Abstract
Many patients discontinue endocrine therapy for breast cancer due to intolerance. Identification of patients at risk for discontinuation is challenging. The minimal important difference (MID) is the smallest change in a score on a patient-reported outcome (PRO) that is clinically significant. We evaluated the association between treatment-emergent symptoms detected by worsening PRO scores in units equal to the MID with discontinuation. We enrolled females with stage 0-III breast cancer initiating endocrine therapy in a prospective cohort. Participants completed PROs at baseline, 3, 6, 12, 24, 36, 48, and 60 months. Measures included PROMIS pain interference, fatigue, depression, anxiety, physical function, and sleep disturbance; Endocrine Subscale of the FACT-ES; and MOS-Sexual Problems (MOS-SP). We evaluated associations between continuous PRO scores in units corresponding to MIDs (PROMIS: 4-points; FACT-ES: 5-points; MOS-SP: 8-points) with time to endocrine therapy discontinuation using Cox proportional hazards models. Among 321 participants, 140 (43.6%) initiated tamoxifen and 181 (56.4%) initiated aromatase inhibitor (AI). The cumulative probability of discontinuation was 23% (95% CI 18–27%) at 48 months. For every 5- and 4-point worsening in endocrine symptoms and sleep disturbance respectively, participants were 13 and 14% more likely to discontinue endocrine therapy respectively (endocrine symptoms HR 1.13, 95% CI 1.02–1.25, p = 0.02; sleep disturbance HR 1.14, 95% CI 1.01–1.29, p = 0.03). AI treatment was associated with greater likelihood of discontinuation than tamoxifen. Treatment-emergent endocrine symptoms and sleep disturbance are associated with endocrine therapy discontinuation. Monitoring for worsening scores meeting or exceeding the MID on PROs may identify patients at risk for discontinuation.
Similar content being viewed by others
Introduction
Although 5–10 years of adjuvant endocrine therapy reduces recurrence and death after early hormone receptor-positive (HR + ) breast cancer, approximately 50% of patients are non-adherent (do not take endocrine therapy as prescribed) or non-persistent (discontinue endocrine therapy early)1,2,3,4,5,6,7,8,9,10,11,12,13,14. While some patients discontinue shortly after initiation, others do so later, with persistence overall declining with time15,16. Risks of recurrence and death are higher among those who are non-adherent or who discontinue endocrine therapy early8,17,18,19.
Common symptoms during endocrine therapy include musculoskeletal discomfort, sleep disturbance, fatigue, anxiety, depression, and endocrine symptoms, such as vaginal dryness and hot flashes10,12,20,21,22,23,24,25,26,27,28,29,30. Side effects are frequently cited as a reason for early endocrine therapy discontinuation and multiple studies have demonstrated associations between symptoms and non-adherence or discontinuation9,10,12,16,23,24,26,27,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45. However, prospective identification of patients at risk for early discontinuation due to intolerance remains a clinical challenge.
Symptoms experienced by patients during endocrine therapy are often under-appreciated by clinicians46. Patient-reported outcomes (PRO) are assessments from patients about their health status without interpretation by a clinician47. The minimal important difference (MID) is the smallest change in a score on a PRO measure that patients perceive as beneficial or harmful and that would affect management48. Prior studies evaluating the association of symptoms with endocrine therapy discontinuation have not used changes in PRO scores meeting or exceeding the MID to identify clinically important symptoms.
We report findings from a clinic-based cohort of women receiving endocrine therapy for early HR + breast cancer who completed PRO measures over 5 years. We aimed to evaluate the association of treatment-emergent symptoms, defined as worsening scores compared to baseline in increments equal to the MID for each PRO measure, with discontinuation of endocrine therapy prior to completing 5 years of treatment. We hypothesized that patient-reported new or worsening symptoms could identify individuals at risk for early discontinuation.
Results
Participant characteristics
Of 321 participants, 140 (43.6%) initiated tamoxifen and 181 (56.4%) an aromatase inhibitor (AI). Seventeen (5.3%) participants received ovarian function suppression (OFS) and 6 (1.9%) enrolled upon switching endocrine therapy agents. The median age at enrollment was 63 years and 65.4% were post-menopausal. The majority of participants were White (83.5%) and lived in zip codes with low neighborhood poverty (85.9%). Participants who initiated tamoxifen were younger than those who initiated an AI (Table 1).
