Abstract
High-entropy alloys (HEAs) have attracted great attention due to their many unique properties and potential applications. The nature of interatomic interactions in this unique class of complex multicomponent alloys is not fully developed or understood. We report a theoretical modeling technique to enable in-depth analysis of their electronic structures and interatomic bonding, and predict HEA properties based on the use of the quantum mechanical metrics, the total bond order density (TBOD) and the partial bond order density (PBOD). Application to 13 biocompatible multicomponent HEAs yields many new and insightful results, including the inadequacy of using the valence electron count, quantification of large lattice distortion, validation of mechanical properties with experiment data, modeling porosity to reduce Young’s modulus. This work outlines a road map for the rational design of HEAs for biomedical applications.
Similar content being viewed by others
Introduction
High-entropy alloys (HEAs) are complex metallic alloys1,2,3,4 comprised of four, five, or more principal components of different concentrations. Such a structure leads to a high mixing entropy that favors the formation of single phase disordered solid solutions at higher temperatures5,6, although the enthalpy also plays a critical role in determining its composition and phase with no long-range-order (LRO)1,7. A significant amount of disorder exist in HEAs when they undergo elemental segregation, precipitation, and chemical ordering, but uncertainty remains regarding the existence and the nature of short-range-orders (SRO)8. The fundamental theory for the formation of HEAs is not fully established despite many theories and modeling efforts since their inception9,10,11,12. Due to the complexity of their compositions and the difficulties for accurate measurements for comparison, most of these efforts are based on different perspectives and quite scattered. They generally used the random solid-solution-model (RSSM) to evaluate their properties although the methods employed could be very different13,14,15. Current theoretical methods for HEAs are spread out and include but not limited to the use of CALPHAD1,12,16, quasi-random-structure (SQS)17,18,19 usually in the framework of density functional theory (DFT) on small-size supercells20, coherent potential approximations (EMTO-CPA) or effective medium theory21,22. Despite a plethora of approaches and methods used, few of them can provide a comprehensive view on their formation and the prediction of their properties due to several obstacles. Firstly, due to relatively large chemical disorder in HEAs, the application of RSSM requires using large supercells. Secondly, the computational demand for accurate large-scale DFT calculations could be prohibitive. Finally, the lack of a non-empirical metric to interpret the results that involve subtle interactions between different metal atoms. By introducing the concept of total bond order density (TBOD) and partial bond order density (PBOD) based on the quantum-mechanical (QM) metrics (see the “Methods” section), we avoid the use of pure geometric parameters in describing the structures, compositions, and properties of HEAs. The new perspective is based on the understanding the nature of metallic bonding, crucially related to the theory of formation of HEAs. Although metallic bonding has been extensively discussed in the field of metallic glasses (MGs)23,24, it has not been thoroughly investigated for HEAs. Metallic bonding is multi-atomic in nature, different from the covalent or ionic bonding where the bond length (BL) is explicitly defined as the distance between the two atoms forming the bond. In MGs, and to a lesser extent in HEAs, the BL can be ambiguous, since all atoms within a certain distance of separation can contribute to metallic bonding. For a fixed distance of separation for a pair of atoms in the model, there could be many possible values of bond order (BO), a measure of the bond strength; and for a specific value of BO, there could be many possible pairs of atoms with the same distance of separation. A theory that is predominately depending on the geometric parameter of BL, or atomic size for interpretation could be oversimplified and problematic. On the other hand, the concept of the TBOD is still applicable as long as the BO values of all the contributing atoms are counted. This point has already been strongly argued for in reference25 for MG. What differentiate HEAs from MGs is that HEAs have a basic crystal lattice (FCC, BCC, or HCP) as its structural backbone. However, based on the RSSM description, HEAs are not strictly crystalline. They possess no LRO and negligible or small SRO with different nearest neighbor (NN) and second nearest neighbor (SNN) pairs. This is the same predicament facing the vague description of the so-called lattice distortion (LD) that will be discussed later. The use of TBOD and its partial components, the PBOD, to explore the theory of formation in HEAs is clearly a novel approach.
As the populations of developed countries continue to age, there is an increasing demand for biocompatible implant materials. Traditionally, stainless steels and titanium alloys have been typically implemented as joint surrogate metals26,27. More recently HEAs5,7 have been proposed as viable candidates due to their favorable properties, such as high strength and ductility, resistance to corrosion, wear, and fatigue. Importantly, HEAs may consist of refractory elements that are mostly non-toxic and hypoallergenic. They favor the structure of a single solid-solution phase in BCC lattice and have been proposed as a new class of metallic biomaterials28. With Mo present, it exhibited excellent biocompatibility compared to the pure Ti28. However, the Young’s modulus of this alloy system is roughly 10 times greater than human bones29. Other challenges including the structural complexity involved at the interfaces with tissues and bones, and other soft matters in an aqueous environment involving body fluids.
