Abstract
We assessed the predictive value of an image analysis-based tumor-infiltrating lymphocytes (TILs) score for pathologic complete response (pCR) and event-free survival in breast cancer (BC). About 113 pretreatment samples were analyzed from patients with stage IIB-IIIC HER-2-negative BC randomized to neoadjuvant chemotherapy ± bevacizumab. TILs quantification was performed on full sections using QuPath open-source software with a convolutional neural network cell classifier (CNN11). We used easTILs% as a digital metric of TILs score defined as [sum of lymphocytes area (mm2)/stromal area(mm2)] × 100. Pathologist-read stromal TILs score (sTILs%) was determined following published guidelines. Mean pretreatment easTILs% was significantly higher in cases with pCR compared to residual disease (median 36.1 vs.14.8%, p < 0.001). We observed a strong positive correlation (r = 0.606, p < 0.0001) between easTILs% and sTILs%. The area under the prediction curve (AUC) was higher for easTILs% than sTILs%, 0.709 and 0.627, respectively. Image analysis-based TILs quantification is predictive of pCR in BC and had better response discrimination than pathologist-read sTILs%.
Similar content being viewed by others
Introduction
Image analysis-based tumor-infiltrating lymphocytes (TILs) quantification methods are being developed to eliminate the substantial reader-to-reader variation in TILs assessment that hinders clinical adoption of TILs as prognostic and chemotherapy response predictive markers in breast and other cancer types1,2,3,4. In melanoma, an image analysis-based assessment of TILs on hematoxylin and eosin (H&E) stained sections separated patients into prognostic cohorts more accurately than pathologist-read stromal TILs (sTILs) scores1. In triple-negative breast cancer (TNBC), high levels of TILs infiltration are also associated with better survival and increased pathologic complete response (pCR) to neoadjuvant (i.e., preoperative) chemotherapy5,6,7,8,9,10. While standardized rules for quantification of TILs in breast cancer have been developed11, the inter-observer variability in results continues to slow the adoption of TILs as routine prognostic and predictive markers12.
We previously showed that digital quantification of TILs using an open-source image analysis software, QuPath, and a convolutional neural network predictor algorithm (CNN11) could stratify patients with TNBC into distinct prognostic cohorts, and high digital TILs was independently associated with improved overall survival after adjustment of clinicopathological factors including stage, age, and histological grade of tumor2.
The S0800 trial was a randomized phase II neoadjuvant chemotherapy trial for patients with stage II and III HER-2-negative breast cancers, including both hormone receptor (HR) positive and negative tumors. Patients were randomly allocated (2:1:1) to three neoadjuvant chemotherapy arms:1 nab-paclitaxel with concurrent bevacizumab followed by AC;2 nab-paclitaxel followed by AC; or3 AC followed by nab-paclitaxel. The sequencing of taxane versus AC had no impact on the pCR rate, but the addition of bevacizumab improved the pCR rate from 21 to 36% (p = 0.019)13. Baseline core needle biopsies and posttreatment surgical resection specimens were collected prospectively for biomarker research. We previously reported that higher baseline immune gene expression14 and higher pathologist-read sTILs score15 were associated with higher pCR rates in this trial.
In the current study, we examined the chemotherapy response predictive and prognostic values of image analysis-based TILs assessment in pretreatment biopsies of the S0800 trial and compared its predictive performance to pathologist-read sTILs scores. We also assessed change in TILs in the subset of patients with residual cancer where paired pre- and post-treatment tissues were available.
Results
For the entire cohort, the mean, median, standard error, and interquartile range of easTILs% were 21.39, 17.02, 1.49, and 21.37%, respectively and of pathologist-read sTILs% were 17.85, 10.00, 1.87, and 17.50%, respectively. Patients with pCR had statistically higher pretreatment mean easTILs% compared to those with residual disease (RD) (median 36.1 vs. 14.8%, p < 0.001) (Fig. 1a). sTILs% was significantly higher in the pCR group compared to the RD group (median 17.5 vs. 8.8%, p = 0.037) (Fig. 1b). When the treatment arms were analyzed separately, significantly higher baseline easTILs% and sTILs% were seen in cancers with pCR in the bevacizumab arm, but not in the combined chemotherapy alone arms (Fig. 1c, d). However, the marker treatment interaction test did not demonstrate statistically significant differential predictive values for easTILs% or sTILs% by treatment type. When we dichotomized easTILs% into high (>19.9%) and low (≤19.9%) categories, the overall pCR rates were 41 and 21% (p = 0.019) in the easTILs% high and low groups, respectively (Fig. 2a). In the bevacizumab arm, the corresponding pCR rates were 59 and 25% (p = 0.012) (Fig. 2b) and in the chemotherapy alone arm the pCR rates were 25 and 17% (p = 0.46) in the easTILs% high and low groups, respectively (Fig. 2c). When we repeated this analysis using pathologist-read sTILs% with a cutoff of 20%, we obtained similar results as with easTILs% (Fig. 2a–c). However, in each comparison, the p values were lower for easTILs% than sTILs% on the same sample set, suggesting a greater ability to identify a difference. In the whole study population, multivariable logistic regression analysis including ER status, treatment arm, and disease type (IBC/LABC), easTILs% either as continuous or as categorical (high vs low) variable remained independently significantly predictive of pCR (continuous easTILs% p < 0.001; easTILs% high category p = 0.035) (Supplementary Tables 1, 2). There was no evidence that prognosis by easTILs% differed by hormone receptor status (Interaction: continuous p = 0.28; categorical p = 0.35).