Scores on PRO measures
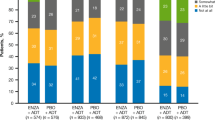
Mean scores for all PRO measures were within one SD of published population means at all time points. The proportion of participants who completed the PROs declined over time (Table 2). Mean changes in PRO scores during the first 24 months were small, and, while statistically significant for worsening endocrine symptoms (p < 0.001), the mean (SD) change in the FACT-ES score from baseline to 24 months was −2.9 (8.3), which is less than 1 MID (Fig. 1). Despite small mean changes, the worst change in the score at any time up to 60 months met or exceeded the MID for each measure in over one-third of participants. Symptom domains with the greatest proportions of participants with score worsening meeting or exceeding the MID at any time up to 60 months were sleep disturbance (54%), endocrine symptoms (53%), sexual problems (48%), and fatigue (46%) (Fig. 2). Treatment-emergent symptoms often developed soon after endocrine therapy initiation (Fig. 3). For example, worsening compared to baseline by at least the MID for sleep disturbance, endocrine symptoms, sexual problems, and fatigue was already observed at 3 months in 27.1%, 26.3%, 23.3%, and 20.3% of participants respectively (Table 3).
Line graphs display median PRO scores at each time point. The size of each dot is proportional to the number of participants who completed the PRO measure at that time point. The numbers of participants who completed the PRO measure at baseline and at 12, 24, 36, 48, and 60 months point are noted under the X-axis at the corresponding time points. The Y-axis denotes the score range for each PRO measure. Bars represent interquartile ranges. P-values summarize overall mean change in PRO scores during the first 24 months compared to baseline with a four-degree-of-freedom test. PRO patient-reported outcomes.
Bar plots display worst change in PRO scores at any time point in follow-up through 60 months. Worst changes are categorized as at least the MID but less than twice the MID, at least twice the MID but less than three times the MID and at least three times the MID for each measure. Only participants with baseline values and at least one follow-up measure are included. The proportions of participants whose worst changes in each PRO scores were less than the MID (i.e. who experienced improvement, no change or worsening less than the MID) are not displayed. The MID was considered to be 4 points for the physical function, depression, anxiety, sleep disturbance, fatigue, and pain interference measures; 8 points for the sexual problems measure; and 5 points for the endocrine symptoms measure. MID minimal important difference, 1 MID at least the MID but less than twice the MID, 2 MID twice the MID but less than three times the MID, 3 MID at least three times the MID, PRO patient-reported outcomes.
Line graphs display percentage of participants with worsening of PRO scores compared to baseline at 3, 6, and 12 months after enrollment. Worsening of PRO scores is categorized as at least the MID but less than twice the MID, at least twice the MID but less than three times the MID and at least three times the MID for each measure. Only participants with baseline values and at least one follow-up measure are included. The percentage of participants whose PRO scores worsened by less than the MID (i.e. who experienced improvement, no change or worsening less than the MID) are not displayed. The MID was considered to be 4 points for the physical function, depression, anxiety, sleep disturbance, fatigue, and pain interference measures; 8 points for the sexual problems measure; and 5 points for the endocrine symptoms measure. MID minimal important difference, 1 MID at least the MID but less than twice the MID, 2 MID twice the MID but less than three times the MID, 3+ MID at least three times the MID, PRO patient-reported outcomes.
Due to the decline in PRO completion rates over time, we performed a sensitivity analysis comparing participants who completed all measures during the first 24 months to those with at least one missing measure during that timeframe. With the exception of differences in the number of concomitant medications and mean baseline fatigue and physical function scores, those with and without missing measures had similar key baseline characteristics. The differences in mean baseline fatigue and physical function between these groups were less than the MID (Table 4).