In this article, we present the results on the electronic structure, interatomic bonding, and the application of TBOD and PBOD in addressing the challenges for fundamental understanding on the theory of formation of HEAs and its potential applications. We would like to point out the special merits of using TBOD and PBOD as key metrics for assessing the fundamental properties of multicomponent alloys (see “Methods” section for detail). They can be directly compared with each other irrespective of their atomic species, composition, or size. Moreover, they can be applied to other materials systems as long as all interatomic bonding between every pair of atoms are included and normalized by the volume of the cell. This characteristic is very different from other techniques based on ground state energies used in the enthalpy evaluation19,30,31. The total energies for different HEAs can be very different because of the difference in the reference energies, although the formation energy for each HEA can explicitly be calculated, which can be quite onerous and time consuming for multi-component HEAs with different compositions. Also, the PBOD resolved from the TBOD have different pairwise components is particularly useful in revealing the details of the interatomic interactions, since TBOD and PBOD are derived from quantum mechanical calculations, not from geometric parameters. Such information cannot be obtained easily based on the calculation of total energies.
In the present work, the electronic structures, interatomic bonding, and mechanical properties of the 13 bioinspired HEAs (Table 1) are investigated through advanced modeling using large supercells yielding many new and insightful results critical to the development and application of biocompatible HEAs.
Results
Electronic structure and interatomic bonding
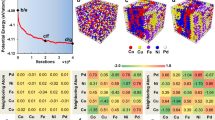
We start the “Results” section with the central part of this paper, the electronic structure, and interatomic bonding. The results for the 13 HEAs constitute a substantial amount of data. Model M3 (TiNbTaZrMo) is chosen as a representative one (Fig. 1) for focused presentation of the results. They are sketch of the supercell structure of 250 atoms (Fig. 1a), total and partial density of states (DOS) (Fig. 1b), partial charge (PC) distribution (Fig. 1c), and results related to interatomic bonding (Fig. 1d, e). Results for other HEAs are presented in (Figs. S1, S2, and S4). The data on TDOS values at the Fermi level EF (N(EF)) and the minimum values at the locations of the deep valley in the conduction band (CB) above EF of the 13 HEAs are listed in Supplementary Table 1. The similarity in the DOS features amongst the 13 HEAs for energies ranging from −5 to +5 eV is because they are all derived from the 3d/4d/5d orbitals of the transition metals in the HEAs.
a Ball and stick sketch of the supercell. b TDOS and atom-resolved PDOS. The zero energy corresponds to the Fermi level EF. The magnitude of DOS at EF, N(EF) correlates with electric conductivity of the alloy. Peaks are marked as O1, O2, and O3 at −0.39, −1.56, and −2.53 eV below EF, and C1 and C2 at 2.70 and 3.98 eV above EF. c Partial charge distribution of 250 atoms in five metals. They can be either positive or negative. d BO vs. “BL” distribution between all atomic pairs among 15 possible types. e Percentage contribution of PBOD to TBOD for each type. Ta–Mo has the largest contributions (12.54%) and Nb–Nb the smallest (2.2%). f FWHM for the two Gaussians fitted to the histogram distribution of the average NN and SNN the interatomic distances. The two FWHMs are 0.233 and 0.269 Å with an average value of 0.250 Å. This provides the quantitative measure of the lattice distortion in HEAs.
An important electronic structural property is the effective charge Q* associated with the so-called PC ΔQ = (Q0–Q*) where Q0 is the charge of the neutral atom also called the valence electron count (VEC) (see “Methods” section). Figure 1c displays the PC distribution of the 250 atoms in M3. Similar plots for other HEAs are shown in Supplementary Fig. 2. Table 2 lists the averaged PC and Q* for each atomic component in the 13 HEAs. We then average them over the HEAs containing that element (bottom of Table 2 and above the row for VEC). As can be seen, Ti, V, Zr, and Hf have a VEC of 4, Nb and Ta have a VEC of 5, and Mo has a VEC of 6. The calculated Q* values range from 4.10 (M3) to 4.37 (M9) for Ti; 4.09 (M1) to 4.25 (M4) for V; 3.67 (M3) to 3.87 (M9) for Zr, and 3.51 (M10, M13) to 3.72 (M9) for Hf, respectively. These Q* values depend on the compositions of the HEAs. The increases in Q* (Ti, Ta, V, and Mo) or decreases in Q* (Nb, Zr, and Hf) beyond their VEC depend on the atomic compositions and specific HEAs involved. The averaged Q* of each type of atoms for all 13 HEAs show significant deviations from VEC (Supplementary Fig. 3). This trend strongly suggests that conventional theories using VEC as the key parameter in their formulation may be inadequate and is the main reason for the failure of using the rule of mixture (ROM) in the explanation of simulation results in other studies32,33. The trend is also particularly true in some more complicated HEAs with specific local structures, such as those involving defects or other microstructures. Since the entire HEA system should be electrically neutral with a zero net charge, a slight deviation in the atomic composition can offer opportunities for fine-tuning their targeted properties. Atom-specific effective charge Q* can also be used as a descriptor in the machine-learning (ML) technique but they are less important than the TBOD and PBOD.
