Abstract
With the reignited push for manned spaceflight and the development of companies focused on commercializing spaceflight, increased human ventures into space are inevitable. However, this venture would not be without risk. The lower gravitational force, known as microgravity, that would be experienced during spaceflight significantly disrupts many physiological systems. One of the most notably affected systems is the musculoskeletal system, where exposure to microgravity causes both bone and skeletal muscle loss, both of which have significant clinical implications. In this review, we focus on recent advancements in our understanding of how exposure to microgravity affects the musculoskeletal system. We will focus on the catabolic effects microgravity exposure has on both bone and skeletal muscle cells, as well as their respective progenitor stem cells. Additionally, we report on the mechanisms that underlie bone and muscle tissue loss resulting from exposure to microgravity and then discuss current countermeasures being evaluated. We reveal the gaps in the current knowledge and expound upon how current research is filling these gaps while also identifying new avenues of study as we continue to pursue manned spaceflight.
Similar content being viewed by others
Introduction
Roughly 60 years ago, Yuri Gagarin made history by becoming the first human to venture into space1. Since that time, international collaboration and technological advancements have allowed humans to venture deeper into space and stay for longer durations than imagined 60 years ago. Both human safety and health during space flight remain the greatest priority when considering further space exploration. Medical examinations done pre-, intra-, and post-spaceflight have demonstrated that exposure to microgravity, defined as forces less than 1 × 10−3 g, negatively impacts the human body and disrupts normal physiological systems, with none more affected than the musculoskeletal system1,2,3. Extensive research has been and continues to be done using in vitro and in vivo models to better understand the effects of microgravity exposure and develop countermeasures to the negative impacts incurred as a result of exposure to microgravity3.
The objective of this article is to review the current knowledge developed over the past decade regarding how real and simulated microgravity affects the cellular and physiological function of the musculoskeletal system. Based on current observations, it is clear that bone and skeletal muscle tissues are highly responsive and adaptive to their mechanical loading environment. However, more musculoskeletal system research is needed to understand how and why the pronounced bone and skeletal muscle mass loss in astronauts occurs in response to microgravity1,2,4. Moreover, we will focus on the multiple cell types of musculoskeletal tissues, along with their crosstalk interactions, and present potential countermeasures to alleviate the negative bone and muscle changes associated with microgravity. We will also discuss future avenues of research and gaps in our knowledge that need addressing as we venture deeper into space.
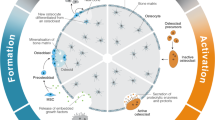
Bone is a complex tissue system that is constantly being remodeled throughout postnatal life to adapt to its mechanical loading environment5,6,7. This continually active process maintains the bone structure, mechanical strength, and function in response to the variable loading that we experience daily2. Under normal physiological loading, bone remodeling maintains homeostasis through bone-forming cells, osteoblasts, and bone-resorbing cells, osteoclasts. Exposure to microgravity disrupts this homeostatic balance and can result in up to a 1% loss in bone mass per month during continual microgravity exposure8. The balance between osteoblasts and osteoclasts is determined partly through osteocyte signaling. Osteocytes are mature osteoblasts that have become embedded within the bone matrix and comprise 90–95% of the total bone cell population6. Osteocytes are suggested to be the main mechanosensitive cell in bone and can detect mechanical signals acting on the bone tissue.
Although bone loss from aging is a common occurrence, the magnitude and speed of bone lost following exposure to microgravity is far greater than that which is typically seen during aging. This is a critical concern for humans when considering the effects of long-duration space flight1,2,3. Exacerbated bone loss leads to a decrease in bone mineral density (BMD), an increase in fracture risk, and a premature osteoporotic phenotype3,9,10. In this review, we will examine the physiological effects that exposure to microgravity has on osteoblasts, osteoclasts, osteocytes, and their progenitor cells and discuss countermeasures to block bone loss resulting from exposure to microgravity.
In addition to bone, exposure to microgravity also affects skeletal muscle. Skeletal muscles made up of individual muscle fibers generate force on our skeletons for movement and postural support. It is well established that skeletal muscle adaptation occurs in response to changes in its mechanical environment. For example, repetitive loading of skeletal muscle tissue leads to skeletal muscle hypertrophy whereas unloading of skeletal muscles under microgravity conditions leads to skeletal muscle atrophy11,12. This observed skeletal muscle atrophy due to exposure to microgravity is problematic because of its rapid onset and severity. Up to a 20% decrease in average skeletal muscle mass over 1 month and up to a 30% decrease in average skeletal muscle strength over 1 month can occur in astronauts exposed to microgravity13. This loss of skeletal muscle mass and function can hinder or prevent astronauts from performing mission tasks, such as performing spacewalks or necessary repairs on the international space station (ISS), as well as increase their risk of injury upon return to higher gravity conditions on Earth or Mars. Therefore, there is a need to better understand the cellular and molecular mechanisms of skeletal muscle atrophy caused by exposure to microgravity so new countermeasures can be developed.
Recently, the interaction between bone and skeletal muscle has become a field of interest in the musculoskeletal community5. Traditionally, bone and skeletal muscle tissue are studied independently, however, it has become apparent that to fully understand how the musculoskeletal system is affected by microgravity, bone and skeletal muscle must be studied together under standard (1 g) and microgravity (<1 g) conditions. It has also become clear that understanding the crosstalk between bone and skeletal muscle necessitates examining other physiological systems known to affect musculoskeletal health, such as the endocrine and nervous systems. These systems influence bone and skeletal muscle tissues under both standard gravity and microgravity. Understanding the interactions of these multiple systems pre-, intra-, and post- spaceflight is essential to gaining a complete understanding of how exposure to microgravity affects the musculoskeletal system. It also guides researchers as to what direction to take in future studies aimed at a better understanding of the effect of microgravity on bone and skeletal muscle.
Studying the effect of microgravity exposure on bone, skeletal muscle, or the interaction of both tissues has not been without challenge or expense. Therefore, as alternatives to using real microgravity, many ground-based analogs that simulate microgravity have been developed and characterized to allow further insight into the effect of microgravity on the musculoskeletal system. In addition, these approaches have led to the development of novel countermeasures to bone and skeletal muscle catabolism in response to microgravity.
Models of Real and Simulated Microgravity
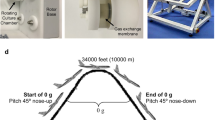
Exposure to microgravity can be examined using both in vitro and in vivo models and exposure to either simulated or real microgravity conditions (Fig. 1). Typical in vitro models examine the influence of microgravity exposure on cells in and on constructs. Clinostats are tube-like structures that are filled with a cell suspension and rotate to simulate exposure to microgravity1,14. Important clinostat parameters include tube volume size and rotation speed which dictate the amount of centrifugal force needed to prevent driving cells toward the vessel walls14. Limitations of clinostats include tube width and smaller sample size and volume. Random positioning machines (RPM) are another type of device used to model and expose cells to simulated microgravity. RPMs consist of two motor-driven frames that allow cells and constructs to be freely rotated, simulating a microgravity environment via gravity vector averaging14,15. Limitations of RPM devices include the size and volume of samples in addition to the significant difficulty of introducing pharmacological agents or other experimental variables during rotation. The last commonly used in vitro model is the rotating wall vessel (RWV). RWVs are slow-rotating, liquid-filled containers developed and approved as a simulated microgravity model by NASA14,16. RWVs are also referred to as rotary cell culture systems (RCCS)4,17. Similar to the clinostat, RWVs rotate constantly. This rotation maintains a constant cell suspension, creating what is known as vector averaged microgravity, where all forces have an equal and opposite force which creates an overall net force of zero on the sample14. The speed at which this rotation occurs is determined is based on the specimen’s specific gravity, vessel fluid density, and viscosity. Since the devlopment of RWVs, alternate models have emerged with various vessel sizes and shapes, including the slow turning lateral vessel (STLV) and the high aspect ratio vessel (HARV)4,17,18. Although the models and devices mentioned above create an environment similar to microgravity conditions, it is important to emphasize that these devices only create a net zero gravity vector, rather than the reduced or near zero gravity experienced in real microgravity.
RCCS, Clinostat, and RPM methods use rotation to create vector averaged forces to simulate microgravity. Hind limb suspension (HLS), spinal injury, and single limb immobilizationvia casting, neurectomy, or skeletal muscle paralysis (SMP) limit the ground reaction forces on the limbs by completely unloading or inducing disuse to simulate microgravity, while only HLS induces a cephalic fluid shift. Bed rest partially unloads the musculoskeletal system and induces a cephalic fluid shift when used in conjunction with head-down tilt. Parabolic flight uses rapid descent to reduce ground reaction forces and momentarily simulate microgravity. Real spaceflight removes ground reaction forces, mechanically unloading the sample or subject, and creates a cephalic fluid shift within the body.
There are two commonly used in vivo microgravity animal models: hindlimb suspension (HLS) and skeletal muscle paralysis (SMP). HLS is a NASA-developed model that exposes the hindlimbs of a rodent to simulated microgravity through tail suspension1,19. This model causes musculoskeletal unloading of the hind limbs, and a cephalic fluid shift similar to that experienced by astronauts during spaceflight1,19. However, a limitation to HLS is that rodents often experience weight loss and increased markers of stress compared to ground control animals, making isolation of gravity-specific affects challenging. Partial weight bearing (PWB) is another model of suspension where the entire body of the animal is suspended, reducing the weight each limb bears. This method is favorable in that it can simulate more precisely the reduced gravitational loads experienced found on the Moon (0.16 g) or Mars (0.38 g). This method significantly reduces bone density and muscle mass20,21. Another model to induce musculoskeletal unloading is via SMP through the use of either Botox or a neurectomy to cause rapid musculoskeletal tissue loss in one of the hindlimbs. A benefit of the unilateral spinal cord injury model (neurectomy), is that atrophy occurs in both the skeletal muscle and bone tissue, as well as a disruption of the cardiovascular and immune systems, both of which are altered in astronauts during long-duration space flight22. Single limb immobilization via casting is a newer model that also causes skeletal muscle and bone loss similar to that experienced during exposure to microgravity environments23. However, this model is still being developed and few studies have been performed to evaluate the efficacy of this model overall. Limb immobilization models of paralysis via casting, Botox, neurectomy, and spinal injury along with PWB, however, do not create the cephalic fluid shift or entirely prevent bone loading from ground reaction forces observed in the HLS model. Although these models of simulated microgravity are an established alternative to exposure to real microgravity exposure, simulated microgravity only mitigates the ground reaction forces, and not the gravitational forces, experienced by the animals24.
The most clinically relevant method to examine the effects of microgravity exposure on the musculoskeletal system is through spaceflight. Unfortunately, experiments conducted in real microgravity are limited due to the infrequency of spaceflights, available capsule space, and extraordinary cost15. While examination of astronauts to evaluate the effects of microgravity on the musculoskeletal system has been performed, the information collected is limited to minimally invasive procedures and does not allow a full examination of the effects of microgravity on the musculoskeletal system. An alternate approach to examine the effects of microgravity exposure on humans is through the use of a head-down tilt bed rest model1,25. The head-down tilt bed rest model simulates microgravity by inducing musculoskeletal disuse and mimics the cephalic fluid shifts observed during spaceflight through the tilted incline. Microgravity can also be simulated during parabolic flights. Parabolic flights provide short-term freefall that simulate exposure to microgravity by transiently minimizing the ground reaction forces26.
Hematopoietic and Bone Mesenchymal Stem Cells
Bone marrow, located in the interior of bones, is home to many different cell types, including bone marrow stromal cells (BMSC) and hematopoietic stem cells (HSCs), with vast differentiation potential as seen in Fig. 227,28,29. Cells within the BMSC population give rise to mesenchymal stem cells (MSCs) which differentiate to bone-forming osteoblasts, cartilage forming chondrocytes, adipose forming adipocytes, or skeletal muscle forming myoblasts30. HSCs that differentiate into immune cells, erythrocytes, and osteoclasts are crucial for the repair of damaged bone tissue and bone turnover. BMSCs which differentiate into osteoblasts, osteocytes, myocytes, or adipocytes are critical for tissue regeneration and homeostasis.