Cumulative probability of discontinuation
Median follow-up was 56.1 months and 204 (63.6%) participants remained on endocrine therapy at the time of data cut-off. Twenty-six (8.1%) participants had discontinued after completing the planned course and 13 (4.0%) due to recurrence. Five (1.6%) switched from the endocrine therapy initiated at enrollment to another agent with less than 6 weeks interruption. Sixty-three (19.6%) participants had stopped endocrine therapy due to side effects/intolerance and 10 (3.1%) due to other reasons besides recurrence, new primary breast cancer, completion of at least 5 years of endocrine therapy or switching agents. Among the 73 participants who discontinued due to side effects/intolerance or other reasons besides recurrence, new primary breast cancer, completion of at least 5 years of endocrine therapy or switching agents, the times at which they discontinued were distributed across the follow-up period. Ten discontinued in the first 3 months (13.7%), 10 in months 3–6 (13.7%), 6 in months 6–9 (8.2%), 9 in months 9–12 (12.3%), 9 in months 12–18 (12.3%), 8 in months 18–24 (11%), and 21 after 24 months (28.8%). Among participants with and without missing PRO measures in the first 24 months, 25.4% and 11.4% respectively discontinued due to side effects/intolerance (Table 4). The cumulative probabilities of discontinuation at 3, 6, 12, 24, 36, and 48 months were 3% [95% confidence interval (CI): 1–5%], 6% (95% CI: 4–9%), 11% (95% CI: 7–14%), 17% (95% CI: 12–21%), 19% (95% CI: 14–23%), and 23% (95% CI: 18–27%), respectively. Figure 4 depicts the time to discontinuation of endocrine therapy for the entire cohort and according to the type of endocrine therapy.
Association of patient-reported outcomes and clinico-demographic factors with endocrine therapy discontinuation
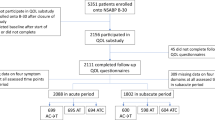
Of the 73 study participants who discontinued endocrine therapy due to side effects/intolerance or due to other reasons besides recurrence, completion of at least 5 years of endocrine therapy or switching agents, 63 completed the PRO measures at the time point immediately before their discontinuation. And, among the 248 participants who did not discontinue endocrine therapy, 190 completed the PRO measures at the time point immediately before they completed follow-up. Therefore, we had complete data for evaluation of the association of PROs with time to discontinuation on 253 (79%) of patients.
In univariate analyses, worsening of multiple symptoms (endocrine symptoms, fatigue, sleep disturbance, and pain interference) plus receipt of AI were associated with endocrine therapy discontinuation. In the final multivariate model, treatment-emergent endocrine symptoms (adjusted HR 1.13, 95% CI 1.02 – 1.25, p = 0.02) and sleep disturbance (adjusted HR 1.14, 95% CI 1.01–1.29, p = 0.03) remained significantly associated with endocrine therapy discontinuation. Additionally, patients receiving AI were twice as likely to discontinue compared to those on tamoxifen (adjusted HR 1.98, 95% CI 1.17–3.33, p = 0.01). Higher stage was associated with lower likelihood of discontinuation (adjusted HR 0.61, 95% CI: 0.43 – 0.87, p = 0.006) (Table 5).
Discussion
In this real-world prospective clinic-based cohort of women with early HR + breast cancer receiving endocrine therapy who completed PRO measures over 5 years, we demonstrated that treatment-emergent symptoms, defined as worsening scores at any time during follow-up compared to baseline, was associated with risk for discontinuing endocrine therapy prior to completing 5 years of treatment. Specifically, new or worsening endocrine symptoms and sleep disturbance were associated with endocrine therapy discontinuation. As the severity of the treatment-emergent symptoms worsened, the risk of discontinuation increased. For every 5-point worsening on the Endocrine Subscale of the FACT-ES measure, the risk of discontinuation was 13% higher, and for every 4-point worsening on the PROMIS sleep disturbance measure, the risk of discontinuation was 14% higher. Worsening of scores meeting or exceeding the MID of on these measures was common, each occurring in approximately half the study population, with approximately half of these participants experiencing worsening by more than twice the MID.
Although multiple factors may contribute to an individual patient’s decision to discontinue endocrine therapy, side effects are a key driver of this decision49. To date, interventions to support adherence and persistence have largely focused on educational or behavioral strategies and have had limited success7,50. We argue that early intervention to mitigate side effects has the potential to enhance endocrine therapy adherence and persistence. The cornerstone to this strategy, however, is comprehensive detection of treatment-emergent symptoms that may drive patients to discontinue therapy. Unfortunately, in routine clinical care, clinicians often underappreciate side effects patients experience during endocrine therapy, and the symptom burden reported by patients often exceeds that detected by clinicians22,46. Our data demonstrate that clinically relevant treatment-emergent symptoms are common during endocrine therapy and that use of PRO measures as an adjunct to routine clinical care can identify symptoms associated with early discontinuation.