The distribution of the BO vs. the bond length (“BL”) for all interatomic pairs up to a “BL” of 4.0 Å in M3 is shown in Fig. 1d. The “BL” (with quotation) is the distance of separation between a pair of atoms with a specific BO value. These BOs are calculated with contributions from all atoms near this particular pair since metallic bonding is multiatomic, not pairwise. The TBOD is determined by the strength of all interatomic bonds and their propensity normalized by the cell volume (see “Methods” section). The contributions from the specific PBOD that are decomposed from the TBOD is depicted in the pie chart (Fig. 1e). Similar BO vs. “BL” plots and pie charts for other HEAs are shown in Supplementary Fig. 4.
The TBOD and PBOD in the 13 biocompatible HEAs are presented in the form of histogram bars (Fig. 2a) and pie chart (Fig. 2b). This complex display requires the detailed analysis to appreciate the information that it contains. Some of the general trends are discussed in the figure caption. Still, we cannot be certain about whether there exists any trends related to their composition, atomic species, and their interatomic interactions in relation to PBOD. This critical issue will require a more penetrating analysis. An interesting issue frequently discussed in the HEA community is the possible existence of SRO and if we can quantify it. A commonsense rule is to consider that a single atom has no SRO, or the short-rang-order-parameter SROP = 0, and a diatomic molecule has the maximum SROP = 1. The SROP in HEAs can be obtained from the root-mean-square (RMS) deviation of [PBOD/TBOD]. First, the ratio of PBOD/TBOD for each of the 13 models are calculated from the data listed in the table of Fig. 2a. Next the RMS value for each model are calculated and listed in Supplementary Table 1. As can be seen, they exclusively depend on the number of atomic components. The 4-component and 5-component HEAs have SROP = 0.11 and 0.07, respectively. The larger the atomic components, the lower the SROP. We thus conclude that SRO in HEAs under the RSSM is very small. Since the TBOD and PBOD are obtained from QM calculations of individual BO values, therefore provide the vitally important details regarding the nature of the formation of HEAs. This is consistent with the notion of order emerging from disorder for a broader class of functionalized HEAs34. It should also be pointed out that the above description is strictly based on the homomgenized RSSM used in the supercell and does not apply to cases where there may involve other factors under differernt assumptions8,35. Experimentally, the actual sample will be much larger and may involve multiphase structures, the presence of impurities or dislocations that may induce some form of SRO beyond the simple notion of number of atomic components. The present method of quantifying SRO could still be applied in such a senario as long as the supercell itself contains far more complicated nonhomogeneoius structures beyond the RSSM.
a Histogram presentation of TBOD and PBOD for 13 models. Observations: (1) M1 has the highest TBOD of 0.0480 of e−/(Å)3 and M9 has the lowest TBOD of 0.0319 of e−/(Å)3. (2) The TBOD does not depend on the number of components (4 or 5). (3) There is no evidence that the size of the atom influences the PBOD. (4) There is some evidence that the Ti–Ta pair has a relatively large PBOD whereas the Nb–Nb pair has a relatively small PBOD. b Pie chart presentation for PBOD except M3 (in Fig. 1e).
Lattice distortion
LD plays a central role in controlling the properties of HEAs36. A large LD could be the harbinger of a possible phase transition or sample inhomogeneity. However, the degree of LD in HEAs is difficult to quantify beyond the geometric parameters (Table 1). We can quantify LD from the BO vs. “BL” data (Fig. 1d and Supplementary Fig. 4) by resolving them into interatomic pairs. For M3, the scattered plot shows that Ti–Zr, Ti–Ti, and Ti–Nb pairs from NN and SNN groups start to merge (Supplementary Fig. 4c). This is a strong evidence of large LD. Similar observations for other HEAs are displayed in Supplementary Fig. 4a–h. The investigations on LD reported in the literature are mostly vague and sometimes misleading. The difficulty in providing a more precise description for LD arises from the lack of a basic reference for the undistorted lattice for HEAs due to the random distributions of the different metallic ions of varying sizes throughout the lattice. In other words, there is no such a thing as an undistorted lattice to be used as a reference. To quantify the notion of LD, the histogram distribution of “BL” of interatomic pairs in M3 is plotted (Fig. 1f). The data are fitted with two Gaussians exhibiting a bimodal distribution for the NNN and SNN pairs. The larger the two FWHM and their sum, the greater the LD. Similar analysis applied to all 13 HEAs are shown in Supplementary Table 1 and Supplementary Fig. 5. The largest (smallest) sum of FWHM is in M8 (M1), which coincides with the supercell volume (Table 1). However, there is little resemblance in the relative ranking of the volume and the sum of FWHM. FWHM is extracted from the distribution of BO values calculated quantum mechanically, whereas volume is a structural parameter that does not involve detailed interactions among the atoms. Thus, LD in HEAs can now be rigorously quantified that are difficult to capture in experiments. For example, neutron scattering may reveal the NN distance, but not the detail of the SNN distance. This trend contributes to the difficulties in discussing the LD from experimental data. In the 4-component HEA such as M1 (TiNbTaV) there are 10 distinctively different NN pairs. In the 5-component HEA M5 (NbTaTiVZr), there are 15 NN pairs. The ab initio modeling can facilitate the interpretation of the experimental data. For example, based on the neutron (synchrotron) diffraction measurements, Lee et al. estimated the NN distance in M136 and M537 to be 2.788 (2.085 Å) and 2.863 (2.866 Å), respectively. These results compare very favorably with the calculated values of 2.786 and 2.854 Å (Supplementary Table 1), respectively. Both results show that adding Zr to M5 from M1, the average NN distance increased by only about 2.2% or 2.4% which is not a large LD envisioned. However, the calculated FWHM values for M1 and M5 (Supplementary Table 1) are 0.211 and 0.379 Å respectively, an increase of almost 80%. Obviously, the sum from NN and SNN FWHM is a more realistic estimation for the observed large LD due to the addition of Zr to M1 (TiNbTaV) observed experimentally.
