Abstract
While glass alteration in liquid water has been widely studied for decades, glass alteration in unsaturated atmosphere (relative humidity, RH < 100%) has been far less examined. However, the understanding of the mechanisms involved in the reactions between glass and water in vapor state is fundamental to several fields such as glass industry, conservation of glasses of the cultural heritage and long-term assessment of nuclear waste glasses. This paper outlines the issues raised by the atmospheric alteration of glass in these fields and attempts to summarize the scientific approaches and findings of the three communities. This short review reveals that atmospheric alteration should not be confused with liquid alteration at high S/V (S = exposed surface of glass and V = volume of solution), because the kinetics and the nature of the alteration products are distinct. Notably, alkalies and non-bridging oxygens may be significantly retained in glass hydrated in unsaturated atmosphere, depending on the glass composition. Future lines of research are drawn to progress in the understanding of the specificities of atmospheric glass alteration.
Similar content being viewed by others
Introduction
Most silicate-based glasses are rich in alkali and alkaline-earth oxides, used as fluxes to lower the melting temperature and viscosity of the glass-forming mixture. As a consequence, these glasses may undergo substantial alteration phenomena involving chemical reaction with water notably in the vapor state, which can impinge on their long-term stability, a matter of concern in the fields of cultural heritage1 (denoted CH in this paper) and of nuclear disposal2.
This paper only focuses on the alteration of glass induced by water in vapor state in unsaturated vapor conditions, i.e., when the relative humidity (RH) is strictly below 100%. To avoid repetitions and lighten the text, the terms “unsaturated atmosphere”, “humid atmosphere”, “unsaturated humidity”, and “atmospheric conditions” are also used. They all refer to the unsaturated vapor conditions. In parallel, the terms “liquid water”, “aqueous conditions”, and “immersion” all refer to the alteration of glass in contact with liquid water or with vapor at 100 RH% (because of the equilibrium with liquid water in this condition). We avoided using the term “weathering” that is defined as glass alteration due to intermittent water, humid air, and/or water contact (see the glossary in ref. 3), encompassing alteration steps in liquid conditions or at 100 RH%.
The problem of chemical reactivity between atmospheric humidity and glass has been addressed for a long time, starting with the pioneering works of Organ4 and of Brill5 for CH glasses preserved in atmospheric conditions, and with the vapor hydration studies of US nuclear waste glass surrogates in the 1980s6. In the glass industry, studies of the chemical attack by atmospheric agents were conducted as early as the 1950s7. However, experimental studies specifically dealing with the alteration in unsaturated vapor conditions are still few in comparison with the studies of glass alteration in aqueous solution, especially in the industrial and nuclear waste management fields. This is because the unsaturated vapor conditions were not considered as distinct from the saturated vapor and liquid conditions in the literature until recently. The only mentioned difference between the two conditions is the very limited volume of water available for reaction, so that unsaturated vapor conditions tend to be confused with the liquid conditions at extremely high S/V8. In the kinetic models of glass dissolution based on affinity laws and modified with the effect of the alteration layer on chemical diffusion, it is expected that a high S/V ratio will induce a rapid saturation of the liquid and hence diminish the dissolution rate at short observation time3,9. Therefore, the forward rate (defined as the initial Si dissolution rate in very dilute conditions) is expected to be the fastest rate at which the glass would react. The dilute liquid conditions have thus been chosen as conservative bounding conditions for studying glass alteration8.
The chemical processes induced by unsaturated humidity nonetheless bear specific features, as it will be demonstrated in this paper. Understanding their deterioration mechanisms is essential to proposing efficient and long term solutions for the conservation of altered CH glasses, and to improving the long-term behavioral simulation programs of nuclear waste storage glasses, to which the altered CH glasses may provide useful naturally aged analogs, as it has been done for glass alteration in liquid water10,11. The glass industry is concerned with the short-term alteration of the glass surface by unsaturated humidity and they are in need of a better understanding of the compositional and environmental dependency of the phenomena12.
The communication of the needs of each group as well as their complementary expertize are essential for progress and to provide answers to the different challenges in the best way in the years to come. In this paper, we propose to put in perspective the different issues, scientific approaches, and findings regarding glass alteration in atmospheric conditions in the three fields. In the second part of the paper, the specific features of the atmospheric alteration of glass, as compared to alteration in liquid phase, are discussed on the basis of the current knowledge. Research prospects are suggested in the last part. This perspective paper was inspired by discussions held at the International Symposium on Glass Degradation in Atmospheric Conditions, which took place in Paris in November, 2017, and gathered specialists of the three communities13.
Signs and issues of glass alteration in humid atmosphere
Glass art objects
Glass art objects are objects entirely made out of glass, or partially (as enamels), which have been produced for utilitarian or artistic purposes and have been preserved in private or museum collections in recognition of their historical and/or artistic value. Since the time of their production, they have existed in atmospheric conditions. For this reason, glass art objects and archeological glasses form two distinct groups, because most of the latter have experienced immersion or buried environments.
Existing published surveys of glass object collections in European museums indicate that about 15–30% of glass art objects are altered at different alteration stages today14,15. These figures, which are based on visual observations, could be largely underestimated as signs of deterioration are not always visible. The low chemical durability of many of these altered objects, primarily dating from the middle-age period to the 19th century, is attributed to the over purification of raw materials and introduction of new recipes leading to glass compositions containing more fluxes (Na2O, K2O, and PbO) and less stabilizer oxides (mainly CaO)1,16. The glass compositions showing detectable signs of atmospheric glass degradation are classified as unstable and the other as stable in the CH field. Beyond general trends such as the content in alkalies and stabilizer oxides, the chemical stability in atmosphere could not be correlated to any precise parameter of the chemical composition but is rather thought of as a property of the overall glass composition1.
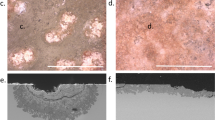
The determining roles of glass composition and humidity on the extent and kinetics of the atmospheric degradation have been verified in laboratory experiments on synthetic glasses representative of historic glass compositions by aging them in an accelerated way or in ambient atmosphere17,18,19,20. The main macroscopic manifestations are always the same, independently of the composition, and very specific1,21,22. Koob21 has identified five stages in an order of increasing alteration, which have built a consensus in the CH community1. Stage 1 is defined by the presence of a white haze, droplets, and/or crystals due to the recurrent formation of salts on the glass surface, which can be removed to recover the initial appearance of the glass. Stages 2 and 3 are related to “crizzling”, an irreversible and specific character of atmospheric glass degradation consisting in microcracks of the glass surface, mainly attributed to the partial drying of the hydrated layer1,5. Stage 3, for which the cracks, initiated during the stage 2, are uniformly visible across the surface is qualified as “excipient crizzling”. At an advanced stage, crizzling can result in surface delamination (stage 4), causing the loss of CH material and the exposure of new glass surface that allows the extent of the alteration possibly until complete disintegration (stage 5). An example of crizzled glass artefacts of the CH is depicted in Fig. 1 with the glass composition given in Table 1. They belong to the stage 4, where loss of matter starts by the fall of surface flakes.
Set of two degraded glass objects of the Musée des Arts Décoratifs, Paris, showing the advanced stage of crizzling (stage 4). a, b Degraded glass ewer, inv. 23431 (H. 19 cm). c, d Fragment of a glass support of oil and vinegar flasks, inv. 22,747 (H. ~5 cm). ©C2RMF, A. Maigret. b and c optical microscopy images showing the formation of small flakes of hydrated glass and their detachment from the surface. e–h SEM images (back-scattered electrons) of the edge of a resin-coated and polished sample of the glass fragment. The alkalies (K, Na) are depleted in the hydrated layer, explaining the contrast on SEM images, but their depletion is partial (Table 1). ©C2RMF, F. Alloteau. The pink color is due to the atmospheric alteration through the oxidation of manganese in the hydrated layer (solarization phenomenon). The micrometric holes may have been induced by the polishing step, they reveal the mechanical fragility of the hydrated layer and probable submicrometric porosity. Scale bars are b and c 1000 µm, e and g 10 µm, f and h 1 µm. Panels c–e are reproduced with permission from ref. 13, Editions Hermann, 2019.
The cross-sections of crizzled glasses show homogenous hydrated layers with different density from the bulk glass at the SEM scale19,20, and of which thicknesses range between 1 and several 10 µm. Microcracks penetrate in the hydrated layer, intersect, and many of them pass into the pristine glass, often redirecting parallel to the surface. Microcracks have a very detrimental effect on glass durability, especially in cyclic climates. They may allow capillary condensation of water at the interface with the pristine glass during humid periods, extending glass hydration. Furthermore, they are the seat of salts crystallization during dry periods, which may induce stress and cause in-depth propagation of the cracks23,24. Note that stable glass compositions have also been observed to be subject to this atmospheric deterioration, most often as a result of poor storage: prolonged exposure to high humidity, fluctuating environment and physical creation of a “microclimate”, where moisture is trapped25,26. It is important to emphasize that these manifestations rarely occur on archeological glasses (from buried environment)27,28, or glasses retrieved from underwater.