Exposure to microgravity leads BMSCs to preferentially differentiate to adipogenic lineage cells as opposed to the osteogenic or myogenic lineage cells31. This decreases their capacity to maintain healthy bone and skeletal muscle tissue as well as to respond and adapt to the mechanical environment. Increased commitment of BMSCs to the adipogenic lineage also reduces the ability of bone and skeletal muscle tissue to regenerate and recover from injury. Additionally, unloading can decrease the population and function of BMSCs32. These effects are similar to what is seen with aging, and thus, studying the effects of exposure to microgravity can be useful for understanding the effects of aging.
In vitro studies of the effects of microgravity on stem cell physiology
Data on how HSCs respond to microgravity is limited, as few studies have been conducted on HSCs in vitro. Mechanical loading increases HSC differentiation and maturation, suggesting that unloading from exposure to microgravity may prevent these effects33. Indeed, hematopoietic clusters of differentiation-34+ (CD34+) cells, when exposed to microgravity for 11 days or simulated microgravity for 2 days, have decreased proliferation and differentiation34. Microgravity also disrupts the migration potential and cell cycle progression of these cells35. Myeloid progenitor cell counts increase while erythroid progenitor cell counts decrease in microgravity. Differentiation towards macrophage lineage cells increases. Furthermore, simulated microgravity decreases erythrocyte proliferation and increases apoptosis which could have negative effects on tissue oxygen levels36.
Exposure to simulated microgravity decreases BMSC differentiation to osteoblasts which can have negative effects on bone formation and bone volume37. Simulated microgravity decreases BMSC cell proliferation, changes cell nucleus size, and reduces expression of cytoskeletal and musculoskeletal proteins38. Progression of the cell cycle is also disrupted, and the effects of growth factors such as insulin-like growth factor 1 (IGF-1) are attenuated39,40,41,42,43. These changes will likely lead to the decreased osteoblastic differentiation seen in microgravity. For example, collagen type I-alpha1 (Col1a1), a gene that is indicative of osteoblastic differentiation and increases as differentiation occurs, decreased after 7 days in simulated microgravity44,45. Additionally, phosphorylation of focal adhesion kinase (FAK) and proline-rich tyrosine kinase 2 (PYK2), which are required for osteoblastic differentiation and function, are decreased46,47.
Return to standard gravity after spaceflight restores bone volume but does not fully restore osteoblast number, indicating that microgravity has long-term effects on the capacity of BMSCs to differentiate into osteoblasts28. Culturing cells under pro-osteogenic conditions after exposure to microgravity is unable to promote osteoblastic differentiation as an expression of alkaline phosphatase (Alpl), Col1a1, runt-related transcription factor 2 (Runx2), and osteonectin (Sparc), genes associated with bone formation, are decreased37,45. Meanwhile, expression of peroxisome proliferator-activated receptor gamma (Ppar-γ) (adipsin (Cfd), leptin (Lep), and glucose transporter-4 (Glut-4), genes associated with adipogenesis, are increased31.
In vivo studies of the effect of microgravity on stem cell physiology
During exposure to microgravity, there is a decrease in the differentiation of HSCs into red blood cells34,48. Studies of astronauts in spaceflight and animals exposed to simulated microgravity revealed that exposure to microgravity induces a condition called space anemia48,49. This condition is characterized by a reduction in red blood cell mass, hemoglobin mass, and plasma volume. Trabecular bone loss during spaceflight also affects HSCs since hematopoiesis often occurs at locations of greatest trabecular bone volume29. Additionally, the accumulation of adipocytes and increased adipogenesis in the bone marrow after exposure to microgravity likely negatively affect HSC function based on the previous literature50.
In vivo models of simulated microgravity reveal similar effects of microgravity on BMSCs as in vitro models. Overall, human and rodent BMSCs are less osteogenic, differentiating less readily into osteoblasts during exposure to microgravity45. Mice that are mechanically stimulated by whole-body vibrations during exposure to simulated microgravity have an increased population of BMSCs, indicating exposure to mechanical loading during exposure to microgravity has a protective effect on these cells28. However, bone marrow cells of mice returning from exposure to microgravity during 2 weeks of spaceflight surprisingly show an increased differentiation potential upon return to normal gravitational conditions51. It is hypothesized that this occurs because exposure to microgravity hinders BMSC and HSC growth and differentiation, leading to an accumulation of undifferentiated cells and increased numbers of differentiated cells on return to normal gravitational conditions (1 g).
Osteoblasts
Osteoblasts are derived from MSCs of the periosteum and bone marrow and are the primary cells that form bone. Throughout an osteoblast’s differentiation and maturation process, each osteoblast deposits the extracellular matrix and facilitates matrix mineralization. When an osteoblast becomes entrapped in the mineralized extracellular matrix it has produced, it will undergo terminal differentiation into an osteocyte.
Osteoblasts can both sense and adapt to changes in gravitational forces. Under microgravity conditions, changes in the loading environment cause variations in osteoblast proliferation, maturation, and activity and therefore changes in de novo bone formation8,39,52. Exposure to microgravity also results in changes in osteoblastic gene expression, cell morphology, and mineralization ability both in vitro and in vivo. However, specific mechanisms by which microgravity influences osteoblastogenesis remain poorly understood.
In vitro studies of the effect of microgravity on osteoblast cell physiology
Under standard gravity conditions, temporal upregulation of pre-osteoblastic genes Runx2, Col1a1, and activator protein-1 (Ap-1), along with a host of other genes, indicates an increase in osteoblast differentiation. Mature osteoblasts then express, at high levels, All, osterix (Sp7), and osteocalcin (OCN; encoded by Bglap)53. These genes and associated downstream proteins are essential for bone formation, initiating the osteoblastic maturation and bone mineralization cascade. In cells exposed to simulated microgravity by RPM, Prado et al. identified 140 genes in 2T3 preosteoblastic cells that were differentially regulated compared to cells cultured in a standard gravity environment54. Genes associated with osteoblastic differentiation such as Alpl, Runx2, Bglap, Col1a1, bone morphogenic protein-2 (Bmp-2), and genes encoding various integrins, were all significantly downregulated after exposure to simulated microgravity54. This suggests that, due to microgravity, genes associated with osteoblastic differentiation and bone formation are significantly affected and downregulated. In support of these findings, aboard the Foton 10 spaceflight, MG-63 cells, an osteosarcoma cell line, also exhibited downregulation of genes encoding the proteins ALPL, OCN, and COL1A1 when compared to ground-based controls55.
In addition to the downregulation of key osteoblastic genes, Hu et al. observed that cells exposed to simulated microgravity displayed decreased mineralization56. This suggests that exposure to microgravity results in an overall decrease in BMD which is caused, in part, by a decrease in osteoblast number and differentiation during microgravity, which contributes to an imbalance of bone formation and bone resorption54,56,57. How variations in gravitational forces regulate and induce changes in the osteoblastic phenotype remains unclear, but evidence suggests a few possible mechanisms.
One possible mechanism underlying the effects of microgravity on osteoblast differentiation is the downregulation of ras homolog family member A (RHOA) protein. Changes in the cytoskeleton have hinted at a possible mechanism by which variations in the mechanical loading environment are transduced. RHOA regulates cytoskeletal arrangement through upregulation of Rho-associated protein kinase (ROCK) and the formation of actin fibers58. Along with regulating cell size and shape, evidence suggests that the actin stress fibers within the cell have a role in mechanotransduction, relaying mechanical signals to the nuclei and modulating cellular gene expression58,59,60. This evidence has led to more studies investigating the effects of microgravity on cytoskeletal structure, morphology, and composition. Most recently, Nabavi et al. exposed primary osteoblasts isolated from CD1 mice to microgravity for 5 days and observed a decrease in overall microtubule organization, a decrease in focal adhesion size and number, and a decrease in F-actin thickness compared to ground control cells60. Additionally, the nuclei of osteoblasts exposed to microgravity were larger and more irregular in shape compared to ground control cells60. This could indicate the onset of anoikis, a programmed cell death initiated by changes in nuclear morphology. This mechanically stimulated cell apoptosis provides a mechanistic explanation of the decreased bone formation seen during exposure to microgravity. However, in multiple studies, osteoblast apoptosis remained unchanged during exposure to simulated microgravity, though the osteoblasts’ sensitivity to apoptotic factors was drastically increased61,62.
Variations in intracellular Ca2+ concentration ([Ca2+]) have also been observed in cells exposed to microgravity. [Ca2+] is an integral second messenger within osteoblasts that regulates proliferation and differentiation63,64. [Ca2+] also plays an integral role in the regulation of cytoskeletal changes65,66. This relationship suggests that variations in [Ca2+] may, in part, be regulating changes in osteoblast phenotype after microgravity exposure. A study by Sun et al. observed decreases in [Ca2+] in primary mouse osteoblasts after exposure to simulated microgravity by clinorotation65. They also observed a decrease in voltage-sensitive Ca2+ channel activity and a functional decrease in the expression of both ryanodine and inositol 1,4,5-triphosphate receptors, which mediate intracellular Ca2+ release65,67. Additionally, studies by Michaletti et al. have shown significant changes in osteoblastic mitochondrion homeostasis and proteomic pathways after exposure to simulated microgravity using RPM68. Specifically, glycolysis, the Kreb’s cycle, the pentose phosphate pathway, the glycerol-phosphate shuttle, as well the malate-aspartate shuttle, were all significantly reduced by microgravity. Additionally, typical mitochondrial activity appeared dysregulated after simulated microgravity by RPM, which the authors suggest is an adaptive response to ensure sufficient cell energy during microgravity68.
Gap junctions also play a role in the response of bone to its mechanical environment. Gap junctions are membrane-spanning channels, permeable to signaling molecules less than 1 kD in size, that allow cells to communicate with one another69. Connexin 43 (Cx43) is the predominant gap junction protein in bone. Compelling in vitro evidence from our lab, and several others suggests that gap junction intracellular communication (GJIC) contributes to osteoblast and osteocyte mechanotransduction in bone. For instance, mechanical signals regulate Cx43 levels and GJIC between bone cells70,71,72,73,74,75,76. GJIC sensitizes bone cell networks to diverse extracellular signals77,78,79, and mechanically-induced signals are communicated between bone cells via gap junctions80,81,82. More recently Gupta et al. demonstrated that Cx43 affects both Wnt-dependent and Wnt-independent activation of β-catenin in osteoblasts83, both of which play important roles in the response of bone to its mechanical environment. Furthermore, Xu et al. found that in vitro simulated microgravity by RPM affects osteocytic Cx43 levels and gap junction hemichannel activity in a rather complicated manner84. Taken together these findings suggest that osteoblast and osteocyte Cx43 plays a role in the response of bone to microgravity. This is strongly supported by in vivo data described in the next section.
In vivo studies of the effect of microgravity on osteoblast cell physiology
Osteoblasts show similar phenotypic changes in simulated microgravity in vivo as those observed during in vitro studies. In medaka fish exposed to microgravity aboard the ISS, a rapid and significant increase in osteoblastic genes sp7 and bglap were observed 1 day after launch and continued for up to 8 days85. However, osteoblastic genes sp7 and col1a1 were downregulated after 60 days of microgravity exposure, but not significantly86. This resulted in a decrease in osteoblastic activity and a decrease in collagen within the bone matrix, but this was not consistently observed. Overall, these fish did display a decrease in BMD, but this was linked to a significant increase in osteoclast activity, suggesting that while rapid increases in bone formation genes may occur upon microgravity exposure, they quickly return to or decrease below ground-control levels86. Conversely, a study conducted in 23-week-old male C57/BL6 mice flown for 30 days during the Bion-M1 mission observed no significant change in osteoblastic activity, despite observing significant (64%) bone volume loss in the trabecular bone of the femur and decreased mechanical properties87. This finding is not typical, as other reports, which used younger mice and rats, showed decreased osteoblastic activity37,87. This highlights the importance of using skeletally mature animals in future studies of microgravity-induced bone loss to limit confounding variables such as bone growth and maturation.
Osteoblastic apoptosis may also contribute to microgravity-induced bone loss. During 7 days of unloading via HLS, an increase in osteoblast apoptosis and a reduction in the total number of osteoblasts was observed by histological quantification as rapidly as 2 days after unloading began88. Further, a marginal decrease in integrin α5β1 and a nonsignificant decrease in FAK phosphorylation was observed 2 and 4 days after HLS, respectively88. In addition to increases in osteoblastic apoptosis during microgravity exposure, decreases in osteoblastic activity was also evidenced by a twofold decrease in mineralizing surface of the tibial bone after 28 days of HLS89.