Mechanical properties
One of the most important properties of HEAs is the mechanical properties. They are intimately related to the electronic structure and bonding (see “Methods” section). The calculated results of the 13 HEAs include the elastic coefficients, compliance tensor, bulk modulus (K), shear modulus (G), Young’s modulus (E), Poisson’s ratio (η), G/K ratio and estimated Vickers harness (Hv) together with the available experimental data37,38,39,40,41,42,43,44,45,46 is presented in Table 3. The experimental data for refractory BCC HEAs are quite limited. Of the 13 models listed, there are no experimental data for (M4, M7, M11, M12, and M13). M10 has data for Hv only, while M2 and M3 have results for E and Hv only. M5 and M9 lack Hv data. M1 is the only HEA having experimental data in every column to compare with the calculated values. The calculated Hv is based on a formula suggested by Tian et al.47 from the fitted data to MGs and is generally underestimated from the measured data. The experimental data for HEAs have considerable variations due to the uncertainty in the composition and different methods used. Overall, the agreement with the limited experimental data is quite reasonable and consistent, especially for M6 and M9. The calculated Poisson’s ratios for the 13 HEAs range from 0.366 (M10) to 0.402 (M1), which are on the ductile side. We plotted the G vs. K for all the 13 HEAs in Supplementary Fig. 6. The Pugh’s ratio G/K is well within the range of 0.30 and 0.20 with the average around 0.25 which is on the ductile side for metallic systems, somewhere between bulk metallic glass (BMG) and polycrystalline metals48. There is a good correlation between the calculated bulk modulus K and TBOD (Fig. 3), since the TBOD is a single metric that quantifies the internal cohesion. Correlations of TBOD with G and E are less obvious since they involve directional dependence in the strain.
Porosity in TiNbTaZrMo
For the biocompatible HEAs proposed for biomedical applications, the most relevant property is Young’s modulus E, which range from 83.7 GPa for M5 (NbTaTiVZr) to 113.5 GPa for M13 (TiNbMoHfTa). To be compatible with the strengths of bones and joints in a human body, the E needs to be reduced by at least 50%. It is well known that there is a correlation between Young’s modulus and the porosity in porous materials49. We provide some preliminary test calculation by introducing porosity in the simulation for M3 to reduce E. Seven cases of porous models (p1–p6 and px) are constructed from the non-porous model or M3 (p0) by deleting a portion of connected atoms from p0 making sure that the atomic composition is still remain equal partition as in the original model. This is not an easy task but is necessary. The porosity values for each model from p0 to p6 and px are determined by using the PLATON software to be 0.0%, 2.90%, 4.00%, 7.50%, 11.2%, 18.2%, 23.5%, and 30.0%, respectively. These models are shown in Fig. 4a using Crystalexplore17 software.
a Ball and stick models that exhibit different levels of porosity from p0 to px are plotted using the CrystalExplorer17 software. The different level of porosity is achieved by removing a portion of connected metal atoms in an equal proportion starting with p0 (no porosity) up to p6, which has a large single pore. These models are fully relaxed with the cell volume fixed and their mechanical properties are calculated in the same manner as for the 13 HEA models. b Young’s modulus E vs. porosity. The data for porosity values from p1 to p3 contain some fluctuations due to the larger error/volume ratio of the small pores. When the modeling was applied to the model with largest porosity of 34.2% (px), the simulation failed to converge because the ratio of the size of the pore to p0 is too large.
The porosity can reduce Young’s modulus significantly in M3 (Fig. 4b). Figure 4a shows that p6 with porosity of 23.5%, the E was reduced to 60.83 GPa from 102.65 GPa in p0, closer to E values exhibited by human cortical bones typically ranging from 7 to 30 GPa50, but still way too large. The other porosity-dependent mechanical properties also show similar decrease in bulk and shear modulus with the Poisson’s ratio decreases from 0.39 at p0 to 0.30 at p6. This implies that the HEA represented by M3 becomes more brittle as the porosity is increased. However, this point is less clear and requires detailed modeling for biocompatible HEAs in an aqueous environment. Another positive fact is that the introduction of porosity greatly reduce the weight of the materials, an important consideration for biomedical applications. Currently, most biomedical applications involve the use of Ti alloys which is the lightest refractory element in the HEAs.