When utilizing PRO measures in clinical care, the optimal score thresholds beyond which clinical action should be taken are uncertain and may vary depending on the clinical scenario, symptoms, specific measures, and clinical outcomes to which score thresholds are anchored51,52. It is possible that MIDs may vary by factors such as age, race, ethnicity, socioeconomic status, stage, treatment duration or intervention and, moreover, that MIDs may change over time. Although severity thresholds can identify particularly bothersome symptoms and absolute scores on PROs during the course of endocrine therapy have been associated with discontinuation, worsening of scores over time can indicate changes in supportive care needs and thus present a clinical opportunity to provide symptom management9,53,54,55,56. Our findings demonstrate that treatment-emergent symptoms, defined by score worsening compared to baseline meeting or exceeding the MID for the selected measure, can identify clinically significant symptoms associated with endocrine therapy discontinuation. Patients in whom these symptoms are identified should be targeted for enhanced symptom management with the dual goals of improving symptoms and supporting persistence.
Our findings build on previous work demonstrating that treatment-emergent symptoms often present soon after endocrine therapy initiation, indicating that symptom monitoring with PROs has the potential to identify patients at risk for discontinuation early during the course of therapy to whom interventions could be targeted12,26,31. However, as has also previously been reported, endocrine therapy discontinuation in our cohort increased over time and worsening of symptoms meeting or exceeding the MID at any time during 5 years of follow-up was associated with discontinuation15,16,33,45. Most prior studies have focused on associations between baseline symptoms or those that emerge early during endocrine therapy with discontinuation9,10,12,24,26,27,31,32,37,42. Our study is unique in that it demonstrates that treatment-emergent symptoms at any time over 5 years are associated with endocrine therapy discontinuation, supporting ongoing PRO monitoring throughout the course of therapy.
In our study, the cumulative probabilities of endocrine therapy discontinuation at 6, 12, 24, 36, and 48 months, respectively were 6, 11, 17, 19, and 23%. These observed discontinuation rates are lower than reported in many prior studies8,11. It is possible that completing PRO measures motivated patients to continue endocrine therapy. Alternatively, although we did not mandate that clinicians review scores nor implement symptom management interventions, it is possible that greater patient and clinician awareness of symptoms due to the PROs led to better symptom management and, in turn, to enhanced persistence.
Our findings are consistent with prior studies linking symptoms prior to or during receipt of tamoxifen or an AI such as endocrine symptoms and sleep disturbance with non-adherence and early treatment discontinuation10,23,26,27,31,40,42,57. Endocrine symptoms include vasomotor symptoms, weight changes, vaginal or sexual symptoms, mood changes and joint pain30. In patients with breast cancer, endocrine symptoms, such as hot flashes and night sweats, can disrupt sleep58,59. Evidence-based strategies can mitigate the treatment-emergent symptoms that were associated with early discontinuation of endocrine therapy in our cohort. For example, improved sleep hygiene, exercise, and cognitive behavioral therapy can support patients with sleep disturbance60,61,62,63,64,65,66,67. For patients with hot flashes or sweats, medications such as anti-depressants or gabapentin may improve both daytime symptoms and sleep68,69.
As has been previously reported, endocrine therapy discontinuation was more frequent among participants in our study taking an AI than tamoxifen, a finding potentially attributable to frequent musculoskeletal discomfort during AI therapy32,70,71. We also confirmed previous findings demonstrating that individuals with higher stage disease are less likely to discontinue endocrine therapy, a finding potentially explained by greater motivation to take therapy in light of higher recurrence risk33,35,44. In addition to PRO scores, these factors may guide identification of patients at risk for endocrine therapy discontinuation for interventions to support persistence.
Strengths of our study are that we comprehensively assessed common symptoms during endocrine therapy prospectively over 5 years with validated PRO measures in a real-world population. Many previous studies evaluating symptoms during endocrine therapy used cross-sectional or retrospective designs and, of those that were prospective, many did not use validated measures, assessed fewer symptoms or limited symptom assessment to early during the course of therapy10,12,16,23,26,27,31,32,33,37,40,41,42,57,72.