Another mechanism for microgravity-induced bone loss that has been proposed is an increase in cell quiescence/senescence. For example, during a 15-day exposure to microgravity on the STS-131 shuttle, female C57BL/6 J mice exhibited a roughly 6% decrease in bone volume fraction and 11% decrease in bone thickness90. Osteoblasts expressed a roughly threefold increase in cyclin-dependent kinase inhibitor 1A (Cdkn1a/p21) gene expression, a mediator of cell cycle arrest, and a roughly 1.5-fold decrease in cell tumor antigen 53 (Trp53/p53) gene expression, an inducer of cell apoptosis90. Numerous other genes that regulate the cell cycle and cell apoptosis were also differentially regulated. Increases in F-box only protein-4 (Fbxo4) and F-box only protein-31 (Fbxo31), which stimulate cyclin-dependent protein-1 (Ccnd1) and thus the transition from G1 to S phase, were observed as well as decreases in death domain-containing protein (Cradd), death-associated protein kinase-1 (Dapk1), HECT, C2, and WW domain-containing E3 ubiquitin-protein ligase 2 (Hecw2), and mitogen-activated protein kinase-10 (Mapk10), which are all apoptosis-related genes90. These data suggest that microgravity disrupts the osteoblastic cell cycle and that this may be partially responsible for bone loss, which is typically observed during microgravity exposure.
Gap junctions and Cx43 also play an important role in bone adaptation to unloading as would occur in microgravity. Our lab demonstrated that in mice Cx43-deficient bone is less sensitive to HLS-induced unloading91,92, and this involves both arms of bone remodeling93, while Grimston et al. showed similar results for muscle paralysis-induced bone loss94. Taken together these findings suggest that Cx43 deficiency may be protective against microgravity-induced bone loss and that Cx43 may be a novel countermeasure target for the prevention of bone loss due to microgravity. However, as mounting evidence suggests that osteocytes are the primary mechanosensors in bone the focus has shifted to evaluating the role of osteocytic Cx43 in microgravity-induced bone loss, as discussed later in this review.
Osteotropic hormones also contribute to the effects of microgravity on bone. For instance, in humans, bone-specific alkaline phosphatase (BSAP), a marker of osteoblastic differentiation, significantly decreased in early spaceflight (<14 days into a mission) but recovered to preflight levels during the remainder of the mission (60–110 days)95. Parathyroid hormone (PTH), a positive regulator of the Wnt-signaling pathway, calcitonin, and OCN, both osteogenic promoters, were all reduced for the duration of the flight, but again recovered upon return to standard gravity after spaceflight95. Evidence from the NASA Twin Study has shown that protein levels of both OCN and BSAP measured in urine are markedly increased in the twin that experienced space flight (<120 days) compared to the ground-matched twin. These levels decrease with long-duration spaceflight until the subject returns to standard gravity, and in time levels return to basal conditions96. Unfortunately, bone mass measurements were not reported in this study, making it difficult to determine the overall outcome of the data presented. Despite this, these data do suggest that after exposure to microgravity, the body adapts to the reduction in mechanical load by increasing levels of osteotropic factors associated with bone formation in an attempt to slow bone catabolism, a change not always readily observed in animal models.
Osteocytes
Osteocytes are the most abundant bone cells, making up more than 90% of all bone cells6,97. Over the past few decades, evidence has emerged that osteocytes are the main mechanosensory cells in bone, although this concept is still developing6,98,99,100. Osteocytes are post-mitotic, terminally differentiated osteoblasts that have become encapsulated in mineralized bone matrix. Osteocytes express the genes encoding dentin matrix acidic phosphoprotein-1
(Dmp1), matrix extracellular phosphoglycoprotein (Mepe), and phosphate regulating endopeptidase homolog X-Linked (Phex) during early differentiation, which regulate proteins that aid in bone matrix formation and regulation. More mature osteocytes express genes encoding sclerostin (Sost) and fibroblast growth factor-23 (Fgf23), which regulate osteoblast activity and phosphate metabolism, respectively. Osteocytes have also been shown to be the predominant bone cell expressing genes encoding proteins for receptor activator of nuclear factor-kappa-Β ligand (RANKL) and osteoprotegerin (OPG) proteins, which are positive and negative regulators, respectively, of osteoclast formation97,101.
Each osteocyte has numerous dendritic processes that are connected to other osteocytes as well as osteoblasts and osteoclasts, thereby creating a vast communication network called the lacunar-canalicular system6,100. It is through this system that osteocytes are hypothesized to regulate bone homeostasis in response to bone loading or unloading, such as that seen during exposure to microgravity. As the main mechanosensory cells in bone, osteocytes can alter the bone formation and resorption depending on which proteins they secrete. Because of this, osteocytes have become a key focus in research evaluating methods to mitigate the bone loss caused by exposure to microgravity.
In vitro studies of the effect of microgravity on osteocyte cell physiology
In vitro examination of the effects of exposure to microgravity on osteocytes was enabled by the development of cell lines, including MLO-Y4 and OCY454, that possess characteristics of primary osteocytes102,103. These characteristics include dendritic morphology and expression of osteocyte-specific genes102,103. Two caveats that must be considered are that, under most experimental conditions, these cell lines are not encased in a three-dimensional mineral matrix. Additionally, these cells undergo proliferation in vitro whereas available evidence suggests that in vivo osteocytes do not proliferate. Despite these caveats, examination of OCY454 and MLO-Y4 cell lines has greatly enhanced our understanding of osteocyte biology
Studies using OCY454 have demonstrated that the expression of Sost and Tnsrf11, which encode RANKL, are upregulated and expression of the gene encoding OPG is downregulated after exposure to simulated microgravity using an RCCS103,104. These findings demonstrate that osteocytes directly respond to microgravity by expressing genes that promote factors that promote osteoclastic differentiation while simultaneously inhibiting osteoblastic differentiation. In addition to examining specific genes that are differentially regulated during exposure to microgravity, in vitro studies are better suited to determine the mechanisms by which these changes occur. For example, deletion of IGF-1 resulted in a decrease in the osteocytic response to mechanical stimuli105. Other in vitro studies have investigated the role of Cx43 in MLO-Y4 cells exposed to simulated microgravity. Previous work has shown Cx43 is necessary for Prostaglandin E2 (Pge2) release, a key hormone affecting osteoblast and osteoclast activity106. During exposure to microgravity by RPM, Cx43 deficient MLO-Y4 osteocytes show attenuated Pge2 release, altering bone metabolism84. This same study showed increased Cx43 hemichannel activity in response to simulated microgravity, implying increased intracellular communication.
In vivo studies of the effect of microgravity on osteocyte cell physiology
Most, if not all, in vivo studies that exposed animals to microgravity, observed a decrease in bone mass. For example, a study examining the iliac crests of monkeys that were exposed to microgravity for 2 weeks during the Bion-11 space mission found an increase in collagen protein biosynthesis in osteocytes107. This increase resulted in the bone not undergoing the same extent of mineralization that is normally seen under standard gravity. This decrease in mineralization, in combination with direct osteolytic bone resorptive activity, caused a decrease in bone density107. In general, exposure to microgravity induces osteocyte apoptosis. When bones exposed to microgravity are examined, they are found to have more empty osteocyte lacunae than bones not exposed to microgravity. Furthermore, both the canilicular canals and the lacunae are larger, and the lacunae more irregular in shape in mice that undergo spaceflight compared to ground control animals, indicating that osteocytic osteolysis is occurring to a greater degree in the bone that undergoes unloading90. It also indicates that osteocyte morphology is varied during exposure to microgravity. The increase in empty lacunae, as well as change in size and lacunar shape, is attributed to an increase in osteocyte apoptosis and bone resorption87,107. This loss of osteocyte viability may dramatically alter bone and osteocyte mechanosensation during spaceflight. Additionally, osteocyte apoptosis, which precedes osteoclast recruitment during exposure to microgravity, results in upregulation of RANKL in neighboring osteocytes leading to large increases in osteoclast recruitment108,109. This results in greater bone resorption and decreased bone mass108. Changes in bone resulting from exposure to microgravity, such as osteocytic cell death and variations in lacunar shape and density, show similarities with known hallmarks of aged bone. Therefore, it is becoming more widely accepted that exposure to microgravity induces phenotypic changes in bone that resemble bone aging under standard gravitational conditions87,110.
HLS studies have corroborated in vitro observations that the genes encoding sclerostin and RANKL are upregulated when the bone is exposed to microgravity93,111,112,113. These HLS studies have also shown that increases in bone RANKL and decreases in expression of Sost are in part due to increased osteocyte apoptosis. For example, apoptotic osteocytes have been shown to release adenosine triphosphate (ATP) via pannexin1 channels, causing neighboring osteocytes to upregulate RANKL expression114. Similarly, a mouse model of osteoblast and osteocyte-specific Cx43 deficiency, with increased osteocyte apoptosis, is less responsive to the catabolic effects of simulated microgravity exposure93,115. Evidence suggests that bones from these mice have less sclerostin-mediated suppression of bone formation and less cortical osteoclast activity during unloading93,115. This suppression of normal osteocyte-driven bone resorption in osteocyte and osteoblast specific Cx43-deficient bone during prolonged unloading shows that Cx43 is a potential target to help prevent bone loss caused by exposure to microgravity.
Osteoclasts
Osteoclasts, unlike osteocytes and osteoblasts are derived from HSCs116. Exposure to microgravity significantly modulates osteoclast number, viability, gene expression, and activity2. Under normal physiological conditions, osteoclasts resorb bone and suppress osteoblastogenesis through the secretion of specific factors. When exposed to unloading this effect is exacerbated, resulting in an increase in osteoclast activity and overall bone resorption while suppressing bone formation9,60,116. However, there are significant variations in osteoclast viability and function in response to microgravity, likely due to the different microgravity models and osteoclastic cell types used. Despite the significant bone loss observed in both in vitro and in vivo studies when osteoclasts are exposed to microgravity, a lack of understanding still exists regarding whether and how osteoclasts cell autonomously transduce changes in the mechanical environment and how this, in turn, regulates bone homeostasis.
In vitro studies of the effects of microgravity on osteoclast cell physiology
Bone loss resulting from microgravity exposure is closely tied to variations in osteoclast activity. Sambandam et al. observed ~3000 differentially regulated genes in RAW 264.7 cells exposed to simulated microgravity using an RCCS117. There was significant upregulation of key genes encoding osteoclastogenic factors, specifically numerous matrix metalloproteinases and Cathepsin K (Ctsk)117. Along with the observed variation in gene expression, an increase in overall RNA concentration was also observed118. These observations suggest that not only does direct exposure to microgravity induce changes in osteoclast gene expression but microgravity also potentially increases osteoclast number and protein production. Upregulation of these key osteoclastogenic genes has also been linked to greater osteoclast differentiation of osteoclast progenitor cells when exposed to microgravity. For example, Tamma et al. seeded murine bone marrow mononuclear macrophages onto a bonelike biomaterial118. After 10 days of microgravity aboard the Foton-M3, increases in osteoclast size, osteoclast number, and a number of multinucleated cells were observed, indicating that exposure to microgravity induced greater osteoclastic differentiation. Additionally, increases in collagen telopeptides present in the media suggested an increase in osteoclast activity118. This microgravity-induced increased osteoclast activity was corroborated in, RAW264.7 cells60. For example, RAW264.7 cells exposed to microgravity produced a significant increase in resorption pit number and size60. Collectively, these data suggest that exposure to microgravity increases osteoclastogenesis, but the direct mechanism by which a lack of mechanical load causes these phenotypic changes is still relatively unknown.
Exposure to microgravity also sensitizes osteoclast precursors to soluble factors. Sexana et al. observed that brief 24-h exposure to microgravity sensitized osteoclast precursors to RANKL, which stimulates osteoclastogenesis119. Subsequent treatment with RANKL protein caused the formation of giant multinucleated osteoclasts and an increase in mRNA expression of genes encoding triiodothyronine receptor auxiliary protein (Trap) and Ctsk, indicating increased osteoclast maturation. Further RANKL-independent activation of the nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), extracellular signal-regulated kinase (ERK), and mitogen-activated protein kinase-p38 (p38MAPK) protein pathways was also observed. However, osteoclastogenesis was only enhanced with the addition of supplemental RANKL119. This suggests that despite the increased sensitivity to soluble factors, osteoclasts alone cannot activate mechanisms associated with increased osteoclastogenesis, as they do not secrete sufficient amounts of RANKL protein. Drastic changes in cytoskeletal structure and remodeling also occur in osteoclasts exposed to microgravity60. Though it is not fully understood how cytoskeletal changes mediate osteoclastogenesis, a correlative link between the two has emerged over the past 10 years.