a Pretreatment easTILs% by pCR status. b Pathologist-read sTILs% by pCR status. c Pretreatment easTILs% by pCR and treatment status. d Pretreatment sTILs% by pCR and treatment status. TILs tumor-infiltrating lymphocytes, easTILs% = [sum of lymphocytes area/stromal area] × 100, sTILs% pathologist assessment of stromal TILs, RD residual disease, pCR pathologic complete response, C control, chemotherapy alone, B bevacizumab + chemotherapy. Box and whiskers plots are shown with min to max whiskers that go down to the smallest value and up to the largest. Statistical analysis was performed using Mann–Whitney test.
a The whole study population, b Bevacizumab group, c chemotherapy alone group. easTILs% = [sum of lymphocytes area/stromal area] × 100, sTILs% pathologist assessment of stromal TILs, easTILs% high is defined as >19.9% and pathologist sTILs% high is defined as >20%. Error bars represent standard error. Statistical analysis was performed using the Chi-square test.
Pathologist-read sTILs% and digital easTILs% were positively and significantly correlated (r = 0.606, p < 0.0001) (Fig. 3). We compared the predictive performance of the two different scoring systems in receiver-operating characteristic (ROC) analysis in all patients included. The area under the ROC curves (AUCs) were 0.709 (95% CI 0.659–0.879) and 0.627 (95% CI 0.599–0.820) for easTILs% and sTILs%, respectively, although these AUCs are not statistically different (p = 0.11) (Fig. 4).
Kaplan–Meier survival curves evaluating event-free survival (EFS) showed that patients with high easTILs% or high sTILs% had no significant difference in EFS compared to those with low TILs (Fig. 5). There was no difference in EFS comparing chemotherapy alone vs. chemotherapy plus bevacizumab groups (p = 0.90) (Fig. 6b), or when treatment group was further stratified by high and low easTILs% (p = 0.76) (Fig. 6c) or high and low sTILs% (p = 0.47) (Fig. 6d).
EFS by a treatment with or without bevacizumab, b easTILs% high or low and treatment status, c sTILs% high or low and treatment status. easTILs% dichotomized at the value of 19.9%. sTILs% dichotomized at the value of 20%. TILs tumor-infiltrating lymphocytes, easTILs% [sum of lymphocytes area/stromal area] × 100, sTILs% pathologist assessment of stromal TILs, RD residual disease, pCR pathologic complete response, ctrl control group with chemotherapy, bev bevacizumab + chemotherapy group. Statistical analysis was performed using the log-rank test.
When TILs were compared between paired pre- and post-treatment tissues in patients with RD, we found that both easTILs% (pretreatment 20%, posttreatment 10%, p < 0.001) and sTILs% (pretreatment 26%, posttreatment 13%, p = 0.002) were significantly lower in residual cancer tissues compared to baseline (Fig. 7).
Discussion
The association between immune cell infiltration of primary breast cancer and good prognosis has long been recognized, but despite attempts to standardize TILs scoring, inconsistencies in quantification limit the application of this biomarker in routine clinical care. Differences in preanalytical tissue processing contribute some variability to TILs assessment, but most of the variability arises from differences in pathologists’ scoring16,17. Image analysis-based TILs quantification holds promise for a more accurate and standardized assessment of TILs. In the current study, we assessed the predictive performance of a previously described breast cancer TILs quantification image analysis tool CNN11 implemented in QuPath, and compared its performance to pathologist-generated sTILs% results on pretreatment H&E-stained slides.