The current study on porosity simulation is limited to the starting supercell size of 250 atoms. A larger supercell containing 432 atoms (see Supplementary Materials) or even larger and with multiple smaller pores would be necessary for more realistic simulations. Young’s modulus for the 432-atom supercell is 100.77 GPa, slightly <102.65 GPa for the 250-atom supercell (Supplementary Table 2). This exploratory study is to provide the proof-of-concept that supercell modeling can provide the guideline to reduce Young’s modulus in biocompatible HEAs by introducing porosity of varying shapes that can accommodate bone structures in the human body. In doing so, our simulation method may facilitate the discovery of viable means to fabricate HEAs that are good candidates for biomedical applications.
Discussion
In this study, several new results with lasting impacts are revealed. (1) The use of quantum mechanical metrics, TBOD and PBOD, for the HEA research. (2) Accurate ab initio modeling for 13 biocompatible HEAs together with their mechanical properties. (3) Realistic calculation of the atomic charges on every atom showing the inadequacy of using the VEC as a valid parameter in the theory of HEAs. (4) Precise quantification of the degree of LD based on our approach of ab initio simulation. (5) Proof of concept in addressing the porosity effect critical for using HEAs for biomedical applications.
The method and approach we used here can be readily extended to other multi-phase HEAs, or composites with lighter nonmetallic elements, and the presence of defects or other special refractory elements, such as tungsten (W)51 greatly expanding its applicability. There is now a new trend to expand HEAs to composite materials, which contain light elements, such as C, B, N, and O (especially C) to form HEA ceramics32,33. Although the random solid solution model may still hold, the supercell construction for the composites will be much more challenging because of the involvement of non-metallic elements. Other possible extensions of HEAs include the deviation from equal-atomic compositions or even the mixed phases to optimize certain properties. Last but not the least, more realistic modeling for biocompatible HEAs in aqueous solution or body fluid is possible. With the ever-increasing computing power, such large-scale computational modeling in complex materials is very realistic in the near future.
Methods
Supercell construction
The cubic supercells for biocompatible high entropy-alloys (HEAs) in the BCC lattice are constructed based on the random solid solution model (RSSM). The size of the supercell, or the number of atoms N it contains BCC supercell is determined by the simple formula N = 2 × (n)3. In the present work, n is 5 so the supercell has 250 atoms in the cubic cell of length equal to na, where a is the lattice constant of the single atom BCC crystal of a typical transition metal. We believe that a 250-atom supercell may be the minimal size to justify the use the RSSM with sufficient confidence. It should be pointed out that our supercell is different from those used in the SQS structure in the simulations by other researchers which is usually much smaller. To account for the different possible structural configurations, many such so-called “supercells” have to be used. In the present study, we assert that the statistical distribution of random distribution of metals is sufficient, since the supercell is sufficiently large and with periodical boundary conditions and can account for the random distribution of the NN, second NN and also the third NN and beyond for each atom in the model. These two approaches are similar in spirit but different in strategy. We believe the use of large supercells for many HEAs is more efficient, tractable, and conducive for detailed bond analysis and calculation of TBOD. Four or five atomic species of equal percentage are chosen from the following seven refractory elements: Ti, V, Zr, Nb, Mo, Hf, and Ta, and are distributed randomly in the lattice sites of the supercell with periodic boundaries. For the 5-component HEAs, there are 50 atoms each. For the 4-component HEAs, two of them have 62 atoms, and the other two have 63 atoms each. The initial lattice constant for the supercell is obtained from the scaled average of the crystal lattice constant for each atom. To fully comply with the spirit of RSSM, a script is written such that the atomic occupation of each site is completely random with no restriction to their NN atoms and beyond. So for the 4 (5)-component HEA models, the supercell will have 10 (15) possible NN pairs.
Structural relaxation
The initial BCC supercells for HEAs are fully optimized using the Vienna Ab initio Simulation Package (VASP)52,53. VASP is a plane-wave-based DFT method using the pseudopotential. It is very efficient for the structural optimization and elastic properties calculations. In the present work, the PAW-PBE potential for the exchange correlation potential within the generalized gradient approximation (GGA) was used54. We adopt a relatively high energy cutoff of 500 eV. The electronic and ionic force convergences were set at 10−5 eV and 10−3 eV/Å, respectively, with a 2 × 2 × 2 k-point mesh. Additional testing with selected sample for higher k-point showed no discernable difference. The final relaxed structures of the 13 biocompatible HEAs (Table 1) based on which all the properties are evaluated. The sketch of the supercell for M3 is shown in Fig. 1a.