In vivo studies of the effects of microgravity on osteoclast cell physiology
Few studies have examined the effect exposure to microgravity has on osteoclastogenesis in vivo. The few studies that have corroborated the findings were found in vitro. Differential regulation of roughly 6000 genes in bone was observed in medaka fish aboard the ISS compared to ground control medaka fish86. Roughly 150 of those genes were differentially regulated between days 2 and 6 in fish aboard the ISS, indicating these genes are primarily involved in maintaining bone homeostasis in response to the mechanical loading environment86. In the same study, greater expression of tartrate-resistant acid phosphatase (TRAP+) cells in medaka fish exposed to microgravity suggests an increase in osteoclastogenesis as well, resulting in a significant decrease in mineral density of the pharyngeal bone and tooth region. In a different study, pre-osteoclastic cells isolated from C57/B6J female mouse femurs also showed an increase in differentiation potential after 15 days of spaceflight. This increase resulted in roughly 50% more multinucleated osteoclasts within the femur compared to femurs of ground control mice51. Osteoclastogenesis is in part regulated by RANKL, which is secreted by osteoblasts, which also secrete OPG, an inhibitor of RANKL. In a study by Lloyd et al., a single subcutaneous injection of OPG was given to C57BL/6J mice prior to 12 days of spaceflight120. This injection reduced bone loss from microgravity exposure to some degree. This was the result of a significant decrease in osteoclast number and a significant decrease in serum marker triiodothyronine receptor auxiliary protein-5 (TRAP5), a marker of osteoclastic activity120. However, the results of this study may not be definitive for multiple reasons. First, as noted by the authors, the mice used were not skeletally mature making it challenging to determine whether the effects observed were a result of microgravity or interference with the other cell populations responsible for growth, differentiation, and mineralization of the skeleton120. Second, the spaceflight was brief, and, as has been shown in other studies, osteoblastic and osteoclastic activity vary significantly within the first days of microgravity exposure86,119.
Numerous HLS studies have also shown a significant increase in osteoclast activity and number as well as a significant decrease in bone volume after 2–3 weeks of HLS52,109,119,121. The increased osteoclast activity results in an increased marrow cavity volume, decreased cortical thickness, increased trabecular porosity, and a weakening of bone mechanical properties51,122. This was further validated by Blaber et al., who reported female C57BL/6J mice flown aboard the STS-131 shuttle for 15 days had decreased bone volume fraction and diminished bone thickness90. Upon histological evaluation it was determined that a significant increase in TRAP+ osteoclast-covered bone surfaces was evident, suggesting that an increase in osteoclastic activity was, in part, responsible for the decrease in bone mass90.
Skeletal Muscle Cells
Skeletal muscle contractions, unlike cardiac or smooth muscle contractions, are voluntary and act to generate the force on the skeleton for movement and postural support. At the cellular level, skeletal muscle is made up of mature muscle fibers, or myofibrils. Myofibrils are multinucleated myoblasts and can be some of the largest cells in the body, upwards of 100 microns in diameter and 30 cm in length in the human leg123. Each skeletal myofiber is made up of smaller units called myofibrils, which consist of actin (thin) and myosin (thick) filaments that overlap in a repeating structure124. These repeating structures called sarcomeres, comprised of actin and myosin, make up the main functional unit of skeletal muscle. The sarcomeres contract when Ca2+ is released from the sarcoplasmic reticulum, activated by nerve impulses, facilitating actin-myosin cross bridging and contraction125.
Individual skeletal muscle fibers can vary greatly between and within skeletal sites. Myofibers are typically classified based on the identification of myosin isoforms by histological staining for specific myosin ATPases, myosin heavy chain (MHC) constituents, and metabolic enzymes. Although not extensively reviewed here, there are over seven currently recognized myofiber types, ranging from the slowest contractile isoform (Type I ATPase, MHC I, oxidative) to the fastest contractile isoform (Type IIB ATPase, MHC IIx, oxidative)126,127. Skeletal muscles are made up of a hybrid of these individual muscle fibers but are usually classified based on their predominant fiber type. For example, the soleus is considered a slow-twitch oxidative skeletal muscle whereas the gastrocnemius is primarily fast-twitch and glycolytic. These histological and metabolic differences are important to note because they can lead to profound variations in the skeletal tissue and cellular response to disuse, which will be highlighted in subsequent sections.
In vitro studies of the effect of microgravity on skeletal muscle cell physiology
In vitro studies have shed light on the molecular mechanisms in skeletal muscle cells regulated by direct exposure to microgravity. In vitro studies have the advantage of limiting the effects of systemic factors and allowing for examination of molecular mechanisms at the cellular level. Unfortunately, unlike in vivo studies, very few in vitro studies have been undertaken where isolated skeletal muscle cells are directly exposed to real microgravity conditions found during spaceflight. Several studies, although not recent, revealed severe and lasting phenotypic changes to skeletal muscle cells during short-term exposure to real microgravity (5–10 days). For example, primary rat L8 myoblasts, a nontumorigenic cell line, flown for 9 days aboard the STS-45 displayed an inability to fuse into myotubes, even upon returning to standard gravity (1 g)128. Another STS study, where avian skeletal muscle cells were seeded in tissue-engineered constructs, revealed a decrease in myotube cross-sectional area (−12%) and total protein synthesis (−75%) following 9 days of exposure to microgravity during spaceflight12. Interestingly these effects were augmented upon return to standard gravity after spaceflight, with concomitant increases in MHC and fibronectin protein. Finally, a more recent study by Uchida et al., showed that pre-differentiated L6 myoblasts cultured onboard the ISS for 10 days, had reduced myosin content (fast and slow), reduced myotube size, and increased oxidative stress compared to 1 g controls129. These important studies demonstrated that exposure to microgravity during spaceflight directly affects skeletal muscle cell differentiation and metabolism, suggesting skeletal muscle cells may be viable countermeasure targets129. However, the molecular mechanisms underlying these phenotypic changes remain poorly understood.
Ground-based analog studies examining skeletal muscle during simulated microgravity are more suited to study mechanisms of atrophy and have primarily used L6 rat wildtype130 or C2C12 dystrophic mouse131 myoblastic cell lines. Differentiation of these cells has been well characterized. For example, skeletal muscle cell differentiation, or myogenesis, from myoblasts requires the coordinated expression of four key muscle regulatory factors (MRFs). These include myogenic regulatory factor 5 (Myf5), myoblast determination protein 1 (MyoD), myogenin, and muscle regulatory factor 4 (Mrf4)132,133,134,135. For instance, Myf5 and MyoD are expressed in undifferentiated myoblasts and together are necessary for myoblast commitment136,137. Subsequently, myogenin and eventually MRF4 are highly expressed and aid in myoblast fusion and myotube formation138. One of the ways these MRFS are controlled is by exogenous signaling factors in the skeletal muscle microenvironment. For instance, exogenous IGF-1, which has been shown to cause skeletal muscle hypertrophy, upregulates myogenin via the PI3K/Akt signaling axis139. The P13K/AKT signaling axis has emerged as the predominant pathway controlling skeletal muscle metabolism. Reviewed extensively elsewhere140, in brief, the P13K/Akt pathway controls protein synthesis via mammalian target of rapamycin (mTOR) and glycogen synthase kinase 3β (GSK3β), and protein degradation via regulation of the transcription factors of the forkhead family of transcription factor (FOXO). Therefore, many recent ground-based in vitro studies have investigated the regulation of MRFs and P13K/Akt signaling components to understand how unloading directly affects skeletal muscle cell function.
Ground-based studies using a 3D clinostat, RPM, or RCCS have observed that exposure to simulated microgravity in C2C12 and L6 cells blocks myogenesis by decreasing MHC expression (fast and slow twitch), myotube thickness, and myotube fusion index141,142,143. These results suggest that simulated microgravity causes similar phenotypic changes in skeletal muscle as observed during spaceflight. Furthermore, these analog studies, due to the enhanced characterization available on earth, have suggested varying mechanisms for the decreased myogenesis. For example, Baek et al. observed that exposure to simulated microgravity decreases myogenesis by decreasing AKT phosphorylation and increasing phospholipase activity141. This resulted in the forkhead family of transcription factor O1 (FOXO1) stabilization and upregulation of genes associated with muscle degradation by apoptosis and autophagy141. This corroborates results by Harding et al. that showed exposure to simulated microgravity by RCCS increases expression of key skeletal muscle degradation pathways involving FOXO3 and muscle RING-finger protein-1 (MuRF1)144. Other results have demonstrated that simulated microgravity also impairs, via epigenetic changes, genes encoding downstream Akt targets, MyoD, and myogenin129,145. Overall, these results suggest that unloading directly affects the myogenic differentiation program by upregulating genes associated with degradation and downregulating genes associated with skeletal muscle differentiation and protein synthesis.
Another suggested mechanism by which exposure to microgravity may regulate skeletal muscle differentiation is through Ca2+ signaling. Studies have revealed that exposure to simulated microgravity via RPM decreases C2C12 myoblast [Ca2+]i, but there are multiple proposed mechanisms by which this occurs. Damm et al. suggested that this was due to a decrease in levels of transient receptor potential cation channel subfamily C member 1 (TRPC1), a nonspecific plasma membrane cation channel142. The authors also showed that pharmacological inhibition of TRPC1 mimicked simulated microgravity effects by decreasing myoblast proliferation and terminal differentiation. Conversely, Calzia et al. showed that simulated microgravity by RPM induced increases in myoblast reactive oxygen species accumulation and this can be alleviated by intracellular Ca2+ release via pharmacological activation of Ryanodine receptors143. Ryanodine receptors play a vital role in skeletal muscle cell contraction by promoting sarcoplasmic reticulum Ca2+ release. Overall, these results suggest that dysfunctional calcium handling and influx may be a potential cause of decreased myogenesis and skeletal muscle metabolism resulting from exposure to simulated microgravity. Thus, promoting skeletal muscle calcium influx during microgravity exposure may be a key countermeasure for alleviating spaceflight-induced skeletal muscle loss. Next, we will review how microgravity has been shown to affect in vivo skeletal muscle from various preclinical and clinical studies.
In vivo studies of the effect of microgravity on skeletal muscle
Multiple animal species have been used to study how microgravity affects skeletal muscle development and function during spaceflight, though rodents have emerged as the predominate preclinical model. Exposure to spaceflight microgravity in skeletally mature mice and rats results in a rapid loss of soleus and gastrocnemius muscle mass within 2 weeks146,147,148,149,150. This is especially evident in oxidative slow-twitch muscles such as the soleus, compared to glycolytic fast-twitch muscles such as the extensor digitorium longus. Accompanying this decline in soleus muscle mass is a preferential loss of slow-twitch type I fibers and a gain in fast-twitch IIB fibers. Recent data have suggested that this is because fast-twitch muscles, unlike slow-twitch, upregulate protective signaling factors such as IGF-1, and interleukin 6 (IL-6), as well as stress-related genes encoding nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and heat shock protein family A member 1 A (Hspa1a)146,147. Furthermore, these same studies show degradation factors such as FOXO1 and Atrogin-1, part of the previously mentioned PI3K-kinase/AKT/mTOR pathway, are upregulated in skeletal muscles exposed to spaceflight microgravity. Exposure of skeletal muscle to unloading using ground-based analogs results in similar changes tissue-level changes seen during spaceflight. For example, skeletal muscle mass is dramatically decreased (15–20%) within the first weeks following HLS or neurectomy resulting from increased myostatin expression and decreased protein synthesis via the PI3K-kinase/AKT/mTOR pathway (Fig. 3)52,151,152. Surprisingly, unilateral casting or botox treatment during HLS can further exacerbate skeletal muscle mass and autophagy in the gastrocnemius but not the soleus153,154. Similar murine studies have also found evidence that skeletal muscle atrophy in these models is accompanied by an increase in key muscle E3 ubiquitin ligases, such as atrogin-1 and MuRF1, which promotes skeletal muscle degradation155. However, MuRF1 knockout mice have demonstrated resistance to skeletal muscle atrophy induced by denervation or HLS but not exposure to real microgravity156,157,158. This suggests that muscle atrophy resulting from simulated microgravity using ground-based analogs and skeletal muscle atrophy resulting from exposure to spaceflight may occur by divergent mechanisms that produce a similar tissue level phenotype.
