis of dark crusts on the church of Nossa Senhora do Carmo in Rio de Janeiro, Brazil, using chemical, microscope and metabarcoding microbial identification techniques. Int. Biodeterior. Biodegradation 117, 60–67 (2017).
Bacon, C. R., Grove, M., Vazquez, J. A. & Coble, M. A. The Stanford-US Geological Survey SHRIMP Ion Microprobe: a Tool for Micro-scale Chemical and Isotopic Analysis (US Department of the Interior, US Geological Survey, 2012).
Kamjunke, N., Mages, M., Büttner, O., Marcus, H. & Weitere, M. Relationship between the elemental composition of stream biofilms and water chemistry—a catchment approach. Environ. Monit. Assess. 187, 432 (2015).
Eick, M. J., Grossl, P. R., Golden, D. C., Sparks, D. L. & Ming, D. W. Dissolution kinetics of a lunar glass simulant at 25 °C: The effect of pH and organic acids. Geochim. Cosmochim. Acta 60, 157–170 (1996).
Robinet, L., Eremin, K., Coupry, C., Hall, C. & Lacome, N. Effect of organic acid vapors on the alteration of soda silicate glass. J. Non-Cryst. Solids 353, 1546–1559 (2007).
Jantzen, C. M., Trivelpiece, C. L., Crawford, C. L., Pareizs, J. M. & Pickett, J. B. Accelerated Leach Testing of GLASS (ALTGLASS): I. Informatics approach to high level waste glass gel formation and aging. Int. J. Appl. Glass Sci. 8, 69–83 (2017).
Ehrlich, H. Microbes and metals. Appl. Microbiol. Biotechnol. 48, 687–692 (1997).
Augustsson, A. et al. High metal reactivity and environmental risks at a site contaminated by glass waste. Chemosphere 154, 434–443 (2016).
Kunal, A. K. & Mamtani. Antibacterial glass market size, by application (hospitals, food & beverage, military equipment, household/residential), by active ingredient (silver), industry analysis report, regional outlook, price trends, growth potential, competitive market share & forecast, 2016–2023, 70 (Global Market Insights, online, 2016). https://www.gminsights.com/industry-analysis/antibacterial-glass-market-report.
Glass, D. J., Raphael, T., Valo, R. & Eyk, J. v. International Activities in Bioremediation: Growing Markets and Opportunities (Battelle Press, 1995).
Orlando, A., Olmi, F., Vaggelli, G. & Bacci, M. Mediaeval stained glasses of Pisa Cathedral (Italy): their composition and alteration products. Analyst 121, 553–558 (1996).
Lombardo, T. et al. Characterisation of complex alteration layers in medieval glasses. Corros. Sci. 72, 10–19 (2013).
Gentaz, L. et al. Role of secondary phases in the scaling of stained glass windows exposed to rain. Corros. Sci. 109, 206–216 (2016).
Dussubieux, L., Robertshaw, P. & Glascock, M. D. LA-ICP-MS analysis of African glass beads: laboratory inter-comparison with an emphasis on the impact of corrosion on data interpretation. Int. J. Mass Spectrom. 284, 152–161 (2009).
Sterpenich, J. & Libourel, G. Using stained glass windows to understand the durability of toxic waste matrices. Chem. Geol. 174, 181–193 (2001).
Wainwright, M. An Introduction to Environmental Biotechnology, 27 (Springer Science & Business Media, 2012).
Palomar, T. Chemical composition and alteration processes of glasses from the Cathedral of León (Spain). Bol. Soc. Esp. Cerám. V. 57, 101–111 (2017).
Messiga, B. & Riccardi, M. Alteration behaviour of glass panes from the medieval Pavia Charterhouse (Italy). J. Cult. Herit. 7, 334–338 (2006).
Mann, C. et al. Interactions between simulant vitrified nuclear wastes and high ph solutions: a natural analogue approach. MRS Adv. 2, 669–674 (2017).
Jorba, M. P., Dallas, J., Bauer, C., Bahezre, C. & Martin, J. Deterioration of stained glass by atmospheric corrosion and micro-organisms. J. Mater. Sci. 15, 1640–1647 (1980).
Krumbein, W. E., Gorbushina, A. & Palinska, K. in Conservation Commune d’un Patrimoine Commun: 2ème Colloque du Programme Franco-allemand de Recherche Pour la Conservation des Monuments Historiques, 39–45 (Programme franco-allemand de recherche pour la conservation des monuments historiques, 1997).
Michelin, A. et al. Silicate glass alteration enhanced by iron: origin and long-term implications. Environ. Sci. Technol. 47, 750–756 (2013).
Verney-Carron, A., Gin, S. & Libourel, G. A fractured roman glass block altered for 1800 years in seawater: analogy with nuclear waste glass in a deep geological repository. Geochim. Cosmochim. Acta 72, 5372–5385 (2008).
Strachan, D. M., Crum, J. V., Ryan, J. V. & Silvestri, A. Characterization and modeling of the cemented sediment surrounding the Iulia Felix glass. Appl. Geochem. 41, 107–114 (2014).
Acknowledgements
The authors would like to thank Dr. Claire Corkhill (University of Sheffield, UK) for providing the inspiration for this review. Additional thanks are to be made to Dr. Eric Pierce, Dr. Lawrence Anovitz, Dr. Marie Jackson, Dr. David Peeler, Dr. James Neeway, and Dr. Joe Ryan for their thought provoking and insightful conversations on this topic. We would also like to thank Dr. John McCloy for his encouragement during the writing of this review. Finally, we would like to acknowledge Dr. Albert Kruger’s support of the Broborg Hillfort project, which partially funded the research presented in this manuscript. Partial funding for this research (J.L.W.) was provided by the United States National Research Council. Trade names and commercial products are identified in this paper to specify the experimental procedures in adequate detail. This identification does not imply recommendation or endorsement by the authors or by the National Institute of Standards and Technology, nor does it imply that the products identified are necessarily the best available for the purpose. Contributions of the National Institute of Standards and Technology are not subject to copyright. C.I.P., A.E.P., and B.W.A. acknowledge funding from United States Department of Energy Office (US DOE) of Environmental Management, International Programs, and by the US DOE Waste Treatment and Immobilization Plant Project. A portion of this research was performed at the Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory under proposal number 49141. PNNL is a multi-programmed national laboratory operated for DOE by Battelle Memorial Institute operating under Contract No. DE-AC06-76RLO-1830.
Author information
Authors and Affiliations
Contributions
J.L.W., P.T.D., A.E.P., R.J.K., and C.I.P.—literature review and manuscript preparation. B.A.—image acquisition (presented in figures).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Second listed affiliation via a non-funded, appointed research position.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weaver, J.L., DePriest, P.T., Plymale, A.E. et al. Microbial interactions with silicate glasses. npj Mater Degrad 5, 11 (2021). https://doi.org/10.1038/s41529-021-00153-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-021-00153-w
This article is cited by
-
The corrosion mechanism of lead-glazed pottery in Han dynasty
npj Materials Degradation (2024)
-
Biodegradation of materials: building bridges between scientific disciplines
npj Materials Degradation (2023)
-
Genome-wide comparison deciphers lifestyle adaptation and glass biodeterioration property of Curvularia eragrostidis C52
Scientific Reports (2022)
-
Fungal hyphae develop where titanomagnetite inclusions reach the surface of basalt grains
Scientific Reports (2022)
-
The contribution of living organisms to rock weathering in the critical zone
npj Materials Degradation (2022)