A key limitation of our study is that the proportion of participants who completed the PROs declined over time. In our sensitivity analysis, baseline characteristics of participants with and without missing measures were similar with the exception of the number of baseline concomitant medications. Some literature demonstrates an association between polypharmacy and endocrine therapy discontinuation, however, this association has not been consistently demonstrated and no prior literature indicates that a difference in one drug (the difference we observed in the median number of concomitant medications for those with and without missing PROs in the first 24 months) is meaningful73,74,75. Thus, we believe our sensitivity analysis indicates patients with and without missing PRO measures are similar and that our findings are robust despite missing data. Of note, however, the proportion of participants who discontinued endocrine therapy due to side effects/intolerance was higher among participants with missing measures suggesting that patients with greater symptom burden may have been less likely to complete the PROs. If participants with greater symptoms did not complete the PROs, it is possible that the association between treatment-emergent symptoms and endocrine therapy discontinuation is even stronger than we estimated. But, the fact that PRO data was complete for assessing the time to discontinuation for 79% of study participants supports our findings regarding the association between treatment-emergent symptoms and endocrine therapy discontinuation. The extent of missing PRO data in our study speaks to the need for strategies to further engage patients in incorporating PROs into their care. Considerations may include reducing the length or frequency of assessments to limit respondent burden and assessing PROs in conjunction with clinic visits.
Another limitation of our study is that few pre-menopausal participants received OFS and an AI, potentially limiting generalizability of our findings to the many young patients who may receive this therapy in light of recently published survival data. Additionally, few participants enrolled upon switching endocrine therapies. Although some patients who switch due to side effects tolerate the second agent, our findings cannot fully address tolerance after switching10. Additionally, we grouped patients taking tamoxifen and an AI, however, the treatment-emergent symptoms that identify those at risk for discontinuation may differ by drug. It is also possible that we misclassified discontinuation status and reasons for discontinuation based on chart review. We did not confirm endocrine therapy administration dates with pharmacy records or pill diaries, nor did we confirm reasons for discontinuation by patient report. Additionally, our analysis evaluated the association between each individual treatment-emergent symptom with discontinuation, but we did not evaluate whether the overall symptom burden affected the risk of discontinuation. Another limitation of our study is that we assessed pain with the PROMIS pain interference measure, a tool not specific to joint pain that may not have been sensitive enough to detect AI-associated musculoskeletal symptoms. It is possible that had we used a more sensitive and specific measure, we would have identified an association between treatment-emergent musculoskeletal pain and endocrine therapy discontinuation as has been reported previously. Additionally, we did not collect any data regarding comorbidities and, for concomitant medications, we only collected the number of medications (including both over the counter and prescribed medications) at baseline in our study, thus we cannot address any potential associations between specific medication classes and their side effects, nor the associations of any changes in concomitant medications during study participation or of any comorbidities, with time-to-discontinuation of endocrine therapy. Furthermore, our study population was predominantly white and high socioeconomic status which may limit generalizability of our findings to other populations.
Finally, it must be noted that while a change in a score of at least the MID is considered clinically significant, it is not certain that a change of this degree is truly the minimal change that is significant48. If anchored to endocrine therapy discontinuation, it is possible that smaller changes in scores would be clinically significant and had we looked at smaller changes, we may have identified associations between discontinuation and worsening of other symptoms as have previously been reported such as musculoskeletal discomfort, depression, and anxiety10,12,23,24,26,32,37,40,41,42. Further study is needed to determine the optimal PRO measures for symptom assessment during endocrine therapy and the score thresholds to best identify treatment-emergent symptoms associated with discontinuation in diverse populations.
The use of PROs to assess symptoms improves clinical outcomes including hospitalization rates, emergency room utilization, chemotherapy delivery and quality of life, effects likely mediated by enhanced symptom detection and subsequent symptom management54,76. To date, whether use of PROs impacts oral cancer therapy persistence has not been prospectively determined. In this real-world cohort of patients taking adjuvant endocrine therapy for early HR + breast cancer, we found that treatment-emergent endocrine symptoms and sleep disturbance detected via PRO measures were associated with early endocrine therapy discontinuation. This finding is proof of the principle that collecting serial PROs during routine clinical care can identify patients at risk for endocrine therapy discontinuation due to side effects who may benefit from symptom management interventions. To this end, we are prospectively evaluating the feasibility of using recommended symptom management pathways for patients receiving endocrine therapy who have symptoms identified on PROs. Future studies will also be needed to clarify the optimal timing, the optimal PRO domains and MIDs to use when implementing PRO collection to identify patients receiving endocrine therapy at risk for treatment discontinuation. Better symptom detection and management has the potential not only to help patients feel better, but also to enhance endocrine therapy persistence, an effect that could ultimately reduce breast cancer recurrence and mortality.