In humans, exposure to microgravity during spaceflight causes rapid and preferential skeletal muscle volumetric loss and decreases in strength, predominately in the lower limb and trunk muscles. This is likely due to the role of these skeletal muscles in ambulation and postural support under standard gravity. Studies have shown rapid volumetric skeletal muscle loss, as revealed by magnetic resonance imaging (MRI), occurs in the quadriceps (−6%), gastrocnemius (−6%), and posterior back muscles (−10%) following 6-9 days of spaceflight159,160,161. Studies investigating the effects of short-term spaceflight on skeletal muscle using biopsies have demonstrated a preferential loss of fiber cross-sectional area depending on fiber type. For instance, preferential decreases in type II (fast-twitch) versus type I (slow-twitch) fiber cross-sectional area were observed in the soleus and vastus lateralis muscles between pre- and post-flight time points (5–10 days)162,163. Longer duration spaceflight missions abroad the ISS (~6 months) have resulted in greater skeletal muscle loss in the quadriceps (−12%), soleus-gastrocnemius (−13%), and posterior back skeletal muscles (−20%) resulting in a 25–30% loss of calf muscle twitch force161,164,165. This suggests a correlative effect between increased exposure to microgravity and skeletal muscle atrophy, regardless of muscle type. Overall, these data suggest that skeletal muscle mass rapidly decreases before becoming relatively stable after 3–4 months of microgravity exposure and are the result of decreased protein synthesis (~45% decrease) rather than degradation161,166.
Integrative Physiology and Muscle-Bone Crosstalk
Microgravity and ground-based unloading models cause a host of systemic effects that can also directly affect the musculoskeletal system. These include immune cell dysfunction, cardiovascular deconditioning, neural/behavioral changes, lower limb hypoxia, and increases in endocrine factors such as glucocorticoid secretion96,149,167,168,169,170,171,172. Although beyond the scope of this review, it is important to note that these systemic changes also contribute to the negative health consequences of spaceflight and unloading on bone and skeletal muscle. Therefore, a more integrative physiological approach is necessary to combat this unique interplay between multiple organ systems. One way this is being realized is via the notion that direct biochemical cross-talk between bone and skeletal muscle is a major driver of these musculoskeletal changes, since these two distinct organs are in direct physical contact and share common developmental origins. This emerging concept has been referred to as muscle-bone crosstalk and support for this concept has been growing for the past decade. For example, factors produced by bone (osteokines) and regulated by unloading, such as Pge2 and RANKL, have anabolic and catabolic, respectively, effects on skeletal myogenesis, both in vivo and in vitro84,173,174,175. Conversely, many skeletal muscle factors (myokines) increased with unloading, such as IL-6 and myostatin, have detrimental effects on bone by supporting osteoclast formation and inhibiting osteoblast activity176,177,178. Furthermore, the myokine Irisin has been shown to alleviate bone and skeletal muscle loss resulting from HLS when given exogenously179,180. These results suggest that targeting shared biochemical pathways between skeletal muscle and bone may be efficacious in ameliorating musculoskeletal defects associated with unloading and spaceflight. However, these biochemical crosstalk mechanisms are complex, and progress has been hindered by the fact most researchers, until recently have not examined the response of bone and muscle as a unit to unloading. Potential therapies as well as their efficacies for augmenting skeletal muscle and bone loss are presented next.
Microgravity Countermeasure Development
Correcting the homeostatic imbalances resulting from exposure to microgravity in both bone and skeletal muscle remains an important area of research. Most pharmacological approaches target pathways specific to either bone or muscle tissue, while exercise and nutritional treatments target both tissues, as well as other distant organ systems.
Pharmacological treatments for bone loss resulting from exposure to microgravity are becoming more prevalent as clinical treatments for osteoporosis expand and our understanding of how bone maintains homeostasis improves181. Many of the countermeasure targets to augment bone loss due to osteoporosis and spaceflight have initially focused on limiting resorption. Bisphosphonates and anti-RANKL therapies (Denosaumab and OPG-Fc) work directly on bone by limiting the formation and action of bone-resorbing osteoclasts. These therapies currently remain a cost-effective and potent treatment for osteoporosis and have shown efficacy in studies of hindlimb unloading and spaceflight182. For example, treatment of mice with bisphosphonates during HLS preserved bone mass by limiting bone resorption and by transiently stimulating bone modeling183,184. In addition, growing female mice given a single treatment of OPG-Fc antibody (20 mg/kg), a RANKL inhibitor, displayed attenuated bone loss during 12 days of spaceflight aboard STS-108.120 Importantly, the use of similar antiresorptive agents in astronauts have yielded promising results when used during spaceflight. For instance, seven astronauts receiving weekly oral bisphosphonates (alendronate) and combined exercise treatments (treadmill, cycle ergometer, and a resistance exercise device (aRED)) displayed no bone loss, as assessed by bone densitometry (DXA) or quantitative computed tomography (QCT), whereas those who only exercised displayed bone loss.185. This suggests that short-term bisphosphonate treatment, at least with exercise, can protect astronauts from bone loss in space. Denosumab, the human equivalent to OPG-Fc and used to treat osteoporosis, has yet to be tested in astronauts186. More long-term studies (>6 months) with the use of these antiresorptive agents alone during spaceflight are needed to directly determine their long-term safety and efficacy for reducing bone fracture risk.
Anabolic agents, which primarily work to promote bone mass by stimulating osteoblast formation and activity, are also potential countermeasures for bone loss resulting from unloading and spaceflight. One of the primary pathways targeted by these therapies is the WNT/ß-catenin pathway. As reviewed extensively elsewhere187,188, substantial evidence suggests that WNT/ß-catenin pathway activation promotes osteoblast cell lineage differentiation and survival while indirectly inhibiting osteoclast activity. Thus, targeting WNT/ß-catenin could potentially mitigate bone loss during unloading and spaceflight. As mentioned earlier in this review, sclerostin, an inhibitor of WNT/ß-catenin signaling, is upregulated in bone cells after exposure to microgravity and elevated in the serum of individuals on extended bedrest2,96,103,189. Recently, a human sclerostin neutralizing antibody, Romosozumab, was approved for osteoporosis treatment190. Furthermore, this same biologic attenuated mouse bone loss resulting from HLS or spaceflight (STS-135)191,192. Interestingly, Spatz et al. showed that in mice undergoing HLS, neutralizing sclerostin antibody preserved hindlimb bone mass proportional to the amount of unloading in their partial weight-bearing model191. Furthermore, they showed that anti-sclerostin antibodies had no effects on preserving skeletal muscle mass. These results suggest that sclerostin antibody treatment should be combined with exercise in NASA astronauts for maximal effect. Although promising, Romosozumab has resulted in side effects, such as an increased risk of cardiovascular disease10, and therefore more long-term studies on its use on Earth and during spaceflight are needed.
Another promotor of WNT/ß-catenin that has shown promise in mitigating bone loss with unloading is neural EGF-Like Protein 1 (NELL-1). James et al. showed that recombinant NELL-1 (rhNELL-1) induces WNT/ß-catenin activation via the ß1 integrin in osteoblast precursors and osteoclasts in vitro and can prevent ovariectomy-induced bone loss in mice193. Follow-up work by this group showed that increasing the half-life and bone retention of rhNELL-1 by PEGylation and inactive bisphosphonate-linkage can improve bone fracture healing in ground control mice and mitigate bone loss in mice undergoing spaceflight194,195. Clinical evaluation, including long-term follow-up, of rhNELL-1 in osteoporotic patients on Earth, is needed before this treatment can be examined in astronauts.
Other bone loss therapies currently investigated during unloading and spaceflight include bone morphogenic proteins (BMP’s), diet, and exercise regimens. The most commonly used BMP, BMP-2, works by binding multiple transcription factors that upregulate genes such as Runx2 and the gene encoding cyclooxygenase-2 (Cox2)196,197. Additionally, BMPs activate the MAPK pathway and its downstream effectors ERK1/2 and p38198,199. Supplementation with BMPs in mouse models has also been shown to partially alleviate bone loss after exposure to microgravity, but their efficacy in humans is complicated by reports of ectopic bone formation and increased cancer risk200,201,202,203.
Exercise and nutritional therapies have shown only moderate protection from bone loss related to microgravity exposure, with improvements in training regimens and the equipment available to subjects playing a large role in the success of the therapy10,204,205. Astronauts aboard the ISS used the interim Resistive Exercise Device (iRED) until 2004, and now its newer higher loading counterpart (up to 600 pounds), the advanced Resistive Exercise Device (aRED) to perform resistive strength exercises206,207. Although the use of the aRED and adequate nutrition (Vitamin D) can boost bone formation indices to partially offset the enhanced resorption in space3, as mentioned previously, only use of an antiresorptive with exercise can effectively limit bone loss following 4–6 month ISS stays185. Limiting bone loss early and to a high degree is paramount since follow-up DXA and QCT show differential recovery of bone mass up to 1–2 years post-flight208,209. Importantly, long-term changes such as permanent loss of femoral trabecular bone volume fraction and thinner cortices cause irreversible changes to the bone that may affect long-term fracture risk upon return to Earth or during future spaceflight.
Skeletal muscle in astronauts undergoing spaceflight shows significant atrophy and strength deficits in just a few weeks. As mentioned earlier, this has been primarily due to unloading-induced decreases in protein synthesis. Protein synthesis and metabolism are positively controlled via the PI3K-Akt-mTOR pathway140,210,211. In this pathway, IGF-1 activates AKT phosphorylation and mTOR activation, thereby inducing skeletal muscle hypertrophy. In contrast, the soluble inhibitor myostatin, binds to activin receptor type 2, blocking mTOR activity thereby promoting skeletal muscle atrophy. Furthermore, the aforementioned work in this review has shown that real and simulated microgravity upregulate myostatin expression but decreases IGF-1 expression in skeletal muscle141,142,146,212. Therefore, exogenous IGF-1 and neutralizing antibodies targeting myostatin have emerged as viable countermeasure targets for mitigating skeletal muscle loss due to spaceflight.
Initially, IGF-1 overexpression in skeletal muscle by gene transfer or in transgenic mice showed promise in mitigating skeletal muscle degeneration and atrophy due to glucocorticoid use and injury in mice and rat models213,214. However, more recent results using similar approaches during immobilization by casting or HLS have yielded fewer promising results. For example, although overexpression of IGF-1 during murine development increased skeletal muscle size and contractile force at baseline, it failed to prevent significant atrophy with disuse213,215. The use of exogenous IGF-1 was also tested during 10 days of spaceflight in mice flown on STS-77216. Although IGF-1’s influence on spaceflight-induced skeletal muscle atrophy was not reported, it did increase femoral bone mass and strength by increasing periosteal bone apposition.
In contrast to IGF-1, recent results using myostatin inhibitors have yielded more consistent outcomes regarding skeletal muscle atrophy resulting from unloading and spaceflight. For example, weekly treatment of young mice with REGN1033 (a monoclonal antibody against myostatin) eliminated tibialis anterior and gastrocnemius muscle mass changes during casting and attenuated losses with hindlimb unloading217. Similar effects have been reported for skeletal muscle loss in mice and rats exposed to spaceflight. For instance, Smith et al. demonstrated that the use of myostatin inhibitor antibody (YN41), as part of the rodent research-3 mission, alleviated spaceflight-induced hindlimb skeletal muscle atrophy and blocked decreases in grip strength following 6 weeks aboard the ISS218. However, myostatin inhibition had no effect on decreases in trabecular BMD. On the other hand, a recent study has shown that using a dual systemic inhibitor of myostatin and activin A ligand signaling can rescue bone and skeletal muscle loss in wild-type mice following 33 days aboard the ISS.219. Lee et al. showed that using this broader ligand targeting approach, lean mass, hindlimb skeletal muscle mass, and femoral BMD were greater in wild-type mice undergoing 33 days of spaceflight compared to age-matched ground control animals. Importantly, age-matched myostatin knockout mice undergoing the same spaceflight regimen, while displaying skeletal muscle protection against spaceflight-induced atrophy, did not show protection against spaceflight-induced decreases in BMD. This suggests that the targeting of activin A and myostatin collectively is a more promising approach to protect against bone and muscle loss in response to spaceflight-induced microgravity.