The monitoring of humidity at a low level in the display cases or storage spaces is fundamental to slow down the evolution of the degradation phenomenon. Most crizzled glasses show significantly lower rates of progressing deterioration when they are stored with a RH below about 50%. On the other hand, too dry of an atmosphere must be avoided to prevent the glass surface from overdrying and subsequent cracking4,5,25. According to Koob21, the RH should be maintained in the 40–45% range for already deteriorating glasses and 45–50% range for all glass objects in general, and it should never drop below 30%. In addition, associating climate control with circulation of air inside exhibition or storage boxes with small fans is crucial to minimize trapped moisture26. Such display cases exist in some museums, for instance the Corning Museum of Glass (USA), the Grünes Gewölbe Museum (Germany) and the Louvre Museum (France)29.
Pollutants around glass collections are acid gases (CO2 and SO2) and volatile organic compounds (acetic acid, formic acid, and aldehydes) that are found in wood, glue, some plastic, silicone joints, and paints30. The effect of these pollutants on the evolution of the degradation has been demonstrated31 but the control of their concentration in the museum atmosphere remains rare (Grünes Gewölbe Museum).
In addition to the control of the environment, periodic washing of unstable glass with water and soap, or with water–alcohol solution, or with dilute acid solution, has been recommended to remove the salts and neutralize the surface, further contributing to slowing down the alteration if the object is kept under good storage condition following washing1,21,32.
Apart from solutions oriented on consolidation (such as Paraloid B-72 acrylics)33, the few invasive conservation treatments aimed at slowing down atmospheric alteration almost exclusively consist of coating the glass surface with either inorganic34,35 or hybrid organic–inorganic products. The inorganic part allows the adhesion of the coating to the glass and the organic part adds hydrophobicity36,37. Recent evaluation of organo(alkoxy)silane polymers called Orcomers® used to consolidate and protect crizzled enamels indicates promising results in the short term38. Still now, curators and conservators do not have enough perspective to decide on their real long-term efficacy.
Other invasive conservation approaches implying a minimal amount of deposited chemicals have been attempted, with the purpose of stabilizing the hydrated layer with respect to the silicate network hydrolysis (such as in refs. 39,40). In this latter approach, the industrial methods consisting of spraying zinc salt solutions on the surface of flat glass41 have been tested on synthetic unstable glasses subjected to accelerated ageing in climatic chambers. Very good results have been obtained on pristine glasses, but research must continue on pre-altered glasses42.
For preventive conservation in museums, it is important to know the composition of glass objects and their state of degradation. Analyzing all the glass in museums is not possible and visually identifying chemically unstable glass objects is difficult before changes in appearance occur. Thus, detecting the glass alteration at the very beginning of its manifestation appears to be an important issue43. In this respect, quantifiable and portable detection methods have been recently developed in the CH community:
-
The thickness of the hydrated layer can be determined on art objects using optical coherence tomography (OCT) to a few micrometers44,45. Such thicknesses typically correspond to the beginning of the degradation of the glass for which cracks are not yet present or not visible to the naked eye. Similarly, interference patterns observed on IR reflectance spectra have been used as an indicator of glass degradation46.
-
The ions present on the surface of the unstable glass objects can be quantitatively studied using a dedicated ion chromatography analytical protocol. A distinction could be made between stable and unstable glass objects in museum collections based on the sodium, potassium, acetate, and formate concentrations47.
Stained glass
Stained glass windows raise serious preservation issues due to their fragility and direct exposure to adverse weather and outdoor air pollution48. The manifestations of the alteration are close to those of the glass art objects on the internal faces of the buildings and more complex on the external faces due to the wider heterogeneity and amplitude of environmental conditions, including the alternation of atmospheric hydration, liquid water run-off (rain)48,49, sometimes freezing, and exposure to sun (drying). Extensive field studies have been carried out to investigate the rate and products of alteration of medieval stained glass (mainly potassium lime silicate) in sheltered or unsheltered outdoor conditions23,50,51. In sheltered conditions that are in the scope of this paper, potassium and calcium salts (mainly K2CO3, calcite, gypsum, and syngenite) are the most obvious and ubiquitous signs of the alteration, together with the increase of surface roughness23. These salts may form an alteration crust covering the leached layer, which may crack51. Iridescence can also be observed and will be discussed in the next section of this paper23. By EDX profiling of polished cross-sections using careful calibration and statistical analysis52, the depth of the K-depleted layer has been measured as a function of exposure time to the sheltered outdoor conditions50,51. The growth rate of this K-depleted layer could not be clearly related to any variation of T, RH% or pollutant concentration except for SO2. In a few cases, the curious decrease of the depth of this layer has been attributed to the possible occurrence of network dissolution (the silicate products being removed by the polishing of the sample)51. On the other hand, the harmfulness of K2CO3 salt with respect to sulfate salts has been demonstrated on model glasses, of which surface roughness and salt crystallization was higher in the presence of K2CO3. Contrary to sulfates, this salt is basic and deliquescent and thus promotes the network hydrolysis and the leaching of alkalies23,48.
In order to restore and protect this Cultural Heritage from mechanical, chemical deteriorations and vandalism, restoration has been engaged (cleaning, bonding, consolidating, filling gaps, etc.). Consolidating products are used occasionally for glass and paints (Orcomer, Paraloïd B72) and new ones will appear soon (European research projects CONSTGLASS and NANOMATCH)53. These products have shown mixed results, and they tend to be replaced by a more efficient and long-term preservation method through protective glazing54,55.
Commercial glasses
The composition of modern commercial glasses has been optimized in order to meet the requirements of the market, including the hydrolytic resistance in immerged as well as in atmospheric environment, so that only few problems are encountered in usual situations. Like stage 1 deterioration of glass art objects, the common first manifestation of the atmospheric alteration of a commercial soda–lime glass is the appearance of a slight veil on the surface, composed of sodium carbonate and calcium carbonate crystals56,57,58, which can develop with time in a polluted atmosphere containing SO2 into sodium sulfate and calcium sulfate. These crystals are soluble in water (or in acid water) and they are easily removed. This first manifestation is considered as reversible from an optical and mechanical point of view: after cleaning, the glass is very similar to the original glass. However, in case of more advanced alteration, some pits below the crystals (Fig. 2) can develop56: they can be responsible for a decrease of the mechanical properties of the glass. If the glass surface composition is more uniformly modified on a thickness of about 0.1 µm or more, then the alteration may appear as an interference pattern (iridescence). This phenomenon of iridescence is common in glass alteration, but is attributed to several origins: the most spectacular concerns many archeological glasses after burial and is characterized by beautiful rainbow colors due to the presence of many layers of corrosion of constant thickness (less than 1 µm)59. This type of alteration (burial corrosion, sometimes also called “weathering”) is out of the scope of this article. Another type of iridescence concerns atmospheric alteration23. The physical origin of the phenomenon is similar: interferences between light reflected by the surface of the object and light reflected by the interface between the corroded layer and the non-corroded glass. The intensity of the reflected light is low at the beginning of the alteration process, when only one layer is formed, and it is often difficult to observe in nonoptimal light, especially when corrosion salts are present on the surface. A full-blown, well visible, iridescence is the sign of a more advanced atmospheric alteration and it is not clear if it occurs in unsaturated conditions only (RH < 100%) or in conditions with discontinuous liquid water supply. In these latter conditions (as for unsheltered stained glass), a complete restructuration of the alteration layer is observed, with the formation of microscopic laminations that may underlie iridescence49. In any case, iridescence is irreversible and makes the glass no longer marketable.
The hexagonal embedded crystals are calcite. Scale bars are a 2 µm, b 1 µm. Reprinted and adapted from ref. 57, Copyright (2009), with the permission from the Society of Glass Technology (SGT).
Note that in a few special situations, some specific issues may arise:
-
During storage or transport of glass where confinement and non-ventilated atmospheres increase the likelihood of corrosion,
-
With photovoltaic panels: alteration and soiling of the glass protection of the cell significantly decrease the efficiency of the device60,
-
With new glass enamels, the composition of which has to be adapted to recent legislation (without lead oxide flux) while maintaining good chemical stability with respect to atmospheric attack61,
-
When some very sensitive thin layers are to be deposited on glass, they may make visible the beginning of the alteration of the glass surface. For instance, low emissivity stacks based on Ag layers may face adhesion problems if the corrosion of the glass surface is not carefully controlled and limited, especially during storage and transport.
-
In some double-glazing windows fitted with a seal which is not totally vapor tight, water vapor can penetrate between the two glass sheets, and condensate inside on the inner glass surfaces: a rapid (years) alteration begins. This defect is well-known, but it is not strictly related to atmospheric conditions because condensation occurs. Due to the high glass surface/water volume ratio the pH increases quickly. To our knowledge, there is no scientific publication concerning this defect.