Electronic structure and interatomic bonding
For electronic structure and bonding calculations, the in-house developed package, the orthogonalized linear combination of the atomic orbitals (OLCAO) method is used55 with the VASP-relaxed structures as the input. The OLCAO method is another DFT-based method using atomic orbitals for the basis expansion, which are expressed as a linear combination of Gaussian type of orbitals (GTO). The use of GTO enables the efficient evaluation of three center integrals in the analytic form and thus makes the method highly efficient, especially for large complex systems as demonstrated in many recent publications. In the present calculations for HEAs, a more localized minimal basis (MB) set is used, which consists of the core orbitals and the open shell of valence orbitals. Using Ti as an example, the MB has core orbitals of (1s, 2s, 2p, 3s, and 3p) and valence orbitals of (4s, 4p, and 3d).
In addition to the usual electronic structures such as DOS and partial DOS (PDOS), the most important part of the calculation is the effective charges \(Q_\alpha ^ \ast\) and the BO values, ραβ, between each pair of atoms (α, β) based on the Mulliken population analysis scheme56:
In Eqs. (1) and (2), Siα,jβ are the overlap integrals between the ith orbital in the αth atom and jth orbital in the βth atom. \(C_{j\beta }^m\) are the eigenvector coefficients of the mth band for the jth orbital in the βth atom. The PC, ΔQ, or the charge transfer for each atom, is the deviation from neutral atomic charge (Q0) from the effective charge (Q*) or ΔQ = Q0–Q*. The accurate determination of PC is extremely important in interpreting many of the material properties and their functionality, especially in HEAs. This function arises from the multi-component nature of the HEAs, which consists of transition metal elements with different d-electron occupations. The BO value, ραβ, between a pair of atoms in Eq. (2) provides a quantitative measure of the contribution to the metallic bond from atoms α and β with a specific distance of separation. This BO value is affected by the presence of all the nearby atoms which contribute to the BO. The summation of all BO values normalized by the cell volume gives us the TBOD, which is a single metric to assess the internal cohesion in the crystal57. The TBOD can be resolved into partial components or the partial BO density (PBOD) for the different types of atomic pairs, or different groups of atoms in the structural units in the crystal or the supercell.
Mechanical properties
For the elastic and mechanical properties of the HEAs, we used the stress (σi) vs. strain (εj) response analysis scheme58,59 on the fully relaxed structure from VASP. A small strain ε (±0.5%) is applied to the supercell to obtain the elastic coefficients Cij and compliance tensor Sij (i, j = 1, 2, 3, 4, 5, 6) by solving the following set of linear equations:
From the calculated Cij and Sij, other mechanical properties such as the bulk modulus (K), shear modulus (G), Young’s modulus (E), and Poisson’s ratio (η) are obtained using the Voight–Reuss–Hill (VRH) polycrystalline approximation60,61,62. More detailed information on the methods used for mechanical properties are described in the Supplementary Materials S1.
Data availability
All the data in this paper including those in the supplementary materials are freely available by contacting the corresponding author (chingw@umkc.edu).
Code availability
References
Miracle, D. B. & Senkov, O. N. A critical review of high entropy alloys and related concepts. Acta Mater. 122, 448–511 (2017).
Gao, M. C., Yeh, J.-W., Liaw, P. K. & Zhang, Y. High-entropy Alloys: Fundamentals and Applications (Springer, 2016).
Lim, X. Mixed-up metals make for stronger, tougher, stretchier alloys. Nat. News 533, 306 (2016).
Ye, Y., Wang, Q., Lu, J., Liu, C. & Yang, Y. High-entropy alloy: challenges and prospects. Mater. Today 19, 349–362 (2016).
Zhang, Y. et al. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 61, 1–93 (2014).
Li, Z., Pradeep, K. G., Deng, Y., Raabe, D. & Tasan, C. C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 534, 227 (2016).
Santodonato, L. J., Liaw, P. K., Unocic, R. R., Bei, H. & Morris, J. R. Predictive multiphase evolution in Al-containing high-entropy alloys. Nat. Commun. 9, 4520 (2018).
Li, Q. J., Sheng, H. & Ma, E. Strengthening in multi-principal element alloys with local-chemical-order roughened dislocation pathways. Nat. Commun. 10, 11 (2019).
Ding, J., Yu, Q., Asta, M. & Ritchie, R. O. Tunable stacking fault energies by tailoring local chemical order in CrCoNi medium-entropy alloys. Proc. Natl Acad. Sci. USA 115, 8919–8924 (2018).
Sun, X. et al. Phase selection rule for Al-doped CrMnFeCoNi high-entropy alloys from first-principles. Acta Mater. 140, 366–374 (2017).
Zhang, F. et al. An understanding of high entropy alloys from phase diagram calculations. Calphad 45, 1–10 (2014).
Senkov, O. N., Senkova, S. V., Woodward, C. & Miracle, D. B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: microstructure and phase analysis. Acta Mater. 61, 1545–1557 (2013).
LaRosa, C. R., Shih, M., Varvenne, C. & Ghazisaeidi, M. Solid solution strengthening theories of high-entropy alloys. Mater. Charact. 151, 310–317 (2019).
Tian, F. A review of solid-solution models of high-entropy alloys based on ab initio calculations. Front. Mater. 4, https://doi.org/10.3389/fmats.2017.00036 (2017).
Widom, M. Modeling the structure and thermodynamics of high-entropy alloys. J. Mater. Res. 33, 2881–2898 (2018).