Finally, increasing effort has been placed on examining the role of nutrient supplementation and resistive exercise in order to maintain skeletal muscle mass during exposure to microgravity. Unfortunately, the use of the iRED and aRED devices has not been able to ameliorate skeletal muscle mass and strength deficits during shorter and longer duration spaceflight164,181,220. In contrast, bedrest analog studies using amino acid supplementation and/or rehabilitative aerobic and resistive exercises similar to that used on the ISS, have shown the ability to prevent skeletal muscle loss due to lack of mechanical loading221,222,223,224. Skeletal muscle atrophy and functional impairments are more of a concern for ensuring that NASA crewmembers have optimal dexterity, metabolism, and strength capacity for performing in-flight or EVA-based tasks. This is because, upon return to earth, most skeletal muscles, except for some paraspinal muscles, return to their normal composition and size225,226.
It is likely that treatment with both pharmacological agents combined with exercise and nutritional therapy will best treat the catabolic and anabolic changes observed in bone and muscle tissue during exposure to microgravity. In addition, the synergy between the two tissue systems should not be ignored.
Conclusion
In the near future, NASA hopes to enable the return of humans to the moon and prepare for manned missions to Mars. For these missions to be successful, humans will need to be prepared for long-term space exploration and be equipped to handle deep space stressors, such as microgravity. As discussed in this review, exposure to microgravity causes severe negative health effects on the musculoskeletal system. Musculoskeletal tissue is heavily dependent and adaptive to its mechanical loading environment. The lack of mechanical loading of the musculoskeletal system that occurs during exposure to microgravity results in skeletal muscle atrophy and loss of bone mass, putting astronauts at risk for injury during spaceflight and upon normal gravitational reloading.
This review has detailed the current understanding of how exposure to microgravity effects the musculoskeletal system, but it has also revealed gaps in our current understanding that still need to be addressed. With recent evidence revealing the importance of bone and muscle crosstalk, future studies will need to focus on how exposure to microgravity affects the interaction of these tissue systems. Additionally, further interactions between the musculoskeletal system and the endocrine and nervous system need to be evaluated but the implications of microgravity exposure on these systems was not within the scope of this review. Moreover, how the musculoskeletal system responds to reloading after extended periods of exposure to microgravity is also of significant interest. There are still questions as to how much force the musculoskeletal system can generate after exposure to microgravity before sustaining injury. This information will also allow for more effective countermeasures to be developed, mitigating the catabolic effects microgravity exposure has on the musculoskeletal system. These avenues will provide greater insight into how exposure to microgravity affects the musculoskeletal system, its effects on musculoskeletal ability, and how we can counteract the negative effects.
Data availability
No original data was created for this manuscript. For data, please contact the authors of such data.
References
Nagaraja, M. P. & Risin, D. The current state of bone loss research: data from spaceflight and microgravity simulators. J. Cell. Biochem. 114, 1001–1008 (2013).
Grimm, D. et al. The impact of microgravity on bone in humans. Bone 87, 44–56 (2016).
Smith, S. M. et al. Bone metabolism and renal stone risk during international space station missions. Bone 81, 712–720 (2015).
Blaber, E., Marçal, H. & Burns, B. P. Bioastronautics: the influence of microgravity on astronaut health. Astrobiology 10, 463–473 (2010).
Tagliaferri, C., Wittrant, Y., Davicco, M. J., Walrand, S. & Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 21, 55–70 (2015).
Bonewald, L. F. The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 (2011).
Priemel, M. et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-Hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 25, 305–312 (2010).
Willey, J. S., Lloyd, S. A. J., Nelson, G. A. & Bateman, T. A. Space radiation and bone loss. Gravit. Space Biol. Bull. 25, 14–21 (2011).
Cazzaniga, A., Maier, J. A. M. & Castiglioni, S. Impact of simulated microgravity on human bone stem cells: new hints for space medicine. Biochem. Biophys. Res. Commun. 473, 181–186 (2016).
Orwoll, E. S. et al. Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA Bone Summit. J. Bone Miner. Res. 28, 1243–1255 (2013).
Booth, F. W. & Watson, P. A. Control of adaptations in protein levels in response to exercise. Fed. Proc. 44, 2293–2300 (1985).
Vandenburgh, H., Chromiak, J., Shansky, J., Del Tatto, M. & Lemaire, J. Space travel directly induces skeletal muscle atrophy. FASEB J. 13, 1031–1038 (1999).
Williams, D., Kuipers, A., Mukai, C. & Thirsk, R. Acclimation during space flight: effects on human physiology. CMAJ 180, 1317–1323 (2009).
Grimm, D. et al. Growing tissues in real and simulated microgravity: new methods for tissue engineering. Tissue Eng. Part B Rev. 20, 555–566 (2014).
Wuest, S. L., Richard, S., Kopp, S., Grimm, D. & Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. Biomed. Res. Int. 2015, 971474 (2015).
Goodwin, T. J., Milburn Jessup, J. & Wolf, D. A. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. Vitr. Cell. Dev. Biol. 28, 47–60 (1992).
Mitteregger, R., Vogt, G., Rossmanith, E. & Falkenhagen, D. Rotary cell culture system (RCCS): a new method for cultivating hepatocytes on microcarriers. Int J Artif Organs 22(12), 816–22 (1999). PMID: 10654878.
Schwarz, R. P., Goodwin, T. J. & Wolf, D. A. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J. Tissue Cult. Methods 14, 51–57 (1992).
Chowdhury, P., Long, A., Harris, G., Soulsby, M. E. & Dobretsov, M. Animal model of simulated microgravity: a comparative study of hindlimb unloading via tail versus pelvic suspension. Physiol. Rep. 1, 1–11 (2013).
Mortreux, M., Nagy, J. A., Ko, F. C., Bouxsein, M. L. & Rutkove, S. B. A novel partial gravity ground-based analog for rats via quadrupedal unloading. J. Appl. Physiol. 125, 175–182 (2018).
Mortreux, M., Ko, F. C., Riveros, D., Bouxsein, M. L. & Rutkove, S. B. Longitudinal time course of muscle impairments during partial weight-bearing in rats. npj Microgravity 5, 1–7 (2019).
Scott, J. M., Warburton, D. E. R., Williams, D., Whelan, S. & Krassioukov, A. Challenges, concerns and common problems: physiological consequences of spinal cord injury and microgravity. Spinal Cord. 49, 4–16 (2011).
Friedman, M. A., Zhang, Y., Wayne, J. S., Farber, C. R. & Donahue, H. J. Single limb immobilization model for bone loss from unloading. J. Biomech. 83, 181–189 (2019).
Arfat, Y. et al. Physiological effects of microgravity on bone cells. Calcif. Tissue Int. 94, 569–579 (2014).
Zwart, S. R. et al. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 40, 529–537 (2007).
Karmali, F. & Shelhamer, M. The dynamics of parabolic flight: flight characteristics and passenger percepts. Acta Astronaut. 63, 594–602 (2008).
Herranz, R. et al. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13, 1–17 (2013).
Ozcivici, E., Luu, Y. K., Rubin, C. T. & Judex, S. Low-Level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS ONE 5, e11178 (2010).
Özçivici, E. Effects of spaceflight on cells of bone marrow origin. Turk. J. Haematol. 30, 1–7 (2013).
Potier, E., Noailly, J. & Ito, K. Directing bone marrow-derived stromal cell function with mechanics. J. Biomech. 43, 807–817 (2010).
Zhang, C. et al. Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J 32, 4444–4458 (2018).
Basso, N., Bellows, C. G. & Heersche, J. N. M. Effect of simulated weightlessness on osteoprogenitor cell number and proliferation in young and adult rats. Bone 36, 173–183 (2005).
Adamo, L. et al. Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 (2009).
Davis, T. A. et al. Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 60, 69–76 (1996).
Ulbrich, C. et al. The impact of simulated and real microgravity on bone cells and mesenchymal stem cells. Biomed. Res. Int. 2014, 928507 (2014).
Zou, L. X. et al. Simulated microgravity induce apoptosis and down-regulation of erythropoietin receptor of UT-7/EPO cells. Adv. Sp. Res. 46, 1237–1244 (2010).
Basso, N., Jia, Y., Bellows, C. G. & Heersche, J. N. The effect of reloading on bone volume, osteoblast number, and osteoprogenitor characteristics: studies in hind limb unloaded rats. Bone 37, 370–378 (2005).
Gershovich, P. M., Gershovich, J. G., Zhambalova, A. P., Romanov, Y. A. & Buravkova, L. B. Cytoskeletal proteins and stem cell markers gene expression in human bone marrow mesenchymal stromal cells after different periods of simulated microgravity. Acta Astronaut. 70, 36–42 (2012).
Dai, Z. Q., Wang, R., Ling, S. K., Wan, Y. M. & Li, Y. H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 40, 671–684 (2007).
Gershovich, J. G. & Buravkova, L. B. Morphofunctional status and osteogenic differentiation potential of human mesenchymal stromal precursor cells during in vitro modeling of microgravity effects. Bull. Exp. Biol. Med. 144, 608–613 (2007).
Buravkova, L. B. et al. Cultured stem cells are sensitive to gravity changes. Acta Astronaut. 63, 603–608 (2008).
Huang, Y. et al. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J. Biomed. Sci. 16, 1–14 (2009).
Merzlikina, N. V., Buravkova, L. B. & Romanov, Y. A. The primary effects of clinorotation on cultured human mesenchymal stem cells. J. Gravit. Physiol. 11, P193–4 (2004).
Kulterer, B. et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. https://doi.org/10.1186/1471-2164-8-70 (2007).
Zayzafoon, M., Gathings, W. E. & McDonald, J. M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 145, 2421–2432 (2004).
Meyers, V. E., Zayzafoon, M., Gonda, S. R., Gathings, W. E. & McDonald, J. M. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 93, 697–707 (2004).
Touchstone, H. et al. Recovery of stem cell proliferation by low intensity vibration under simulated microgravity requires LINC complex. npj Microgravity 5, 11 (2019).
De Santo, N. G. et al. Anemia and erythropoietin in space flights. Semin. Nephrol. 25, 379–387 (2005).
Serova, L. V., Chelnaia, N. A. & Ivanova, S. I. [Comparative analysis of weightlessness and hypergravity effects on erythropoiesis in male and female mammals]. Aviakosm. Ekol. Med. 27, 54–59 (1993).
Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009).
Blaber, E. A. et al. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 13, 181–201 (2014).
Pan, Z. et al. Effects of hindlimb unloading on ex vivo growth and osteogenic/adipogenic potentials of bone marrow-derived mesenchymal stem cells in rats. Stem Cells Dev. 17, 795–804 (2008).
Rutkovskiy, A., Stensløkken, K.-O. & Vaage, I. J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 22, 95–106 (2016).
Pardo, S. J. et al. Simulated microgravity using the random positioning machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am. J. Physiol. Cell Physiol. 288, C1211–C1221 (2005).
Carmeliet, G., Nys, G. & Bouillon, R. Microgravity reduces the differentiation of human osteoblastic MG-63 cells. J. Bone Miner. Res. 12, 786–794 (1997).
Hu, L. et al. Response and adaptation of bone cells to simulated microgravity. Acta Astronaut. 104, 396–408 (2014).
Xu, L. H., Shao, H., Ma, Y. V. H. V. & You, L. OCY454 osteocytes as an in vitro cell model for bone remodeling under mechanical loading. J. Orthop. Res. 37, 1681–1689 (2019).
Prowse, P. D. H., Elliott, C. G., Hutter, J. & Hamilton, D. W. Inhibition of Rac and ROCK signalling influence osteoblast adhesion, differentiation and mineralization on titanium topographies. PLoS ONE 8, e58898 (2013).
Shafrir, Y. & Forgacs, G. Mechanotransduction through the cytoskeleton. Am. J. Physiol. Cell Physiol. 282, C479–86 (2002).
Nabavi, N., Khandani, A., Camirand, A. & Harrison, R. E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 49, 965–974 (2011).
Bucaro, M. A. et al. The effect of simulated microgravity on osteoblasts is independent of the induction of apoptosis. J. Cell. Biochem. 102, 483–495 (2007).