The atmospheric glass alteration depends strongly on the glass surface composition, which may be different from the bulk composition, due to process parameters62. As an example, the tin side of the float glass, which is tin enriched63, has a much better chemical durability than the non-tinside (called atmosphere side)64. Similarly, the presence of SO2 in the annealing lehr causes surface dealkalization65,66, which improves the durability of the glass. This last point is industrially exploited in dealkalization treatments especially in the case of vials. In addition, the surface composition (and structure) may be modified by specific protective treatments such as those used in dishwashers (with zinc and bismuth, or with polyethyleneimine), the efficacy of this surface modification being clearly demonstrated67,68. The chemical durability in atmosphere also depends on the environment: it has been demonstrated that the presence of deposited dust on the glass surface, such as wind-blown sand69, usually considered as inert, strongly decreases the atmospheric glass durability.
Nuclear glasses
The immobilization of high-level radioactive wastes in borosilicate glasses is considered by many countries as the most effective and adaptable solution for confinement of these wastes among the available technologies. In this concept, the waste elements, amongst them the radionuclides, are fixed by chemical bonds to the oxygens of the glass, so that they are part of the material’s structure and chemistry. After their disposal in a geological repository, these glassy waste forms may be exposed to unsaturated vapor conditions before water may saturate the repository site70. This unsaturated period may last a very long time (several tens of thousands of years) and significantly contribute to the glass ageing6. Therefore, glass alteration in vapor conditions is being investigated in the field of nuclear glasses, although it is still far less studied than glass alteration in liquid water71. The main concerns regarding the vapor hydration of nuclear glasses are: (i) long term (geological time scales) prediction of glass behavior and (ii) the ability to retain radionuclides in the glass matrix. Vapor hydrated nuclear waste glasses are usually characterized by a hydrated layer on the surface and the formation of secondary phases. Some of these secondary phases incorporate radionuclides and studies have shown that these phases may readily dissolve when they are subsequently exposed to aqueous medium. As a consequence, the aqueous alteration of a nuclear waste glass prehydrated in vapor phase releases more radioactivity in solution than a pristine waste glass6,72.
Most of the vapor hydration studies conducted in the nuclear waste glass domain are dedicated to understanding and describing the mechanisms of deterioration, identifying the secondary phases and studying the effect of composition, temperature and humidity on the vapor hydration kinetics. Unlike the glasses in the other two communities, silicates such as zeolites, calcium-silicate-hydrates (CSHs) and phyllosilicates are the most observed secondary phases in nuclear glasses. This maybe because most of the experiments are conducted at high temperatures (>90 °C) which promote the precipitation of silicates, and/or because of compositional differences of the glass. Carbonates are also observed on vapor hydrated nuclear waste glasses, especially on calcium containing glasses. The morphology of the alteration layer may either be a homogeneous hydrated layer or heterogeneously altered zones adjacent to visibly unaltered zones or sometimes both70,73,74,75.
As outlined in the above sections, the stakes and concerns arising due to glass alteration in atmospheric conditions are different in all three communities. Moreover, it can be noticed that the atmospheric alteration phenomena are differently described in the three fields. Indeed, the observation methods are not the same, so that different scales in space and time are investigated. For instance, the CH community describes a “stage 1” with the formation of salts on the glass surface. A hydrated layer has certainly formed at this stage already, but it cannot be detected by visual observation or optical microscopy that are the common methods used in museums. In the nuclear glass field, altered samples are systematically observed with a SEM (or any instrument with greater spatial resolution), and the presence of a hydrated layer is always reported. Moreover, the accelerated ageing methods using high temperatures and RH preclude a proper comparison between the observations in CH (ambient conditions) and in the nuclear glass field (accelerated ageing), because the influences of T and RH are not well-known and it is currently impossible to rely a given alteration stage, as used in the CH, to any given T, RH and time conditions in accelerated experiments. High T and RH as well as their time evolution (notably the cooling and/or drying ramps) may also modify the manifestations of the alteration. It is probably the case for crizzling, which is described as an important, ubiquitous feature of degraded glass objects in the CH, while it is not as systematic in the other fields (by considering that “cracks”, “fracturing” or “mechanical breakdown” of the alteration layer relate to similar phenomena as crizzling). Crizzling displays a characteristic pattern of cracks intersecting at right angles, as it is expected for the release of tensile stresses in a mechanically fragile layer76,77. The magnitude of these stresses and the mechanical properties of the layer depend on the glass composition and on the details of the RH variation history (cycling and ramp rates), so that the characteristics of the layer breakdown are probably diverse. Notably, the minimum thickness at which it cracks may probably vary. All these elements make it difficult to properly compare the studies and descriptions reported in the different fields. However, all three communities are dedicated to better understand the phenomena and mechanisms by which glass alters in humid conditions. The primary goal is to either preserve glass objects from further alteration (CH glasses) or prevent alteration altogether (glass industry) or to predict the long term behavior of a glass matrix exposed to the vapor phase (nuclear waste glasses). The following section of this article discusses the insights on the specific features and mechanisms of glass alteration in unsaturated vapor phase gained from studies conducted in all three communities.
Mechanisms of glass alteration in humid atmosphere
Various protocols have been used to control RH and temperature in glass alteration experiments: (i) desiccator with or without saline solutions18,19,78, (ii) climatic chamber64,75,79,80,81,82, (iii) hermetic box enclosing a saline solution and placed in an oven83, (iv) teflon-lined autoclave in an aluminum container (to homogenize T)70,84, with samples placed on a teflon holder above a saline solution, and placed in an oven. Humidity conditions can be unsaturated (less than 100% RH) or saturated (100% RH), although we focus on the unsaturated conditions in this paper. Tables 2 and 3 draw up the list of the main studies reported in the scientific literature on atmospheric glass alteration to our knowledge, with the main glass compositions and alteration conditions.
The RH determines the number of layers of water molecules that build on the glass surface. This number reaches 3–4 layers at 60% RH, then it increases exponentially when approaching 100% RH85. Signs of glass hydration, as the presence of a hydrated layer of a few µm, are observed at RH beyond 50% and are absent or negligible below this value19,73. As mentioned above, a value less than 50% is generally recommended for the preventive conservation of CH glasses.
Considering the presence of a thin water film in equilibrium with vapor on the glass surface, the question of the specificity of atmospheric glass alteration can be formulated as “is glass alteration in unsaturated atmosphere like alteration in liquid water at extremely high S/V ratio?” (where S is the total glass surface exposed to the solution and V is the volume of the solution).
In immersion conditions, two chemical processes referred to as “glass leaching” lead to the dealkalization of the hydrated layer and to the increase of the solution pH:
-
The ionic exchange between protons and alkali ions, which implies Na+/H+ interdiffusion86:
-
The acid–base reaction of non-bridging oxygens (NBO) with water, which implies molecular water diffusion and dissociation onto the NBO site87:
In immersion and static conditions (no water renewal), the rate of the pH increase due to glass leaching rises with the S/V ratio. Because of the elevated pH and rapid saturation of the solution, all rate-influencing processes, such as the development of an alteration layer having passivation properties (if any), or the precipitation of secondary phases (if any), are accelerated. For instance, for the SON68 glass (the inactive surrogate of the French R7T7 nuclear glass, of complex soda–lime aluminoborosilicate composition), the residual alteration rate is attained more quickly with an increase of the S/V ratio88. However, despite the diversity of glass compositions and experimental conditions (set-up, T, %RH and duration, characterization techniques…) found in the literature regarding the atmospheric alteration and summarized in Tables 2, 3, experimental results gathered so far indicate that glass is differently attacked in vapor conditions and in immersion conditions, so that the atmospheric alteration phenomenology (rate, chemical nature of the alteration layer and products) cannot be accounted for by an extreme S/V ratio. In the following sections, we try to point out these differences to capture the specificities of glass atmospheric alteration and draw possible lines of further research.
Kinetics of atmospheric alteration and chemical durability
Very few experimental studies have attempted to determine the alteration rates in atmospheric conditions. In all cases, the alteration rate is defined as the growth rate of the hydrated layer, the thickness of which being measured directly on glass monoliths by SEM or TEM of cross-sections, or by TOF-SIMS profiling, or by FTIR in transmission mode using the absorbance of the (Si–)OH stretching band at 3595 cm−1 that was linearly linked to thickness after SEM and SIMS calibration70,73,74,79,82,84,89,90. Alternatively, the fractional weight gain of crushed glass has also been used to measure the extent of hydration73, and one study made use of nuclear reaction in-depth analysis (NRA)91.
The chemical durability refers to the dependency of the alteration rate on the chemical composition of the glass. As mentioned earlier, it is a property of the overall glass composition. The chemical durability in atmospheric conditions may be drawn from the chemical durability in liquid conditions in the extent to which the chemical processes are similar between both conditions. Regarding liquid conditions, general trends are well known. For most glasses of the CH and glass industry (commercial glasses), the main chemical parameters are the content in alkali and alkaline-earth oxides and their relative proportions, and the content in Al2O3. For instance, replacing Na2O by CaO improves the chemical durability, until some amount of around 10 mol% CaO92. K2O-bearing silicate glasses are less durable, this observation being attributed to the lower field strength of K+ compared with Na+ and hence the weaker K–NBO bond, and to the larger size of K+ ions. Both parameters are the main origin of the distinction between stable and unstable glass compositions of CH16,51,93. The addition of oxides such as Al2O3, ZrO2, or ZnO also improves the chemical durability by increasing the relative stability of the glass against dissolution (less soluble dissolution products, depending on the pH)92. B2O3 may also be favorable, especially when substituted for the alkali oxide94,95,96. Other effects than thermodynamics also play a role, such as the glass molecular structure, which may favor the densification of the alteration gel by condensation of the Si–OH groups97,98, and the network topology, which may increase the activation energy of the network hydrolysis99.