Sanchez, J. M., Vicario, T., Albizuri, J., Guraya, T. & Garcia, J. C. Phase prediction, microstructure and high hardness of novel light-weight high entropy alloys. J. Mater. Res. Technol. 8, 795–803 (2019).
Zunger, A., Wei, S. H., Ferreira, L. G. & Bernard, J. E. Special quasirandom structures. Phys. Rev. Lett. 65, 353–356 (1990).
Yang, Y., Wang, W., Gan, G.-Y., Shi, X.-F. & Tang, B.-Y. Structural, mechanical and electronic properties of (TaNbHfTiZr)C high entropy carbide under pressure: ab initio investigation. Physica B: Condens. Matter 550, 163–170 (2018).
Curtarolo, S. et al. The high-throughput highway to computational materials design. Nat. Mater. 12, 191–201 (2013).
Chen, H. L., Mao, H. H. & Chen, Q. Database development and Calphad calculations for high entropy alloys: challenges, strategies, and tips. Mater. Chem. Phys. 210, 279–290 (2018).
Tian, F., Varga, L. K., Shen, J. & Vitos, L. Calculating elastic constants in high-entropy alloys using the coherent potential approximation: current issues and errors. Comput. Mater. Sci. 111, 350–358 (2016).
Huang, S., Tian, F. & Vitos, L. Elasticity of high-entropy alloys from ab initio theory. J. Mater. Res. 33, 2938–2953 (2018).
Miller, M. & Liaw, P. K. Bulk metallic glasses: an overview. in T. Egami (ed), Atomistic theory of metallic liquids and glasses, Chapter 2 (Springer Science & Business Media, 2008).
Pelletier, J.-M. & Qiao, J. in J. D. Musgraves, J. Hu, L. Calvez (eds), Springer Handbook of Glass 617–643 (Springer, 2019).
Ching, W.-Y. in J. D. Musgraves, J. Hu, L. Calvez (eds), Springer Handbook of Glass 1097–1130 (Springer, 2019).
Gibon, E. et al. The biological response to orthopaedic implants for joint replacement: Part I: metals. J. Biomed. Mater. Res. Part B 105, 2162–2173 (2017).
An, Y. H. & Draughn, R. A. Mechanical Testing of Bone and the Bone–Implant Interface (CRC Press, 1999).
Todai, M. et al. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 129, 65–68 (2017).
Diao, H. Y., Feng, R., Dahmen, K. A. & Liaw, P. K. Fundamental deformation behavior in high-entropy alloys: an overview. Curr. Opin. Solid State Mater. Sci. 21, 252–266 (2017).
Yang, K., Oses, C. & Curtarolo, S. Modeling off-stoichiometry materials with a high-throughput ab-initio approach. Chem. Mater. 28, 6484–6492 (2016).
Troparevsky, M. C., Morris, J. R., Kent, P. R., Lupini, A. R. & Stocks, G. M. Criteria for predicting the formation of single-phase high-entropy alloys. Phys. Rev. X 5, 011041 (2015).
Sarker, P. et al. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 9, 4980 (2018).
Harrington, T. J. et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 166, 271–280 (2019).
Dragoe, N. & Bérardan, D. Order emerging from disorder. Science 366, 573–574 (2019).
Wei, S. et al. Natural-mixing guided design of refractory high-entropy alloys with as-cast tensile ductility. Preprint at 1911.10975 (2019).
Lee, C. et al. Lattice distortion in a strong and ductile refractory high-entropy alloy. Acta Mater. 160, 158–172 (2018).
Lee, C. et al. Temperature dependence of elastic and plastic deformation behavior of a ductile refractory high-entropy alloy. Sci. Adv. (2019) (in press).
Couzinie, J. P., Senkov, O. N., Miracle, D. B. & Dirras, G. Comprehensive data compilation on the mechanical properties of refractory high-entropy alloys. Data Brief. 21, 1622–1641 (2018).
Yao, H. W. et al. NbTaV-(Ti,W) refractory high-entropy alloys: experiments and modeling. Mater. Sci. Eng.: A 674, 203–211 (2016).
Wang, S. P. & Xu, J. (TiZrNbTa)-Mo high-entropy alloys: dependence of microstructure and mechanical properties on Mo concentration and modeling of solid solution strengthening. Intermetallics 95, 59–72 (2018).
Wang, S. P. & Xu, J. TiZrNbTaMo high-entropy alloy designed for orthopedic implants: as-cast microstructure and mechanical properties. Mater. Sci. Eng. C 73, 80–89 (2017).
Dirras, G. et al. Elastic and plastic properties of as-cast equimolar TiHfZrTaNb high-entropy alloy. Mater. Sci. Eng. A 654, 30–38 (2016).
Gorsse, S., Nguyen, M. H., Senkov, O. N. & Miracle, D. B. Database on the mechanical properties of high entropy alloys and complex concentrated alloys. Data Brief. 21, 2664–2678 (2018).