Bucaro, M. A. et al. Bone cell survival in microgravity evidence that modeled microgravity increases osteoblast sensitivity to apoptogens. Ann. N. Y. Acad. Sci. 1027, 64–73 (2004).
Tong, J. et al. Pulsed electromagnetic fields promote the proliferation and differentiation of osteoblasts by reinforcing intracellular calcium transients. Bioelectromagnetics 38, 541–549 (2017).
Hu, F. et al. Elevation of extracellular ca2+ induces store-operated calcium entry via calcium-sensing receptors: a pathway contributes to the proliferation of osteoblasts. PLoS ONE 9, e107217 (2014).
Sun, Z. et al. Simulated microgravity inhibits L-type calcium channel currents partially by the up-regulation of miR-103 in MC3T3-E1 osteoblasts. Sci. Rep. 5, 8077 (2015).
Morrell, A. E. et al. Mechanically induced Ca2+ oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 6, 6 (2018).
Sun, Z. et al. Simulated microgravity reduces intracellular‐free calcium concentration by inhibiting calcium channels in primary mouse osteoblasts. J. Cell. Biochem. 120, 4009–4020 (2019).
Michaletti, A., Gioia, M., Tarantino, U. & Zolla, L. Effects of microgravity on osteoblast mitochondria: a proteomic and metabolomics profile. Sci. Rep. 7, 1–12 (2017).
Donahue, H. J., Qu, R. W. & Genetos, D. C. Joint diseases: from connexins to gap junctions. Nat. Rev. Rheumatol. 14, 42–51 (2017).
Alford, A. I., Jacobs, C. R. & Donahue, H. J. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone 33, 64–70 (2003).
Ziambaras, K., Lecanda, F., Steinberg, T. H. & Civitelli, R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J. Bone Miner. Res. 13, 218–228 (1998).
Cheng, B. et al. PGE2 is essential for gap junction-mediated Intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology 142, 3464–3473 (2001).
Cheng, B. et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J. Bone Miner. Res. 16, 249–259 (2001).
Cherian, P. P. et al. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J. Biol. Chem. 278, 43146–43156 (2003).
Li, X. et al. Connexin 43 is a potential regulator in fluid shear stress-induced signal transduction in osteocytes. J. Orthop. Res. 31, 1959–1965 (2013).
Thi, M. M., Kojima, T., Cowin, S. C., Weinbaum, S. & Spray, D. C. Fluid shear stress remodels expression and function of junctional proteins in cultured bone cells. Am. J. Physiol. Cell Physiol. 284, C389–403 (2003).
Chung, D. J. et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J. Cell Sci. 119, 4187–4198 (2006).
Saunders, M. M. et al. Gap junctions and fluid flow response in MC3T3-E1 cells. Am. J. Physiol. Cell Physiol. 281, C1917–C1925 (2001).
Saunders, M. M. et al. Fluid flow-induced prostaglandin E2 response of osteoblastic ROS 17/2.8 cells is gap junction-mediated and independent of cytosolic calcium. Bone 32, 350–356 (2003).
Yellowley, C. E., Li, Z., Zhou, Z., Jacobs, C. R. & Donahue, H. J. Functional gap junctions between osteocytic and osteoblastic cells. J. Bone Miner. Res. 15, 209–217 (2010).
Taylor, A. F. et al. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am. J. Physiol. Cell Physiol. 292, 545–552 (2007).
Jørgensen, N. R. et al. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J. Bone Miner. Res. 15, 1024–1032 (2000).
Gupta, A. et al. Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell. Signal. 28, 1048–1057 (2016).
Xu, H. et al. Biological responses of osteocytic connexin 43 hemichannels to simulated microgravity. J. Orthop. Res. 35, 1195–1202 (2017).
Chatani, M. et al. Acute transcriptional up-regulation specific to osteoblasts/osteoclasts in medaka fish immediately after exposure to microgravity. Sci. Rep. 6, 39545 (2016).
Chatani, M. Microgravity promotes osteoclast activity in medaka fish reared at the international space station. Sci. Rep. 5, 14172 (2017).
Gerbaix, M. et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci. Rep. 7, 2659 (2017).
Dufour, C., Holy, X. & Marie, P. J. Skeletal unloading induces osteoblast apoptosis and targets α5β1-PI3K-Bcl-2 signaling in rat bone. Exp. Cell Res. 313, 394–403 (2007).
Allen, M. R. & Bloomfield, S. A. Hindlimb unloading has a greater effect on cortical compared with cancellous bone in mature female rats. J. Appl. Physiol. 94, 642–650 (2003).
Blaber, E. A. et al. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS ONE 8, 61372 (2013).
Lloyd, S. A., Loiselle, A. E., Zhang, Y. & Donahue, H. J. Evidence for the role of connexin 43-mediated intercellular communication in the process of intracortical bone resorption via osteocytic osteolysis. BMC Musculoskelet. Disord. 15, 122 (2014).
Lloyd, S. A., Lewis, G. S., Zhang, Y., Paul, E. M. & Donahue, H. J. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J. Bone Miner. Res. 27, 2359–2372 (2012).
Lloyd, S. A., Loiselle, A. E., Zhang, Y. & Donahue, H. J. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone 57, 76–83 (2013).
Grimston, S. K. et al. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J. Bone Miner. Res. 26, 2151–2160 (2011).
Smith, S. M. et al. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir Space Station. J. Bone Miner. Res. 20, 208–218 (2005).
Garrett-Bakelman, F. E. The NASA Twins Study: a multidimensional analysis of a year-long human spaceflight. Science https://doi.org/10.1126/science.aau8650 (2019).
Bonewald, L. F. & Johnson, M. L. Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 (2008).
Cowin, S. C. On mechanosensation in bone under microgravity. Bone 22, 119S–125S (1998).
Webster, D. J., Schneider, P., Dallas, S. L. & Müller, R. Studying osteocytes within their environment. Bone https://doi.org/10.1016/j.bone.2013.01.004 (2012).
Main, R. P. Osteocytes and the bone lacunar-canalicular system: insights into bone biology and skeletal function using bone tissue microstructure. Int. J. Paleopathol. https://doi.org/10.1016/j.ijpp.2017.05.002 (2017).
Xiong, J. & O’Brien, C. A. Osteocyte RANKL: new insights into the control of bone remodeling. J. Bone Miner. Res. 27, 499–505 (2012).
Bonewald, L. F. Establishment and characterization of an osteocyte-like cell line, MLO- Y4. J. Bone Miner. Metab. 17, 61–65 (1999).
Spatz, J. M. et al. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J. Biol. Chem. 290, 16744–16758 (2015).
Goyden, J. et al. The effect of OSM on MC3T3-E1 osteoblastic cells in simulated microgravity with radiation. PLoS ONE 10, e0127230 (2015).
Tian, F., Wang, Y. & Bikle, D. D. IGF-1 signaling mediated cell-specific skeletal mechano-transduction HHS Public Access. J. Orthop. Res 36, 576–583 (2018).
Cherian, P. P. et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell 16, 3100–3106 (2005).
Rodionova, N. V., Oganov, V. S. & Zolotova, N. V. Ultrastructural changes in osteocytes in microgravity conditions. Adv. Sp. Res. 30, 765–770 (2002).
Cabahug-Zuckerman, P. et al. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J. Bone Miner. Res. 31, 1356–1365 (2016).
Aguirre, J. I. et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21, 605–615 (2006).
Hemmatian, H., Bakker, A. D., Klein-Nulend, J. & van Lenthe, G. H. Aging, osteocytes, and mechanotransduction. Curr. Osteoporos. Rep. 15, 401–411 (2017).
Robling, A. G. et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 (2008).
Kennedy, O. D., Laudier, D. M., Majeska, R. J., Sun, H. B. & Schaffler, M. B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 64, 132–137 (2014).
Moriishi, T., Fukuyama, R., Ito, M., Miyazaki, T. & Maeno, T. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS ONE 7, 40143 (2012).
McCutcheon, S., Majeska, R. J., Spray, D. C., Schaffler, M. B. & Vazquez, M. Apoptotic osteocytes induce RANKL production in bystanders via purinergic signaling and activation of pannexin channels. J. Bone Miner. Res 35, 966–977 (2020).
Loiselle, A. E., Paul, E. M., Lewis, G. S. & Donahue, H. J. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J. Orthop. Res. 31, 147–154 (2013).
Ikeda, K. & Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 159, 1–8 (2016).
Sambandam, Y. et al. Microarray profile of gene expression during osteoclast differentiation in modelled microgravity. J. Cell. Biochem. 111, 1179–1187 (2010).
Tamma, R. et al. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 23, 2549–2554 (2009).
Saxena, R., Pan, G., Dohm, E. D. & McDonald, J. M. Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J. Bone Miner. Metab. 29, 111–122 (2011).
Lloyd, S. A. et al. Osteoprotegerin is an effective countermeasure for spaceflight-induced bone loss in mice. Bone 81, 562–572 (2015).
Dohke, T. et al. Regional osteoporosis due to osteoclast activation as a trigger for the pain-like behaviors in tail-suspended mice. J. Orthop. Res. 35, 1226–1236 (2017).
Lloyd, S. A. et al. Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J. Bone Miner. Res. 29, 1118–1130 (2014).
Biga, L. M. et al. Anatomy and Physiology (OpenStax/Oregon State University, 2017).
Frontera, W. R. & Ochala, J. Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int. 96, 183–195 (2015).
MacIntosh, B., Gardniner, P. & McComas, A. Skeletal Muscle: Form and Function (Human Kinetics, 2006).
Pette, D. & Staron, R. S. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 115, 359–372 (2001).
Scott, W., Stevens, J. & Binder-Macleod, S. A. Human skeletal muscle fiber type classifications. Phys. Ther. 81, 1810–1816 (2001).
Kulesh, D. A. et al. Space shuttle flight (STS-45) of L8 myoblast cells results in the isolation of a nonfusing cell line variant. J. Cell Biochem. 55, 530–544 (1994).
Uchida, T. et al. Reactive oxygen species upregulate expression of muscle atrophy-associated ubiquitin ligase Cbl-b in rat L6 skeletal muscle cells. Am. J. Physiol. Cell Physiol. 314, C721–C731 (2018).
Yaffe, D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl Acad. Sci. USA 61, 477–483 (1968).
Yaffe, D. & Saxel, O. R. A. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727 (1977).
Braun, T., Buschhausen-Denker, G., Bober, E., Tannich, E. & Arnold, H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 8, 701–709 (1989).
Braun, T., Bober, E., Winter, B., Rosenthal, N. & Arnold, H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 9, 821–831 (1990).
Pinney, D. F., Pearson-White, S. H., Konieczny, S. F., Latham, K. E. & Emerson, C. P. Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell 53, 781–793 (1988).
Wright, W. E., Sassoon, D. A. & Lin, V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56, 607–617 (1989).
Dedieu, S., Mazères, G., Cottin, P. & Brustis, J. J. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int. J. Dev. Biol. 46, 235–241 (2002).
Rudnicki, M. A. et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351–1359 (1993).
Bentzinger, C. F., Wang, Y. X. & Rudnicki, M. A. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect. Biol. 4, a008342 (2012).
Xu, Q. & Wu, Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J. Biol. Chem. 275, 36750–36757 (2000).
Schiaffino, S. & Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 1, 4 (2011).
Baek, M. O. et al. Simulated microgravity inhibits C2C12 myogenesis via phospholipase D2-induced Akt/FOXO1 regulation. Sci. Rep. 9, 14910 (2019).
Damm, T. B. et al. Calcium‐dependent deceleration of the cell cycle in muscle cells by simulated microgravity. FASEB J. 27, 2045–2054 (2013).
Calzia, D. et al. Characterization of C2C12 cells in simulated microgravity: possible use for myoblast regeneration. J. Cell. Physiol. 235, 3508–3518 (2020).
Harding, C. P. & Vargis, E. Muscle atrophy marker expression differs between rotary cell culture system and animal studies. Biomed. Res. Int. 2019, 2042808 (2019).
Furukawa, T. et al. Simulated microgravity attenuates myogenic differentiation via epigenetic regulations. NPJ Microgravity 4, 11 (2018).
Allen, D. L. et al. Effects of spaceflight on murine skeletal muscle gene expression. 106, 582–595 (2009).
Sandonà, D. et al. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS ONE 7, e33232 (2012).
Ohira, Y. et al. Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J. Appl. Physiol. 73, 51S–57S (1992).