Although the studies are scarce, it is possible to compare the alteration rates in atmosphere and in immersion for a few glass compositions. Important results can then be noticed:
(i) The kinetics of glass alteration in liquid and static conditions (static referring to no circulation of liquid) generally separates into three successive regimes71. The initial dissolution regime corresponds to the initial glass dissolution rate (or forward rate) in very dilute conditions with respect to the glass elements. The rate-drop regime corresponds to the formation and densification of the alteration layer, and the residual regime is related to the constant residual glass dissolution rate resulting from the rate equality between diffusion through, and dissolution of, the dense alteration layer56. As mentioned above, high S/V conditions accelerate the advent of the residual regime.
In atmospheric conditions, in general, the hydration rate increases with the %HR until 100%, but it stays lower than the dissolution rate in hydrothermal or liquid conditions in the initial dissolution regime89,91. An exception is however provided by a specific composition of mixed-alkali lime silicate glass identified as an unstable glass composition of the CH. Its atmospheric hydration rate (80 °C, 85% RH, 6 days) was very fast, and even exceeded by a factor 10 the initial dissolution rate measured for the same glass at 80 °C (pure water, S/V = 50 m−1, 6 days, rate measured from the Na normalized mass loss)79. Comparing the atmospheric hydration rate and the dissolution rate in the residual regime (high S/V), is only possible in the case of the SON68 glass composition for which numerous and accurate measurements have been performed. For this glass, the atmospheric hydration rate is higher than the residual rate at the same temperature (residual dissolution regime at very high S/V)100. Indeed, the residual dissolution rate is about 2 × 10−4 g m−2 d−1 (90 °C, pH = 9 buffered with KOH, S/V = 10 m−1, 8000 m−1 or 200 000 m−1, 1-year experiments)88,101, while the vapor hydration rate measured after 512 days (90 °C, 92% RH) is about 3 × 10–3 g m−2 d−184, i.e., higher by one order of magnitude.
(ii) In some cases, glasses that classify as the most durable in aqueous phase become the least durable in vapor phase: such an order reversal occurs in a series of Mg-rich75, and another series of Fe-rich73, sodium aluminoborosilicate glasses.
The existence of such glasses hydrating rapidly in vapor phase (i), or of reverse order in glass durability (ii), demonstrates that the dissolution at high S/V cannot be extrapolated to the hydration of glass in unsaturated vapor conditions. This is probably because in vapor conditions, the attacking water does not correspond to an aqueous saturated solution, and because the nature of the gel/hydrated layer and secondary phases are distinct as will be discussed below.
Considering the time and temperature dependency of the kinetics, studies dealing with aluminoborosilicate glass compositions for nuclear wastes generally find a decrease of the hydration rate with time, and a square root time dependency is established in several cases73,84. Moreover, the temperature dependency of the rate follows an Arrhenius law (Fig. 3)84. Therefore, for the SON68 glass, it is concluded that the vapor hydration rate is controlled by a reactive diffusion process of H2O, the activation energy of which being close to that determined in Si saturated solution, although the diffusion coefficient is higher by two orders of magnitude102.
a Alteration layer thickness measured by FTIR in transmission mode with SEM calibration, as a function of the square root of time, for various conditions from 35 °C, 95% RH (slowest kinetics) to 125 °C, 95% RH (highest kinetics). b Arrhenius plot of the H2O apparent diffusion coefficient deduced from the alteration layer thickness Alt by the equation \(Alt = 2\sqrt {\frac{{D_{H2O} \times t}}{\pi }}\). c Alteration products observed on glass powder altered at 125 °C and 95% RH for 593 days and their identification by Raman spectroscopy. In addition to calcite, apatite, tobermorite and powellite identified by Raman, phyllosilicates are also present on the surface of the grains. d Left: SEM image (secondary electrons) of the edge of the glass monolith altered at 125 °C and 95% RH for 593 days in an autoclave with D218O saline solution, and right: 18O/16O TOF-SIMS profile of the same sample. The D2O apparent diffusion coefficient deduced by fitting the TOF-SIMS profile was 2.3 × 10−19 m2 s−1, in good agreement with the H2O diffusion coefficient evaluated in (b) of 5.3 × 10−19 m2 s−1. Scale bars are c 10 µm, d 1 µm. Reprinted from ref. 84, Copyright (2018), with permission from ELSEVIER.
One study deals with the hydration kinetics of a soda–magnesia–lime silicate glass under unsaturated conditions using ion beam analysis techniques. The depth of hydration (H+ profile by NRA) increased at the square root of time in all T and %RH conditions over the 10-day period, again pointing out a diffusion-controlled process for this composition91.
However, in the unstable mixed-alkali lime composition of the CH mentioned above, the hydration rate (at 85% RH) increased with time over the 6-day experimental period at 80 °C and over the 9-month experimental period at 40 °C, while the temperature had an accelerating effect beyond that predicted by the Arrhenius law (considering a high activation energy of 85 kJ/mol). Thus in this case, a self-accelerated mechanism involving several processes controlled the kinetics90.
Overall, the glass composition appears to have a critical effect in determining the rate-controlling process in the first few months of alteration, during which a hydrated layer forms. Like in immersion conditions, the composition also closely controls the properties of the hydrated layer, leading to largely differing hydration kinetics in glasses of different compositions.
Chemical nature of the alteration layer
As expected considering a high S/V ratio, secondary phases form more rapidly in atmospheric conditions than in immersion conditions89. The first and most commonly observed crystalline alteration products are salts of leached modifier cations reacted with an acid gas, such as sulfates and carbonates (i). In more specific conditions, zeolites, phyllosilicates and silicate hydrates are also encountered (ii)100,103,104. However, the only alteration product that is always present is an amorphous hydrated layer, with variable thickness (iii). We describe these three components in the following.
(i) The presence of alkali- or alkaline earth-salts indicates that modifier ions and non-bridging oxygens have reacted with water either by ionic exchange or by water dissociation, and the released cations and hydroxyl anions have migrated to the surface, driven by the precipitation of salts. This general process is often called interdiffusion, although this term should be strictly restricted to Na+/H+ exchange. In the case of calcite, a salt that is always observed in atmospheric alteration of Ca-bearing glasses whatever their overall composition, the crystals are often embedded in the superficial hydrated glass layer, suggesting that their growth has partly occurred in the layer57,90,105. Together with calcite, the alkali salts are the most visible part of the alteration in the first stages, inducing the misleading (to our opinion) idea that interdiffusion is the first and rate-limiting process of the alteration. In this respect, it is important to emphasize that these alkali salts do not generally hold all the alkalies of the alteration layer. Indeed, many studies report that alkalies are significantly retained in the hydrated layer5,64,73,75,79,80,84. It is generally supposed that this retention is due to the saturation of the water film that cancels the chemical gradient, and that Na+ is released but present in the pore water75,84. Dissolution experiments of glass samples pre-altered in vapor conditions show a spike in alkali release that supports this idea84, although the alkalies released in the solution account for less than 30% of the alkalies initially present in the hydrated layer in the case of the unstable mixed alkali lime glass of CH79. Regarding this latter glass, a part of the retained alkalies are still bound to non-bridging oxygens, as discussed later. Note that the retention of a small concentration of alkalies in the leached layer has also been noticed and emphasized in immersion conditions, with the authors even suggesting that some Na concentration in the leached layer is necessary to continue the dissolution process106.
(ii) Silicate-bearing secondary phases, mainly zeolites, but also phyllosilicates and C–S–H, are observed in vapor hydration tests (VHT) at high temperature (125 °C or more). These VHT experiments were the first to demonstrate an acceleration of the rate due to Si and Al consumption that destabilized the passivating alteration layer6,70. An alteration resumption, or the retention of a high dissolution rate close to the forward rate, has then been observed in immersion conditions at high S/V and at high temperature or high pH (>150 °C or >pH 11 at 6500 m−1 for SON68), and associated to the formation of silicate phases100,107. However a few differences are to be noted again between both conditions. First, the temperature effect seems particularly important in vapor conditions. For the SON68 glass, at temperatures below 125 °C, no Si-bearing phase appears in unsaturated conditions, while zeolites do form at 90 °C in aqueous solution at high S/V and alkaline pH (pH ~11.5 with NaOH or KOH in solution)103,104. This might indicate a higher activation barrier for the nucleation and growth of these phases in the unsaturated medium, although an effect of the high solution concentration in alkalies is also probable. The marked influence of temperature also extends to the hydration processes, with different activation energies reported on different temperature ranges74,84, or a strongly non-Arrhenian behavior90. Secondly, the secondary phases may not be exactly the same in both conditions. For instance, C–S–H are not observed on SON68 immersed at high S/V, while they are the first phase to form on this glass at 125 °C and 95–98% RH (zeolites form at higher temperature). In Mg- and Al-rich aluminoborosilicate glasses, Mg-smectites precipitate in unsaturated (50 °C, 95 %RH)75 as well as in aqueous high S/V conditions at pH > 9 (50 °C)108, but the nature of these smectites is distinct. Their Al and Mg content is higher in saturated (tri-octahedral smectites) than in unsaturated conditions (di-octahedral smectites). This difference may be related to the order reversal in glass durability between these glasses with distinct Al2O3/MgO ratio. The authors proposed that these differences stem from the different solution chemistry of the aqueous phase and poral water75.