Lin, C. M., Juan, C. C., Chang, C. H., Tsai, C. W. & Yeh, J. W. Effect of Al addition on mechanical properties and microstructure of refractory AlxHfNbTaTiZr alloys. J. Alloy. Compd. 624, 100–107 (2015).
Ye, Y. X. et al. Evaluating elastic properties of a body-centered cubic NbHfZrTi high-entropy alloy—a direct comparison between experiments and ab initio calculations. Intermetallics 109, 167–173 (2019).
Juan, C. C. et al. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 62, 76–83 (2015).
Tian, Y. J., Xu, B. & Zhao, Z. S. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 33, 93–106 (2012).
Greaves, G. N., Greer, A., Lakes, R. S. & Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 10, 823 (2011).
Kováčik, J. Correlation between Young’s modulus and porosity in porous materials. J. Mater. Sci. Lett. 18, 1007–1010 (1999).
Bowen, P. K. et al. Biodegradable metals for cardiovascular stents: from clinical concerns to recent Zn-alloys. Adv. Healthc. Mater. 5, 1121–1140 (2016).
El-Atwani, O. et al. Outstanding radiation resistance of tungsten-based high-entropy alloys. Sci. Adv. 5, eaav2002 (2019).
Kresse, G. & Furthmuller, J. Vienna ab-initio simulation package (VASP): The guide (VASP Group, Institut fur Materialphysik, Universitat Wien, Sensengasse 8, 2002).
Kresse, G. & Furthmüller, J. Software vasp, vienna, 1999. Phys. Rev. B 54, 169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Ching, W.-Y. & Rulis, P. Electronic Structure Methods for Complex Materials: The Orthogonalized Linear Combination of Atomic Orbitals (Oxford University Press, 2012).
Mulliken, R. S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 23, 1833–1840 (1955).
Dharmawardhana, C., Misra, A. & Ching, W.-Y. Quantum mechanical metric for internal cohesion in cement crystals. Sci. Rep. 4, 7332 (2014).
Nielsen, O. & Martin, R. M. First-principles calculation of stress. Phys. Rev. Lett. 50, 697 (1983).
Yao, H., Ouyang, L. & Ching, W. Y. Ab initio calculation of elastic constants of ceramic crystals. J. Am. Ceram. Soc. 90, 3194–3204 (2007).
Voigt, W. Lehrbuch der Kristallphysik, Nachdruck, 1. Auflage, Teubner (Berlin, Leipzig, B.G. Teubner, 1928).
Reuss, A. Account of the liquid limit of mixed crystals on the basis of the plasticity condition for single crystal. Z. Angew. Math. Mech. 9, 49–58 (1929).
Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 65, 349 (1952).
Acknowledgements
This research used the resources of the National Energy Research Scientific Computing Center supported by DOE under Contract No. DE-AC03-76SF00098 and the Research Computing Support Services (RCSS) of the University of Missouri System. We thank C. Lee, Prof. V. Keppens and B. L. Musico for the helpful discussion on experimental measurements of elastic properties using neutrons and ultrasonics. The project is partially supported by DOE-NETL grant DE-FE0031554 (R.S. and W.-Y.C.). S.S. was supported in part from funds provided by the University of Missouri-Kansas City, School of Graduate Studies. P.K.L. is supported by the National Science Foundation (DMR-1611180 and 1809640) and Department of Energy (FE0008855 and DE-FE-0011194) with Drs. J. Mullen, V. Cedro, R. Dunst, S. Markovich, G. Shiflet, and D. Farkas as program managers and the U.S. Army Office Project (W911NF-13-1-0438 and W911NF-19-2-0049) with the program managers, Drs. M.P. Bakas, S. N. Mathaudhu, and D.M. Stepp.
Author information
Authors and Affiliations
Contributions
W.-Y.C., R.S., P.L. and M.Z. conceived the project. W.-Y.C. and S.S. performed the calculations, W.-Y.C. drafted the paper. S.S. made all figures. J.B. compiled the references. All authors participated in the discussion and interpretation of the results. All authors edited and proofread the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ching, WY., San, S., Brechtl, J. et al. Fundamental electronic structure and multiatomic bonding in 13 biocompatible high-entropy alloys. npj Comput Mater 6, 45 (2020). https://doi.org/10.1038/s41524-020-0321-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-020-0321-x
This article is cited by
-
Ab initio study of mechanical and thermal properties of GeTe-based and PbSe-based high-entropy chalcogenides
Scientific Reports (2023)
-
Corrosion and Biocompatibility Properties of TiZrNbCrV, TiZrNbFeCr, and TiZrNbFeV High Entropy Alloys Produced through Mechanical Alloying
Journal of Materials Engineering and Performance (2023)
-
Simultaneous Twinning and Microband-Induced Plasticity of a Compositionally Complex Alloy with Interstitial Carbon at Cryogenic Temperatures
High Entropy Alloys & Materials (2023)
-
Catalytic Potential and Utility of High Entropy Alloys
Transactions of the Indian National Academy of Engineering (2023)
-
Enthalpy induced phase partition toward hierarchical, nanostructured high-entropy alloys
Nano Research (2022)