Our digital TILs metric, easTILs%, quantifies TILs density within the area of invasive cancer, counting both stromal and intratumoral lymphocytes correlated closely and significantly with pathologist-assessed sTILs%. Higher easTILs% was associated with a higher probability of pCR to neoadjuvant chemotherapy in the entire study population and in the bevacizumab plus chemotherapy arm of the trial. easTILs% high tumors also had a numerically higher pCR rate than easTILs% low cancers in the chemotherapy alone cohort, but this has not reached statistical significance. Marker treatment interaction test was not significant for differential treatment benefit by easTILs%. In multivariable analysis, easTILs% remained prognostic of pCR after adjustment for disease type, hormone receptor status, and randomization to bevacizumab. easTILs% had higher AUC than sTILs%, indicating a better discriminating ability.
We also examined pre- and post-treatment changes in easTILs% in paired samples in patients with residual disease. Cases with pCR were not included in this analysis because of the inability to consistently define the tumor bed for digital analysis, and we also previously demonstrated that in posttreatment tissues with pCR, the immune infiltration is largely resolved15. In the earlier analysis, based on pathologist-read sTILs%, we observed a trend to lower sTILs% in residual cancers compared to paired pretreatment tissues, but this difference has not reached statistical significance. In the current analysis, digital easTILs% was statistically significantly decreased in posttreatment tissues, consistent with a greater quantitative ability to detect differences with digital assessment.
Despite the promising performance of our digital TILs quantification method, there are several caveats. While QuPath is an open-source software, high-quality results require substantial human quality control because tissue pre-fixation time, fixation protocols, and microtome technique can change cell features and cause artifacts on tissue sections leading to poor performance of the classifier. False-positive TILs signal can be generated by apoptotic bodies, neutrophils, tissue artifact, and low-grade tumors with monotonously uniform nuclei2,18.
In summary, we demonstrated that a machine learning-derived digital measure of TILs correlates closely with pathologist-assessed sTILs score and is predictive of pCR in breast cancer. Digital TILs quantification had better outcome discrimination than pathologist-read stromal TILs score.
Methods
Patient cohorts and tissue preparation
Of the 215 patients registered in the S0800 trial, 134 patients had formalin fixed paraffin embedded pretreatment core needle biopsy tissues, 63 patients had posttreatment surgical resection tissues, including 59 paired pre- and post-treatment tissues, with written informed consent for future research (Fig. 8). Hematoxylin and eosin (H&E) stained full sections were used for TILs assessment. We were able to successfully generate digital TILs scores on 113 pre- and 31 post-treatment tissues, including 31 paired specimens. The remaining samples were excluded due to quality control failure, including lack of tumor on the section or artifact with ink or stains on tissue that interfered with image analysis; we also excluded slides where more than 10% of cells were misclassified according to the pathologist’s review (Fig. 9f). Patient characteristics of the S0800 trial population and the digital TILs quantification subpopulation were similar (Table 1). This study was approved by the Yale Cancer Center Human Investigations Committee. Two pathologists generated the pathologist-read sTILs% scores, which were defined as the percentage of invasive cancer stromal area occupied by mononuclear inflammatory cells as described in our previous report15. The reporting recommendations for tumor marker prognostic studies (REMARK) were followed19. This research is the result of data collected in clinical trial NCT00856492.
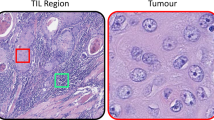
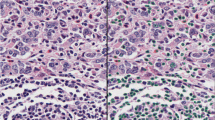
a Invasive breast cancer region delineated at low magnification. b Cell classification mask applied within invasive breast cancer region. c Higher magnification image of invasive breast cancer within the delineated region. d Cell classification mask applied to the region of invasive breast cancer. e Representative image of invasive breast cancer within the delineated region. f Representative image of the case with inaccurate cell classification. g Representative image of invasive breast cancer within the delineated region. h Cell classification mask applied to the region of invasive breast cancer. Color code of cell classification mask: tumor cells (red), tumor-infiltrating lymphocytes (purple), fibroblasts (green), and others (yellow). Scale bar of (a, b): 1 mm; scale bar of (c–h): 20 μm.