(iii) The amorphous hydrated layer also shows compositional and structural differences between both alteration conditions. This was revealed for three alkali–lime silicate glasses representative of CH glass compositions, aged in controlled temperature (40 and 80 °C) and RH (85% RH) as monoliths and as powders109. The structure of their hydrated layer was characterized by solid state NMR and Raman spectroscopy of the powders. SEM images are shown in Fig. 4 and NMR spectra in Fig. 5. In the two compositions classified as unstable (mixed alkali lime and potassium lime), the hydrated layer was very thick and partially retained alkalies and NBOs, embedded in a dense network of hydrogen bonds90,109. Specific signatures of the presence of NBOs are the very high chemical shift contributions (+14 to +10 ppm) in the 1H MAS NMR spectra, which are assigned to Si–OH groups with strong H-bonding and short O–H–NBO distances. These contributions exist in crystalline alkali silicate hydrates110, and in hydrous glasses obtained by melting glass with water at high pressure111 and are therefore referred to as “hydrate-type” in this paper. To the best of our knowledge, they have never been found in alteration gels produced in liquid phase (see ref. 112. for instance). Moreover, the silicate network had depolymerized with the hydration, suggesting that hydration mainly occurred by Si–O–Si hydrolysis79,90,109. This hydrolysis was particularly fast, possibly due to the retention of alkalies and NBOs that may have locally formed catalytic OH− species. On the contrary, in the soda-lime composition classified as stable, the hydrated layer was thinner, more extensively sodium depleted with a thick crust of carbonate salts on the top. The high 1H chemical shift contributions were absent and the network had repolymerized109. In this latter composition, the water reaction with the NBO sites has been followed by alkali migration to the surface and salt crystallization, while significant silanol condensation took place in the hydrated layer. Consequently, the hydrated layer was probably denser and more passivating against water penetration, in relation to the better chemical durability of this glass.
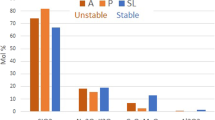
(wt%): Glass A, 71.3 SiO2, 0.8 Al2O3, 0.8 MgO, 5.0 CaO, 11.0 Na2O, 11.0 K2O; Glass P, 75.8 SiO2, 0.3 Al2O3, 0.2 MgO, 2.0 CaO, 1.5 Na2O, 20.2 K2O; Glass SL, 66.8 SiO2, 2.5 Al2O3, 3.0 MgO, 7.5 CaO, 18.0 Na2O, 2.1 K2O. In the field of Cultural Heritage glasses, the compositions A and P are considered as unstable and the composition SL is considered as stable against atmospheric degradation. SEM images (secondary electrons except d back-scattered electrons) of the surface (a, c, e) and of the edge (b, d, f) of glass plates altered at 40 °C, 85% RH for 6 months. The alteration layer thickness is indicated on the images. The salts are mainly Ca carbonates (A, P, and SL), Na, Ca, H-mixed carbonates (A and SL), deliquescent K-carbonates (P), and K, Na, Ca-sulfates (P). Scale bars are a 10 µm, b 1 µm, c 10 µm, d 500 nm, e 1 µm, f 500 nm. Reproduced with permission from ref. 109, Editions Hermann, 2019.
1H and 29Si MAS NMR spectra of the A, P, and SL glass powders altered at 40 °C, 85% RH for 6 months (see the caption of Fig. 4 for the compositions of glasses A, P, and SL). a, c, e 1H NMR spectra acquired by varying the echo delay (TE) allow to filter the contribution of hydrated species with strong homonuclear dipolar coupling such as water. Si–OH contributions with very high chemical shift (about 14 ppm) exist in the A and P altered powders and are assigned to silanols groups H-bonded to non-bridging oxygens, as in crystalline alkali silicate hydrates. Their presence is consistent with the partial retention of alkalies in the hydrated layer. b, d, f the black and pink lines are the 29Si MAS NMR spectra of the pristine and altered glass respectively. These spectra show the depolymerization of the silicate network for the glasses A and P (loss of Q4 and growth of Q2, Q3 contributions), while the SL glass network has repolymerized. 1H → 29Si CP MAS spectra are also given in blue. 1H MAS NMR spectra are reproduced with permission from ref. 109, Editions Hermann, 2019.
Therefore, the hydrated layer produced in atmospheric conditions shows a wider range of compositions, structures, and H-bonding strengths, from hydrate-type (depolymerized, with NBOs) to gel-type (repolymerized, with no NBOs). The extent of retention of alkalies and NBOs in hydrate-type environments strongly depends on the glass composition and seems to be linked with the glass chemical durability in atmosphere. These recent results on alkali lime silicate compositions109 very interestingly connect to similar previous findings on nuclear waste compositions. Indeed, Abrajano et al.73 stated that “The available data suggest that nuclear waste glass reacts following two distinct processes: one where hydration and dealkalization occur parabolically with time, and one where the depletion of mobile elements from the glass is restricted to the near surface region and occurs at a rate which becomes nearly zero with time. In the latter case, it appears as though hydration could be occurring independently from the depletion of mobile cations”. The latter mechanism may correspond to the Si–O–Si fast hydrolysis in hydrate-type environment observed by Alloteau et al.90,109. This mechanism adds to the interdiffusion mechanism and seems specific to atmospheric conditions, although a similar effect has also been suggested in liquid conditions but with much lower concentrations of alkalies113. When this mechanism prevails, it induces an increase of hydration rate and corresponding decrease of chemical durability. Its occurrence may not be systematic: for instance, in the case of medieval stained glass, careful experiments with D218O and nano-SIMS imaging put in evidence only the interdiffusion mechanism83. On the other hand, it may be severely dominating with dramatic consequences, as in the case of the mixed-alkali lime glass of CH aged at 80 °C and 85% RH, where a thick hydrated, depolymerized layer with same composition as the pristine glass, except the additional water, was the only alteration phase to be observed79. How the dominance of this additional mechanism depends on the composition, temperature, and alteration time is totally unknown and requires specific studies.
Summary, discussion, and future prospects
The unsaturated water vapor significantly reacts with the glass surface, especially when RH approaches 100%. Compared to liquid water, the atmospheric alteration seems to show a similar general dependency on glass composition, notably on the content in alkali, but with an amplified effect24, and some inversion has been observed. Glass compositions recognized as unstable in the cultural heritage are particularly sensitive to unsaturated humidity attack. Even with the stable soda lime silicate compositions, it has been stated that compared to corrosion in liquid water, corrosion in humid atmosphere renders more severe degradation, ie, thicker reaction layers64, partly owing to the apparition of salts.
Moreover, many results indicate that the atmospheric alteration of glass is distinct from the alteration in immersion at high S/V. One cause may be the distinct solution chemistry of the adsorbed water film and poral water, such as extreme pH and saturation, equivalent to an extremely high S/V not reachable in immersion. We also think that another cause is the existence of a mechanism that has not been encountered in liquid alteration studies, allowing glass hydration without dealkalization. This mechanism may be described as the case where the Si–O–Si bond hydrolysis becomes equal or more rapid than the water reaction with the NBOs. Alternatively, the latter reaction may occur releasing the modifier ions, but these are trapped in the dense network of hydrogen bonds and may be adsorbed back on the NBO sites, so that the ongoing Si–O–Si hydrolysis appears more rapid. This situation is related to the existence of Si–OH groups strongly H-bonded to NBOs well evidenced in 1H MAS NMR spectra, called hydrate-type environment in this paper. Such a mechanism reveals different chemical properties for the water reacting in atmospheric conditions. Presumably, these modified properties are related to the adsorbed, or bound, state of nearly all the water molecules in unsaturated conditions, which diminishes their solvation properties114. In their study on the unstable mixed-alkali lime silicate glass of the CH, Alloteau et al. suggest that in these conditions of lower solvation by water, the alkalies are retained next to NBOs in the inverse order of their hydration energy, K+ > Na+ » Ca2+. The Ca2+ ions are thus more efficiently solvated and extracted out of the hydrated layer to form salts (calcite), while the silanol sites left behind may condense and contribute to densify the alteration layer, passivating the glass90. On the contrary to Ca2+, K+ ions are more retained next to NBOs, promoting fast network hydrolysis in hydrate-type environment. If these hypotheses were confirmed, then they would provide alternative explanations of the roles of CaO and K2O on glass chemical durability in atmosphere.