Sung, M. et al. Spaceflight and hind limb unloading induce similar changes in electrical impedance characteristics of mouse gastrocnemius muscle. J. Musculoskelet. Neuronal Interact. 13, 405–411 (2013).
Harrison, B. C. et al. Skeletal muscle adaptations to microgravity exposure in the mouse. J. Appl Physiol. 95, 2462–2470 (2003).
Augusto, V., Padovani, C. R. & Rocha Campos, G. E. Skeletal muscle fiber types in C57Bl6J mice. Braz. J. Morphol. Sci. 21, 89–94 (2004).
Zhang, D., Liu, M., Ding, F. & Gu, X. Expression of myostatin RNA transcript and protein in gastrocnemius muscle of rats after sciatic nerve resection. J. Muscle Res. Cell Motil. 27, 37–44 (2006).
Speacht, T. L., Krause, A. R., Steiner, J. L., Lang, C. H. & Donahue, H. J. Combination of hindlimb suspension and immobilization by casting exaggerates sarcopenia by stimulating autophagy but does not worsen osteopenia. Bone 110, 29–37 (2018).
Ellman, R. et al. Combined effects of botulinum toxin injection and hindlimb unloading on bone and muscle. Calcif. Tissue Int. 94, 327 (2014).
Foletta, V. C., White, L. J., Larsen, A. E., Leger, B. & Russell, A. P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflug. Arch. 461, 325–335 (2011).
Cadena, S. M. et al. Skeletal muscle in MuRF1 null mice is not spared in low-gravity conditions, indicating atrophy proceeds by unique mechanisms in space. Sci. Rep. 9, 9397 (2019).
Labeit, S. et al. Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J. Biomed. Biotechnol. 2010, 693741 (2010).
Bodine, S. C. et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 (2001).
LeBlanc, A. et al. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. Neuronal Interact. 1, 157–160 (2000).
Akima, H. et al. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci. Sport. Exerc. 32, 1743–1747 (2000).
LeBlanc, A. et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 89, 2158–2164 (2000).
Edgerton, V. R. et al. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J. Appl. Physiol. 78, 1733–1739 (1995).
Widrick, J. J. et al. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J. Physiol. 516, 915–930 (1999).
Trappe, S. et al. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 106, 1159–1168 (2009).
Fitts, R. H. et al. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 588, 3567–3592 (2010).
Stein, T. P., Leskiw, M. J., Schluter, M. D., Donaldson, M. R. & Larina, I. Protein kinetics during and after long-duration spaceflight on MIR. Am. J. Physiol. Metab. 276, E1014–E1021 (1999).
Prisby, R. D., Behnke, B. J., Allen, M. R. & Delp, M. D. Effects of skeletal unloading on the vasomotor properties of the rat femur principal nutrient artery. J. Appl. Physiol. 118, 980–988 (2015).
J, Yang. et al. Blocking glucocorticoid signaling in osteoblasts and osteocytes prevents mechanical unloading-induced cortical bone loss. Bone 130, 115108 (2020).
Lescale, C. et al. Hind limb unloading, a model of spaceflight conditions, leads to decreased B lymphopoiesis similar to aging. FASEB J. 29, 455–463 (2015).
Moffitt, J. A., Henry, M. K., Welliver, K. C., Jepson, A. J. & Garnett, E. R. Hindlimb unloading results in increased predisposition to cardiac arrhythmias and alters left ventricular connexin 43 expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R362 (2013).
Norsk, P., Asmar, A., Damgaard, M. & Christensen, N. J. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J. Physiol. 593, 573 (2015).
Ronca, A. E. et al. Behavior of mice aboard the International Space Station. Sci. Rep. 9, 1–14 (2019).
Tominari, T. et al. Hypergravity and microgravity exhibited reversal effects on the bone and muscle mass in mice. Sci. Rep. 9, 1–10 (2019).
Bonnet, N., Bourgoin, L., Biver, E., Douni, E. & Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Invest. 129, 3214–3223 (2019).
Mo, C. et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle 14, 1507–1516 (2015).
Wu, Q., Zhou, X., Huang, D., JI, Y. & Kang, F. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell. Physiol. Biochem. 41, 1360–1369 (2017).
Qin, Y. et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J. Biol. Chem. 292, 11021–11033 (2017).
Axmann, R. et al. Inhibition of interleukin‐6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 60, 2747–2756 (2009).
Colaianni, G. et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 7, 1–16 (2017).
Colaianni, G. et al. The myokine irisin increases cortical bone mass. Proc. Natl Acad. Sci. USA 112, 12157–12162 (2015).
Lang, T. et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 3, 8 (2017).
Tosteson, A. N. A., Burge, R. T., Marshall, D. A. & Lindsay, R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am. J. Manag. Care 14, 605–615 (2008).
Zhao, Y. et al. Effects of hindlimb unloading and bisphosphonates on the serum proteome of rats. Bone 41, 646–658 (2007).
Barou, O. et al. Bisphosphonate effects in rat unloaded hindlimb bone loss model: three-dimensional microcomputed tomographic, histomorphometric, and densitometric analyses. J. Pharmacol. Exp. Ther. 291, 321–328 (1999).
Leblanc, A. et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 24, 2105–2114 (2013).
Brown, J. P. et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J. Bone Miner. Res. 24, 153–161 (2009).
Glass, D. A. & Karsenty, G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr. Top. Dev. Biol. 73, 43–84 (2006).
Duan, P. & Bonewald, L. F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 77, 23–29 (2016).
Spatz, J. M. et al. Serum sclerostin increases in healthy adult men during bed rest. J. Clin. Endocrinol. Metab. 97, E1736 (2012).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016).
Spatz, J. M. et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J. Bone Miner. Res. 28, 865–874 (2013).
Macnabb, C., Patton, D. & Hayes, J. S. Sclerostin antibody therapy for the treatment of osteoporosis: clinical prospects and challenges. J. Osteoporos. 2016, 6217286 (2016).
James, A. W. et al. NELL-1 in the treatment of osteoporotic bone loss. Nat. Commun. 6, 7362 (2015).
Shi, J. Systemic Therapy of Inactivated-Bisphosphonate-Conjugated PEGylated NELL-1 (BP-NELL-PEG) for Spaceflight-Induced Osteoporosis (University of California, 2019.
Tanjaya, J. et al. The effects of systemic therapy of PEGylated NEL-Like protein 1 (NELL-1) on fracture healing in mice. Am. J. Pathol. 188, 715–727 (2018).
Jang, W. G. et al. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 287, 905–915 (2012).
Susperregui, A. R. G. et al. Noncanonical BMP signaling regulates cyclooxygenase-2 transcription. Mol. Endocrinol. 25, 1006–1017 (2011).
Cao, X. & Chen, D. The BMP signaling and in vivo bone formation. Gene 357, 1–8 (2005).
Reilly, G. C., Golden, E. B., Grasso-Knight, G. & Leboy, P. S. Differential effects of ERK and p38 signaling in BMP-2 stimulated hypertrophy of cultured chick sternal chondrocytes. Cell Commun. Signal. 3, 3 (2005).
Zerath, E. et al. Effects of BMP-2 on osteoblastic cells and on skeletal growth and bone formation in unloaded rats. Growth Horm. IGF Res. 8, 141–149 (1998).
Ko, F. C. et al. Treatment with a soluble bone morphogenetic protein type 1A receptor (BMPR1A) fusion protein increases bone mass and bone formation in mice subjected to hindlimb unloading. JBMR 1, 66–72 (2017).
Carragee, E. J. et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J. Bone Joint Surg. 95, 1537–1545 (2013).
Rihn, J. A. et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 9, 623–629 (2009).
Smith, S. M. et al. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J. Bone Miner. Res. 27, 1896–1906 (2012).
Sibonga, J. et al. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 128, 112037 (2019).
Loehr, J. A. et al. Musculoskeletal adaptations to training with the advanced resistive exercise device. Med. Sci. Sport. Exerc. 43, 146–156 (2011).
Schneider, S. M. et al. Training with the International Space Station interim resistive exercise device. Med. Sci. Sport. Exerc. 35, 1935–1945 (2003).
Lang, T. F., Leblanc, A. D., Evans, H. J. & Lu, Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J. Bone Miner. Res. 21, 1224–1230 (2006).
Dana Carpenter, R., LeBlanc, A. D., Evans, H., Sibonga, J. D. & Lang, T. F. Long-term changes in the density and structure of the human hip and spine after long-duration spaceflight. Acta Astronaut. 67, 71–81 (2010).
Gao, Y., Arfat, Y., Wang, H. & Goswami, N. Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front. Physiol. 9, 235 (2018).
Elkina, Y., von Haehling, S., Anker, S. D. & Springer, J. The role of myostatin in muscle wasting: an overview. J. Cachexia. Sarcopenia Muscle 2, 143 (2011).
Lalani, R. et al. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J. Endocrinol. 167, 417–428 (2000).
Stevens-Lapsley, J. E. et al. Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am. J. Physiol. Endocrinol. Metab. 299, E730–E740 (2010).
Schakman, O. et al. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 146, 1789–1797 (2005).
Pierno, S. et al. Paracrine effects of IGF-1 overexpression on the functional decline due to skeletal muscle disuse: molecular and functional evaluation in hindlimb unloaded MLC/mIgf-1 transgenic mice. PLoS ONE 8, e65167 (2013).
Bateman, T. A. et al. Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor-I on rat long bones. Bone 23, 527–535 (1998).
Latres, E. et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice.Skelet. Muscle 5, 34 (2015).
Smith, R. C. et al. Inhibition of myostatin prevents microgravity-induced loss of skeletal muscle mass and strength. PLoS ONE 15, e0230818 (2020).
Lee, S. J. et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl Acad. Sci. USA 117, 23942–23951 (2020).
English, K., Lee, S., Loehr, J., Ploutz–Snyder, R. & Ploutz–Snyder, L. Isokinetic strength changes following long-duration spaceflight on the ISS. Aerosp. Med. Hum. Perform. 86, 68–77 (2015).
English, K. L. et al. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am. J. Clin. Nutr. 103, 465–473 (2016).
Paddon-Jones, D. et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J. Clin. Endocrinol. Metab. 89, 4351–4358 (2004).
Konda, N. N. et al. A comparison of exercise interventions from bed rest studies for the prevention of musculoskeletal loss. NPJ Microgravity 5, 12 (2019).
Ploutz-Snyder, L. L. et al. Integrated resistance and aerobic exercise protects fitness during bed rest. Med Sci. Sport. Exerc. 46, 358–368 (2014).
Rittweger, J. et al. Sarcolab pilot study into skeletal muscle’s adaptation to longterm spaceflight. NPJ Microgravity 4, 1–9 (2018).
Burkhart, K., Allaire, B. & Bouxsein, M. L. Negative effects of long-duration spaceflight on paraspinal muscle morphology. Spine 44, 879–886 (2019).
Acknowledgements
Drs. Friedman and Buettmann were supported by the Translational Research Institute through Cooperative Agreement NNX16AO69A. Dr. Donahue’s work was supported by NIH (NIAMS) grant R01 AR068132-20, NASA grant 80NSSC18K1473, and National Space Biological Research Institute grant NSBRI/NASA, MA02802.
Author information
Authors and Affiliations
Contributions
O.J.J. contributed to the conception, writing, production, editing, and accountability of the manuscript. E.G.B., M.A.F., R.C.D., and G.A.H. contributed to the conception, writing, editing, and accountability of the manuscript. H.J.D. contributed to the conception, editing, approval, and accountability of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juhl, O.J., Buettmann, E.G., Friedman, M.A. et al. Update on the effects of microgravity on the musculoskeletal system. npj Microgravity 7, 28 (2021). https://doi.org/10.1038/s41526-021-00158-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-021-00158-4
This article is cited by
-
Modeled microgravity unravels the roles of mechanical forces in renal progenitor cell physiology
Stem Cell Research & Therapy (2024)
-
Muscle stiffness indicating mission crew health in space
Scientific Reports (2024)
-
Effects of Simulated Microgravity on Rat Reproductive System: Potential Benefits of Vitamin D3 Intervention
Reproductive Sciences (2024)
-
How are cell and tissue structure and function influenced by gravity and what are the gravity perception mechanisms?
npj Microgravity (2024)
-
Investigating the effects of chronic low-dose radiation exposure in the liver of a hypothermic zebrafish model
Scientific Reports (2023)