Digital image analysis
The Aperio ScanScope CS2 platform (Leica Biosystems, Wetzlar, Germany) was used to scan H&E-stained whole slides at 20x magnification and a pixel size of 0.4986 µm × 0.4986 µm. The QuPath version 0.1.2 open-source image analysis software (https://qupath.readthedocs.io/en/stable/) was used for digital data generation1,2,20. A convolutional neural network algorithm (CNN11) with eight hidden layers (maximum iterations: 100) that was previously trained to assign cells into one of four categories (i) tumor cells, (ii) lymphocytes, (iii) stromal cells, and (iv) other cells on stained sections was used to digitally quantify TILs1,2,21. The intensity of H&E staining varied from slide to slide and therefore, we recalibrated the H&E stain estimates for each digitized slide using the “estimate stain vectors” command in QuPath to produce normalized staining for each slide (Fig. 9). Watershed cell detection22 was used to segment the cells in the images with the following settings: Detection image: hematoxylin OD; requested pixel size: 0.5 µm; background radius: 8 µm; median filter radius: 0 µm; sigma: 1.5 µm; minimum cell area: 10 µm2; maximum cell area: 400 µm2; threshold: 0.1; maximum background intensity: 2. Cell expansion: 5 µm. To enhance classification accuracy, we also added smoothed object features at 25 and 50 µm radius to supplement the measurements of individual cells. The CNN11 tissue annotation consists of cell assignment to one of the four cell types described above, calculation of invasive tumor area (mm2), and calculation of area occupied by each cell type within the tumor area (mm2). The CNN11 algorithm has been deposited on GitHub. To digitally quantify TILs, we used the following formula: easTILs% = [sum of lymphocytes area (mm2)/stromal area (mm2)] × 100 where the stromal area (mm2) is the sum of all invasive tumor region areas (mm2) minus the sum of tumor cell area (mm2). easTILs%, therefore, represents the density of TILs per stromal area within invasive cancer and is the digital equivalent of pathologist scoring of stromal infiltrating lymphocytes as recommended by the International Immuno-Oncology Biomarker Working Group on Breast Cancer11. A subtle difference between sTILs% and easTILs% is that easTILs% includes intratumoral infiltrating lymphocytes, whereas sTILs% excludes these. Inflammatory infiltrates in the stroma of noninvasive lesions and normal breast structures were excluded from both the digital and pathologist-read TILs scores.
Statistical analysis
All available specimens were used in this study, and the sample size was defined by tissue availability. The primary clinical outcome measure was pCR (ypT0/is ypN0). The Mann–Whitney test was used to investigate the association between pCR and easTILs% and sTILs%. Pearson correlation coefficient was used to assess the correlation between pathologist-read sTILs% with paired easTILs%. We also dichotomized easTILs% into low and high categories using our previously published optimal cut point of 19.9%2 and compared pCR rates in the two groups using the Chi-square test. The secondary clinical endpoint was EFS, defined as the time from registration to progression prior to surgery, recurrence post-surgery, or death from any cause. Patients without an event were censored at the time of the last known follow-up. The Kaplan–Meier method and log-rank test were used to plot and compare survival curves implemented in the GraphPad Prism software (GraphPad Software Inc., San Diego, CA) and IBM SPSS Statistics for Macintosh version 26 (IBM Corp., Armonk, N.Y., USA). The two control arms were combined to compare to the bevacizumab arm. The predictive performance of easTILs% and sTILs% were compared using ROC analysis and AUC values implemented in R (version 4.1.0). The difference in AUC was tested using the DeLong test. Change in easTILs% in paired pre- and post-treatment samples was compared by Wilcoxon matched-pairs signed rank test. In all statistical analysis, the level of significance was set at p < 0.05. Multivariable logistic regression models were used to examine predictive factors (ER status, treatment arm, disease type, and easTILs%) for pCR jointly.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data from which the results of this study are calculated are available upon request. The CNN11 algorithm is deposited on GitHub: https://github.com/Yalaibai/Automated_QuPath_TIL_-Classifier_for-TNBC.git. Digitalized images used in this study were deposited into the National Institutes of Health National Cancer Institute The Cancer Imaging Archive (TCIA) at https://doi.org/10.7937/awa3-sc85. The clinical data is deposited on National Cancer Institute NCTN/NCORP Data Archive (https://nctn-data-archive.nci.nih.gov) under NCT00856492-D1.
References
Acs, B. et al. An open source automated tumor infiltrating lymphocyte algorithm for prognosis in melanoma. Nat. Commun. 10, 5440 (2019).
Bai, Y. et al. An open-source, automated tumor-infiltrating lymphocyte algorithm for prognosis in triple-negative breast cancer. Clin. Cancer Res. 27, 5557–5565 (2021).
Saltz, J. et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 23, 181–93.e7 (2018).
Le, H. et al. Utilizing automated breast cancer detection to identify spatial distributions of tumor-infiltrating lymphocytes in invasive breast cancer. Am. J. Pathol. 190, 1491–1504 (2020).
Wimberly, H. et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer.Cancer Immunol. Res. 3, 326–332 (2015).
Loi, S. et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 31, 860–867 (2013).
Adams, S. et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 32, 2959–2966 (2014).
Loi, S. et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann. Oncol. 25, 1544–1550 (2014).
Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 19, 40–50 (2018).
Loi, S. et al. Tumor- infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early- stage triple-negative breast cancers. J. Clin. Oncol. 37, 559–569 (2019).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2015).
Kos, Z. et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 6, 17 (2020).
Nahleh, Z. A. et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab- paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res. Treat. 158, 485–495 (2016).
Li, X. et al. Immune profiling of pre- and post-treatment breast cancer tissues from the SWOG S0800 neoadjuvant trial. J. Immunother. Cancer 7, 88 (2019).
Pelekanou, V. et al. Tumor-infiltrating lymphocytes and PD-L1 expression in pre- and posttreatment breast cancers in the SWOG S0800 phase II neoadjuvant chemotherapy trial. Mol. Cancer Ther. 17, 1324–1331 (2018).
Klauschen, F. et al. Scoring of tumor-infiltrating lymphocytes: from visual estimation to machine learning. Semin. Cancer Biol. 52, 151–157 (2018).
Masucci, G. V. et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I - pre-analytical and analytical validation. J. Immunother. Cancer 4, 76 (2016).
Amgad, M. et al. Report on computational assessment of tumor infiltrating lymphocytes from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 6, 16 (2020).
McShane, L. M. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl Cancer Inst. 97, 1180–1184 (2005).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Bishop, C. M. Neural Networks for Pattern Recognition (Oxford Univ. Press, 1995).
Malpica, N. et al. Applying watershed algorithms to the segmentation of clustered nuclei. Cytometry 28, 289–297 (1997).
Acknowledgements
Susan Komen Foundation Leadership Award (SAC160076) and Breast Cancer Research Foundation Investigator Award (BCRF-21–133) (to L.P.); National Institutes of Health/National Cancer Institute grants U10CA180888 and U10CA180819; and in part by Genentech (Roche), Abraxis BioScience (Celgene), Helomics™. This work was supported in part by a grant from the Breast Cancer Research Foundation to DLR.
Author information
Authors and Affiliations
Contributions
Y.B., D.L.R., K.R.M.B., and L.P. conceived and designed the study. Z.N., W.B., P.S., A.T., A.G., G.H., V.P., and L.P. contributed to the acquisition of the data. Y.B., K.F., Y.L., X.W., W.W., W.B., and K.R.M.B. contributed to the analysis of the data. Y.B., S.S., S.B., W.B., D.L.R., L.P., and K.R.M.B. contributed to the interpretation of the data. Y.B. and K.F. are co-first authors. Y.B., K.F., W.B., L.P., and K.R.M.B. wrote the manuscript. All authors reviewed, revised, and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
L.P. has received consulting fees and honoraria from Pfizer, AstraZeneca, Merck, Novartis, Bristol-Myers Squibb, Genentech, Eisai, Pieris, Immunomedics, Seattle Genetics, Clovis, Syndax, H3Bio, and Daiichi. D.L.R. reports grants and personal fees from Amgen, grants and personal fees from AstraZeneca, personal fees from Cell Signaling Technology, grants and personal fees from Cepheid, personal fees from Danaher, personal fees from Fluidigm, personal fees from GSK, grants and personal fees from Konica Minolta, grants and personal fees from Lilly, personal fees from Merck, personal fees from Monopteros, personal fees from NanoString, grants and personal fees from NextCure, personal fees from Odonate, personal fees from Paige.AI, personal fees from Regeneron, personal fees from Roche, personal fees from Sanofi, personal fees from Ventana, and personal fees from Verily outside the submitted work. A.K.G. is a co-founder of Sinochips Diagnostics, serves as a scientific advisory board member to Biovica, Clara Biotech, and Sinochips Diagnostics, and receives research funding from Predicine and VITRAC Therapeutics. V.P. is currently a Bayer Pharmaceuticals-US employee. P.S. has received consulting fees from Novartis, Merck, Seattle Genetics, Exact Biosciences, Epic Biosciences, Immunomedics, and AstraZeneca. P.S. had received research funding from Novartis, Celgene, Bristol-Myers Squibb, and Merck. A.T’s spouse is an employee of Eli Lilly. K.R.M.B. is on the Scientific Advisory Board of CDI Labs as a non-financial interest. The above and other authors declare no other competing financial or non-financial interests. This research was conducted with support from Genentech.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fanucci, K.A., Bai, Y., Pelekanou, V. et al. Image analysis-based tumor infiltrating lymphocytes measurement predicts breast cancer pathologic complete response in SWOG S0800 neoadjuvant chemotherapy trial. npj Breast Cancer 9, 38 (2023). https://doi.org/10.1038/s41523-023-00535-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00535-0