Interestingly, recent studies of soda lime silicate glass surfaces by sum frequency generation spectroscopy (SFG), a vibrational spectroscopy technique spatially selective of the extreme surface, have put in evidence the existence of hydrous species thermodynamically more stable than physisorbed water molecules, and associated with slow hydrogen-bonding dynamics115,116. These species could be related to the Si–OH groups H-bonded to the NBOs, or hydrate-type environment of this paper. Whatever these species are, such discoveries indicate that the chemistry of water reacting with the glass surface is probably more complex than previously thought.
In addition, the atmospheric alteration is characterized by its sensitivity to the surface structure and defects, being either compositional gradient, internal stress, scratches, dust and environmental particles, or alteration salts produced in the first alteration stage. Defects cause differential local humidity conditions due to capillary forces, building up heterogeneity in the alteration front. Other surface characteristics (composition and stress) determine the chemical water-surface reactivity and influence the first alteration stage.
To our sense, future research should be carried out open-mindedly with the idea that chemical properties of the glass-water system are modified with respect to the immersion conditions. Whatever the unsaturated or saturated conditions, the very first step of glass alteration is the adsorption of water at the surface and its penetration in the glass. Then, interdiffusion (encompassing two processes as mentioned above) and network hydrolysis take place at the same time but with different rates according to the conditions (T, pH). This is well-known and written everywhere about glass alteration, but we often find in the literature the hierarchical order of (1) interdiffusion and (2) network hydrolysis. In the case of unsaturated conditions, it seems that one should instead consider the order (1) network hydrolysis and (2) interdiffusion, especially with the compositions classified as unstable in the CH field.
How the alkali retention and fast hydrolysis mechanism in hydrate-type environment depends on composition and temperature and how it may be related to special chemical properties of water in bound state are suggested lines of research. In a similar way than in aqueous alteration studies, the stability and evolution of the hydrated layer should be studied as a function of environmental parameters, keeping in mind the specific chemistry of unsaturated conditions. Concepts and ideas from the fields of interfaces and confined space chemistry will probably be useful to enrich our description of these alteration phenomena and improve predictions and control strategies. In parallel, it is also necessary to conduct research on the development of suitable geochemical models that would consider these modified water properties, to satisfactorily describe experimental results and predict glass behavior in the long term.
References
Kunicki-Goldfinger, J. J. Unstable historic glass: symptoms, causes, mechanisms and conservation. Rev. Conserv. 9, 47–60 (2008).
Werme, L. et al. Chemical corrosion of highly radioactive borosilicate nuclear waste glass under simulated repository conditionso Title. J. Mater. Res. 5, 1130–1146 (1990).
Bates, J. K. et al. High-Level Waste Borosilicate Glass: A Compendium of Corrosion Characteristics. Vol. 1. DOE-EM-0177 (1994).
Organ, R. M. The safe storage of unstable glass. Mus. J. 56, 255–272 (1957).
Brill, R. H. Crizzling—a problem in glass conservation. Stud. Conserv. 20, 121–134 (1975).
Bates, J. K., Seitz, M. G. & Steindler, M. J. The relevance of vapor phase hydration aging to nuclear waste isolation. Nucl. Chem. Waste Manag 5, 63–73 (1984).
Godron, Y. Bibliographie raisonnée de l’attaque, par les agents atmosphériques, des verres utilisés dans le bâtiment. Verres Réfract. 30, 495–650 (1976).
Bates, J. K. et al. High-Level Waste Borosilicate Glass: A Compendium of Corrosion Characteristics. Vol. 2. DOE-EM-0177 (1994).
Frugier, P. et al. SON68 nuclear glass dissolution kinetics: current state of knowledge and basis of the new GRAAL model. J. Nucl. Mater. 380, 8–21 (2008).
Sterpenich, J. & Libourel, G. Using stained glass windows to understand the durability of toxic waste matrices. Chem. Geol. 174, 181–193 (2001).
Verney-Carron, A., Gin, S. & Libourel, G. A fractured roman glass block altered for 1800 years in seawater: analogy with nuclear waste glass in a deep geological repository. Geochim. Cosmochim. Acta 72, 5372–5385 (2008).
Chopinet, M. H. & Lehuédé, P. Les problèmes d’altération rencontrés sur des verres industriels. Verre 16, 20–27 (2010).
Collective book. Glass Atmospheric Alteration—Cultural Heritage, Industrial and Nuclear Glasses. (Hermann, 2019).
Cobo del Arco, B. Survey of the national museums of Scotland glass collection. in Conservation of Glass and Ceramics (ed. Tennent, N. H.) 229–238 (James & James, 1999).
Oakley, V. Vessel glass deterioration at the Victoria and Albert museum: surveying the collection. Conserv 14, 30–36 (1990).
Iliffe, C. J. & Newton, R. G. Using triangular diagrams to understand the behaviour of medieval glasses. Verres Refract. 30, 30–34 (1976).
Rodrigues, A., Fearn, S. & Vilarigues, M. Historic K-rich silicate glass surface alteration: behaviour of high-silica content matrices. Corros. Sci. 145, 249–261 (2018).
Rodrigues, A., Fearn, S., Palomar, T. & Vilarigues, M. Early stages of surface alteration of soda-rich-silicate glasses in the museum environment. Corros. Sci. 143, 362–375 (2018).
Roemich, H., Wittstadt, K. & Maas-Diegeler, G. Accelerated weathering and long-term experiments - conclusions for preventive conservation of glass objects. in Glass Atmospheric Alteration - Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 25–35 (Hermann, 2019).
Wittstadt, K., Maas-Diegeler, G., Hiller-König, W. & Grieb, H. Crizzling—exploring degradation and simulation on model glasses. in Glass Atmospheric Alteration—Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 185–195 (Hermann, 2019).
Koob, S. P. Conservation and Care of Glass Objects. (Archetype Publications, 2006).
Biron, I. Le matériau verre et les objets du patrimoine. Origine et manifestation des problèmes rencontrés. in Conservation, Restauration du Verre. Actualité et Problématiques Muséales—Trélon 28 Septembre 2007 13–23 (Écomusée de l’Avesnois, 2007).
Gentaz, L., Lombardo, T., Chabas, A., Loisel, C. & Verney-Carron, A. Impact of neocrystallisations on the SiO2–K2O–CaO glass degradation due to atmospheric dry depositions. Atmos. Environ. 55, 459–466 (2012).
Verità, M. Ancient glass and modern glass: long and short term glass weathering. in Glass Atmospheric Alteration—Cultural Heritage, Industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 73–80 (Hermann, 2019).
Bailly, M. La conservation-restauration du verre: Bilan et perspective. in Conservation, Restauration du Verre. Actualité et Problématiques Muséales—Trélon 28 Septembre 2007 (ed. Écomusée de l’Avesnois) 59–68 (2007).
Koob, S. P. Atmospheric conditions that promote or inhibit crizzling in glass objects. in Glass Atmospheric Alteration—Cultural Heritage, Industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 169–174 (Hermann, 2019).
Bellendorf, P. et al. Archaeological glass: the surface and beyond. Glass and Ceramics Conservation 2010: Interim Meeting of the ICOM-CC Working Group, October 3–6, 2010, Corning, New York, USA 137–144 (2010).
Jackson, C. M., Greenfield, D. & Howie, L. A. An assessment of compositional and morphological changes in model archaeological glasses in an acid burial matrix. Archaeometry 54, 489–507 (2012).
Barbe, F. & Le Roux, J. Presentation and conservation of the collection of chemically unstable Limoges painted enamels in the Louvre Museum. in Glass Atmospheric Alteration—Cultural Heritage, Industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 111–117 (Hermann, 2019).
Robinet, L. The role of organic pollutants in the alteration of historic soda silicate glasses. PhD thesis (University of Edinburgh, 2006).
Robinet, L., Hall, C., Eremin, K., Fearn, S. & Tate, J. Alteration of soda silicate glasses by organic pollutants in museums: mechanisms and kinetics. J. Non Cryst. Solids 355, 1479–1488 (2009).
Davison, S. & Newton, R. G. Conservation and Restauration of Glass. (Elservier, 2003).
Koob, S. P. Crizzling glasses: problems and solutions. Eur. J. Glas. Sci. Technol. A 53, 225–227 (2012).
Dal Bianco, B. et al. Investigation on sol–gel silica coatings for the protection of ancient glass: Interaction with glass surface and protection efficiency. J. Non-Cryst. Solids 354, 2983–2992 (2008).
Carmona, N., Villegas, M. A. & Fernández Navarro, J. M. Sol–gel coatings in the ZrO2–SiO2 system for protection of historical works of glass. Thin Solid Films 515, 1320–1326 (2006).
Newton, R. G. & Seddon, A. Organic coatings for medieval glass. in The Conservation of Glass and Ceramics (ed. Tennent, N. H.) 66–71 (James & James, 1999).
De Ferri, L., Lottici, P. P., Lorenzi, A., Montenero, A. & Vezzalini, G. Hybrid sol–gel based coatings for the protection of historical window glass. J. Sol. Gel Sci. Technol. 66, 253–263 (2013).
Richter, R. Evaluation and re-evaluation of a conservation concept for crizzling enamels. in Glass Atmospheric Alteration—Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 175–184 (Hermann, 2019).
Ryan, J. Chemical stabilisation of weathered glass surfaces. V A Conserv. J. 16, 6–9 (1995).
Hiebert, M. Characterizing the effect of atomic layer deposited coatings for the prevention of glass alteration in museum collections. (University of Maryland, USA, 2019).
Rullier, R. Procédé de Traitement des Surfaces de Verre. Patent FR 2269500A1. (1975).
Alloteau, F. et al. Study of a surface treatment based on zinc salts to protect glasses from atmospheric alteration: mechanisms and application to ancient glass objects in museum. in Glass Atmospheric Alteration—Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 196–206 (Hermann, 2019).
Kunicki-Goldfinger, J. J. & Kierzek, J. Ultraviolet blue fluorescence of 18th century central European glass: an indicator for curators and conservators. Glass Technol. 43C, 111–113 (2002).
Kunicki-Goldfinger, J. J., Targowski, P., Góra, M., Karaszkiewicz, P. & Dzierżanowski, P. Characterization of glass surface morphology by optical coherence tomography. Stud. Conserv. 54, 117–128 (2009).
Targowski, P. et al. Optical coherence tomography for noninvasive investigation of E.A. structure and properties of historic glass. in The Art of Collaboration: Stained-Glass Conservation in the Twenty-First Century. 127–134 (Harvey Miller Publishers, 2010).
Bouquillon, A. et al. Méthodes portables non destructives d’analyse de l’altération des verres au plomb. in Technè hors-série (ed. Centre de Recherche et Restauration des Musées de France) 103–113 (2008).
Verhaar, G., Van Bommel, M. R. & Tennent, N. H. The development of an ion chromatography protocol for detecting the early stages of glass degradation. in Recent Advances in Glass and Ceramics Conservation (eds Roemich, H. & Fair, L.) 123–133 (ICOM-CC, 2016).
Lombardo, T., Gentaz, L. & Loisel, C. Technique de l’Ingénieur. Altération des verres—Cas des vitraux du Moyen Âge. Re242 V1, (2015).
Lombardo, T. et al. Characterisation of complex alteration layers in medieval glasses. Corros. Sci. 72, 10–19 (2013).
Melcher, M. & Schreiner, M. Statistical evaluation of potash-lime-silica glass weathering. Anal. Bioanal. Chem. 379, 628–639 (2004).
Melcher, M. & Schreiner, M. Leaching studies on naturally weathered potash-lime–silica glasses. J. Non-Cryst. Solids 352, 368–379 (2006).
Melcher, M. & Schreiner, M. Evaluation procedure for leaching studies on naturally weathered potash-lime-silica glasses with medieval composition by scanning electron microscopy. J. Non-Cryst. Solids 351, 1210–1225 (2005).
Edaine, J., Loisel, C., Geronazzo, D. & Pallot-Froissard, I. EU-Project CONSTGLASS N° 044339. Conservation materials for stained glass windows—assessment of treatments, studies on reversibility and performance of innovative restoration strategies and products. Part I of IV. (2011).
Loisel, C. & Pallot-Frossard, I. Stained-glass: how to take care of a fragile heritage? in 9th Forum for the Conservation and Technology of Historic Stained-Glass (ed. ICOMOS) 183 (2015).
Bernardi, A., Becherini, F. & Verità, M. Conservation of stained-glass windows with protective glazing: main results from the European VIDRIO research program. J. Cult. Heritage 14, 527–536 (2013).
Chopinet, M. H. et al. Soda-lime-silica glass containers: chemical durability and weathering products. Adv. Mater. Res. 39–40, 305–310 (2008).
Verità, M., Falcone, R., Sommariva, G., Chopinet, M. H. & Lehuédé, P. Weathering of the inner surface of soda-lime-silica glass containers exposed to the atmosphere. Eur. J. Glas. Sci. Technol. Part A 50, 65–70 (2009).
Lombardo, T., Chabas, A. & Lefèvre, R. Weathering of float glass exposed outdoors in an urban area. Glass Technol. 46, 271–276 (2005).
Raman, C. V. & Rajagopalan, V. S. Colours of stratified media-I—ancient decomposed glass. Proc. Indian Acad. Sci.—Sect. A 11, 469–482 (1940).
Reiß, S., Urban, S., Jacob, K., Krischok, S. & Rädlein, E. Investigation of the influence of a commercial glass protector on float glass surfaces by X-ray photoelectron spectroscopy. Phys. Chem. Glasss 58, 99–108 (2017).
Rädlein, E. & Brokmann, U. Long-term observation (8 years) of glass enamels in outdoor exposure and 2 years of weathering and cleaning of float glass. in Glass Atmospheric Alteration—Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 97–105 (Hermann, 2019).
Mellott, N. P., Brantley, S. L., Hamilton, J. P. & Pantano, C. G. Evaluation of surface preparation methods for glass. Surf. Interface Anal. 31, 362–368 (2001).
Takeda, S. Oxygen and silver diffusion into float glass. J. Non-Cryst. Solids 352, 3910–3913 (2006).
Bange, K. et al. Multi-method characterization of soda-lime glass corrosion, Part 2. Corrosion in humidity. Glass Sci. Technol. 75, 20–33 (2002).
Stella, A. & Verità, M. EPMA analysis of float glass surfaces. Mikrochim. Acta 114–115, 475–480 (1994).
Yamamoto, Y. & Yamamoto, K. Precise XPS depth profile of soda-lime-silica float glass using C 60 ion beam. Opt. Mater. 33, 1927–1930 (2011).
Nunes de Carvalho, J., Cleaver, J. A. S., Kirkby, N. F. & Holmes, P. A. An experimental study of the effect of zinc treatment on float glass. Eur. J. Glass Sci. Technol. A 55, 14–22 (2014).
Hahn, K. Protection of glassware in the automatic dishwashing process—a detergent manufacturer’s insight and experiences. in Glass Atmospheric Alteration—Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 209–214 (Hermann, 2019).
Reiß, S., Grieseler, R., Krischok, S. & Rädlein, E. The influence of Sahara sand on the degradation behavior of float glass surfaces. J. Non-Cryst. Solids 479, 16–28 (2018).
Chaou, A. A. et al. Vapor hydration of a simulated borosilicate nuclear waste glass in unsaturated conditions at 50 °C and 90 °C. RSC Adv. 5, 64538–64549 (2015).
Gin, S. et al. An international initiative on long-term behavior of high-level nuclear waste glass. Mater. Today 16, 243–248 (2013).
Bates, J. K., Ebert, W. L. & Gerding, T. J. Vapor hydration and subsequent leaching of transuranic-containing SRL and WV glasses. DE90 002261 (1990).
Abrajano, T. A., Bates, J. K. & Mazer, J. J. Aqueous corrosion of natural and nuclear waste glasses II. Mechanisms of vapor hydration of nuclear waste glasses. J. Non-Cryst. Solids 108, 269–288 (1989).
Neeway, J. J. et al. Vapor hydration of SON68 glass from 90 °C to 200 °C: a kinetic study and corrosion products investigation. J. Non-Cryst. Solids 358, 2894–2905 (2012).
Narayanasamy, S. et al. Influence of composition of nuclear waste glasses on vapor phase hydration. J. Nucl. Mater. 525, 53–71 (2019).
Léang, M., Giorgiutti-Dauphiné, F., Lee, L. T. & Pauchard, L. Crack opening: From colloidal systems to paintings. Soft Matter 13, 5802–5808 (2017).
Lahlil, S., Xu, J. & Li, W. Influence of manufacturing parameters on the crackling process of ancient Chinese glazed ceramics. J. Cult. Heritage 16, 401–412 (2015).
Robinet, L., Eremin, K., Coupry, C., Hall, C. & Lacome, N. Effect of organic acid vapors on the alteration of soda silicate glass. J. Non Cryst. Solids 353, 1546–1559 (2007).
Alloteau, F. et al. New insight into atmospheric alteration of alkali-lime silicate glasses. Corros. Sci. 122, 12–25 (2017).
Walters, H. V. & Adams, P. B. Effects of humidity on the weathering of glass. J. Non-Cryst. Solids 19, 183–199 (1975).
Fearn, S., McPhail, D. S. & Oakley, V. Investigation of the corrosion of seventeen-century façon de venise glass using advanced surface analysis techniques. in Annales du 16e Congrès de L’association Internationale Pour l’histoire du Verre, Londres, 7–13 Septembre 2003 375–379 (AIHV, 2005).
Fearn, S., McPhail, D. S., Hagenhoff, B. & Tallarek, E. TOF-SIMS analysis of corroding museum glass. Appl. Surf. Sci. 252, 7136–7139 (2006).
Sessegolo, L. et al. Long-term weathering rate of stained-glass windows using H and O isotopes. npj Mater. Degrad. 2, 17 (2018).
Bouakkaz, R., Abdelouas, A. & Grambow, B. Kinetic study and structural evolution of SON68 nuclear waste glass altered from 35 to 125 °C under unsaturated H2O and D2O18 vapour conditions. Corros. Sci. 134, 1–16 (2018).
Asay, D. B. & Kim, S. H. Evolution of the Adsorbed Water Layer Structure on Silicon Oxide at Room Temperature. J. Phys. Chem. B 109, 16760–16763 (2005).
Doremus, R. H. Interdiffusion of hydrogen and alkali ions in a glass surface. J. Non Cryst. Solids 19, 137–144 (1975).
Smets, B. M. J. & Lommen, T. P. A. The leaching of sodium containing glasses: ion exchange or diffusion of molecular water? J. Phys. 43, C9-649–C9-652 (1982).
Gin, S., Frugier, P., Jollivet, P., Bruguier, F. & Curti, E. New insight into the residual rate of borosilicate glasses: effect of S/V and glass composition. Int. J. Appl. Glass Sci. 4, 371–382 (2013).
Abrajano, T. A., Bates, J. K. & Byers, C. D. Aqueous corrosion of natural and nuclear waste glasses I. Comparative rates of hydration in liquid and vapor environments at elevated temperatures. J. Non Cryst. Solids 84, 251–257 (1986).
Alloteau, F. et al. Temperature-dependent mechanisms of the atmospheric alteration of a mixed-alkali lime silicate glass. Corros. Sci. 159, 108129 (2019).
Cummings, K., Lanford, W. A. & Feldmann, M. Weathering of glass in moist and polluted air. Nucl. Instrum. Methods Phys. Res. B 136–138, 858–862 (1998).
Paul, A. Chemical durability of glasses; a thermodynamic approach. J. Mater. Sci. 12, 2246–2268 (1977).
Verità, M. Venetian soda glass. in Modern Methods fo Analysing Archeological and Historical Glass (ed. Janssens, K.) 515–533 (John Wiley & Sons, 2013).
Bunker, B. C., Arnold, G. W., Day, D. E. & Bray, P. J. The effect of molecular structure on borosilicate glass leaching. J. Non Cryst. Solids 87, 226–253 (1986).
Ledieu, a, Devreux, F., Barboux, P., Sicard, L. & Spalla, O. Leaching of borosilicate glasses. I. Experiments. J. Non Cryst. Solids 343, 3–12 (2004).
Devreux, F., Ledieu, A., Barboux, P. & Minet, Y. Leaching of borosilicate glasses. II. Model and Monte-Carlo simulations. J. Non Cryst. Solids 343, 13–25 (2004).
Sicard, L., Spalla, O., Né, F., Taché, O. & Barboux, P. Dissolution of oxide glasses: a process driven by surface generation. J. Phys. Chem. C 112, 1594–1603 (2008).
Cailleteau, C. et al. Insight into silicate-glass corrosion mechanisms. Nat. Mater. 7, 978–983 (2008).
Mascaraque, N., Bauchy, M. & Smedskjaer, M. M. Correlating the network topology of oxide glasses with their chemical durability. J. Phys. Chem. B 121, 1139–1147 (2017).
Fournier, M., Gin, S. & Frugier, P. Resumption of nuclear glass alteration: state of the art. J. Nucl. Mater. 448, 348–363 (2014).
Gin, S., Beaudoux, X., Angéli, F., Jégou, C. & Godon, N. Effect of composition on the short-term and long-term dissolution rates of ten borosilicate glasses of increasing complexity from 3 to 30 oxides. J. Non Cryst. Solids 358, 2559–2570 (2012).
Chave, T., Frugier, P., Ayral, A. & Gin, S. Solid state diffusion during nuclear glass residual alteration in solution. J. Nucl. Mater. 362, 466–473 (2007).
Gin, S. et al. The fate of silicon during glass corrosion under alkaline conditions: A mechanistic and kinetic study with the International Simple Glass. Geochim. Cosmochim. Acta 151, 68–85 (2015).
Ribet, S. & Gin, S. Role of neoformed phases on the mechanisms controlling the resumption of SON68 glass alteration in alkaline media. J. Nucl. Mater. 324, 152–164 (2004).
Chinnam, R. K., Fossati, P. C. M. & Lee, W. E. Degradation of partially immersed glass: A new perspective. J. Nucl. Mater. 503, 56–65 (2018).
Scholze, H. Chemical Durability of Glasses. J. Non-Cryst. Solids 52, 91–103 (1982).
Michelin, A. et al. Silicate glass alteration enhanced by iron: Origin and long-term implications. Environ. Sci. Technol. 47, 750–756 (2013).
Thien, B. M. J., Godon, N., Ballestero, A., Gin, S. & Ayral, A. The dual effect of Mg on the long-term alteration rate of AVM nuclear waste glasses. J. Nucl. Mater. 427, 297–310 (2012).
Alloteau, F. et al. Alteration mechanisms of ancient glass objects exposed to the atmosphere. in Glass Atmospheric Alteration - Cultural Heritage, industrial and Nuclear Glasses (eds Biron, I., Alloteau, F., Lehuédé, P., Majérus, O. & Caurant, D.) 13–24 (Hermann, 2019).
Xue, X. & Kanzaki, M. Proton distributions and hydrogen bonding in crystalline and glassy hydrous silicates and related inorganic materials: Insights from high-resolution solid-state nuclear magnetic resonance spectroscopy. J. Am. Ceram. Soc. 92, 2803–2830 (2009).
Le Losq, C., Cody, G. D. & Mysen, B. O. Alkali influence on the water speciation and the environment of protons in silicate glasses revealed by 1H MAS NMR spectroscopy. Am. Mineral. 100, 466–473 (2015).
Angeli, F., Gaillard, M., Jollivet, P. & Charpentier, T. Influence of glass composition and alteration solution on leached silicate glass structure: a solid-state NMR investigation. Geochim. Cosmochim. Acta 70, 2577–2590 (2006).
Scholze, H. Chemical durability of glasses. J. Non Cryst. Solids 52, 91–103 (1982).
Collin, M. et al. Molecular dynamics simulations of water structure and diffusion in a 1 nm diameter silica nanopore as a function of surface charge and alkali metal counterion identity. J. Phys. Chem. C 122, 17764–17776 (2018).
Sheth, N. et al. Probing hydrogen-bonding interactions of water molecules adsorbed on silica, sodium calcium silicate, and calcium aluminosilicate glasses. J. Phys. Chem. C 122, 17792–17801 (2018).
Luo, J., Banerjee, J., Pantano, C. G. & Kim, S. H. Vibrational sum frequency generation spectroscopy study of hydrous species in soda lime silica float glass. Langmuir 32, 6035–6045 (2016).
Acknowledgements
The International Symposium on Glass Degradation in Atmospheric Conditions was held in Paris at the Center for Research and Restoration of the French Museums (C2RMF) on November 15th−17th, 2017. A hundred people participated to the symposium with the presentation of 27 conferences and 11 posters. The proceedings of this symposium have been published in the collection La Nature de l’Œuvre, Hermann edition, in August 2019. The authors are very grateful to all the institutions, laboratories and companies for their financial support that allowed the organization of the conference and publication of their acts: the French Ministry of Culture, the C2RMF, the Foundation of the Cultural Heritage Science (FSP), the company Reckitt Benckiser, the Center for Atomic Energy and Alternative Energies (CEA Marcoule), Paris Science et Lettres University (PSL University), the Institute of Research of Chimie Paris (IRCP), the Research Laboratory of Historical Monuments (LRMH-CRC, USR 3224) and the French National Center of Scientific Research (CNRS).
Author information
Authors and Affiliations
Contributions
O.M. organized the design and co-writing of the paper, wrote the sections “Mechanisms of glass alteration in humid atmosphere” and “Summary” and contributed to the other sections. P.L. wrote the section “Signs and issues of glass alteration in humid atmosphere/Commercial glasses”. I.B. wrote the section “Signs and issues of glass alteration in humid atmosphere/Glass art objects”. S.N. wrote the section “Signs and issues of glass alteration in humid atmosphere/Nuclear glasses”. F.A. carried out the experimental characterization of crizzled glass displayed in Fig. 1 and produced the experimental results shown in Fig. 4 and Fig. 5. D.C. contributed to all the paper sections by careful reading and comments. All authors contributed to the design of the paper, discussed the relevant literature, and contributed to conceive the novel opinions presented in the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Majérus, O., Lehuédé, P., Biron, I. et al. Glass alteration in atmospheric conditions: crossing perspectives from cultural heritage, glass industry, and nuclear waste management. npj Mater Degrad 4, 27 (2020). https://doi.org/10.1038/s41529-020-00130-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-020-00130-9
This article is cited by
-
The atypical hues of the Santa Cruz blue-and-white cargo: non-invasive analysis of glaze defects and color variations in mid-Ming porcelain
Heritage Science (2023)
-
Alteration of medieval stained glass windows in atmospheric medium: review and simplified alteration model
npj Materials Degradation (2023)
-
A review of glass corrosion: the unique contribution of studying ancient glass to validate glass alteration models
npj Materials Degradation (2023)
-
Research on the degradation of ancient Longquan celadons in the Dalian Island shipwreck
npj Materials Degradation (2022)
-
Borosilicate glass alteration in vapor phase and aqueous medium
npj Materials Degradation (2022)