Methods
Study population
We enrolled women with HR + stage 0-III breast cancer-initiating adjuvant endocrine therapy with tamoxifen or an AI to a clinic-based prospective cohort between March 2012 and December 2016 at Johns Hopkins (ClinicalTrials.gov Identifier: NCT01937052, registered September 3, 2013). The study cohort was a convenience sample with candidate participants identified by screening medical oncology provider clinic schedules and by provider referral. Pre-menopausal women could receive concurrent OFS. Participants could enroll when first initiating endocrine therapy or upon switching from one agent to another. All participants signed written informed consent. The study was approved by the Johns Hopkins IRB.
Discontinuation
Endocrine therapy discontinuation was defined as stopping the endocrine therapy initiated at enrollment prior to completing 5 years of treatment for at least 6 weeks due to side effects/intolerance or other reasons besides recurrence, new primary breast cancer, completion of at least 5 years of endocrine therapy or switching to another type of endocrine therapy after less than 6 weeks off therapy. Participants were allowed to continue endocrine therapy beyond 5 years. Endocrine therapy discontinuation and reason for discontinuation were determined by chart review. Reasons for discontinuation were classified as: completed treatment, discontinued due to distant metastases, discontinued due to loco-regional recurrence, discontinued due to side effects/intolerance, discontinued tamoxifen to transition to AI and discontinued due to other reasons. No patient discontinued due to a new primary breast cancer.
Patient-reported outcomes
PROs were collected at baseline and 3, 6, 12, 24, 36, 48, and 60 months using the online PatientViewpoint website77,78,79. Measures included the Patient-Reported Outcome Measurement Information System (PROMIS) Version 1.0 pain interference, fatigue, depression, anxiety, physical function, and sleep disturbance short forms; the Endocrine Subscale of the Functional Assessment of Cancer Therapy - Endocrine Symptom (FACT-ES) measure; and the Medical Outcomes Study Sexual Problems (MOS-SP) scale30,80,81,82,83,84,85,86,87. Treatment-emergent symptoms were defined as worsening of scores at any time compared to baseline in increments meeting or exceeding the MID for each measure. PROMIS measures are scored with a T-score metric for which 50 represents the mean in the United States (US) population and 10 is the standard deviation (SD). Higher scores on PROMIS measures indicate more of the outcome measured. PROMIS measures have been validated in early-stage cancer patients with a MID of 3–5 points80,81,82,83,88. We considered the midpoint of this range (4 points) to be the MID. Endocrine Subscale FACT-ES scores range from 0–76 with lower scores indicating more endocrine symptoms. The mean and SD on the Endocrine Subscale of the FACT-ES in women with early breast cancer are 59 and 9.7 respectively30. In accordance with the distribution-based, Effect Size method of identifying a MID for a PRO measure, we used 0.5 SD to define a medium effect size as a conservative estimate of the MID on the Endocrine Subscale of the FACT-ES and rounded this estimate to 5 points48. MOS-SP scores range from 0–100 with higher scores indicating more sexual problems. The reported mean MOS-SP score for women with early breast cancer ranges from approximately 20–36 with SD approximately 27–3128,85,87. As has previously been done, due to some uncertainty about the mean and SD on the MOS-SP in the literature, we used the distribution-based, Empirical Rule Effect Size method to guide the identification of the MID for the MOS-SP. Based on this method, the SD was assumed to be one-sixth of 100 and the estimated MID was calculated by dividing this number by half, yielding an estimated MID of 8 points28,89. A summary of PRO scores was available to clinicians at the time of follow-up clinic visits.
Statistical analysis
Clinico-demographic characteristics of the participants and PRO scores over time are presented descriptively using mean (SD), median (range or interquartile range), and proportions. We used Fisher’s exact test to compare categorical measures, t-test to compare means and Wilcoxon rank-sum test to compare medians between subgroups of the study population as appropriate. Mean changes in PRO scores in the first 24 months compared to baseline were estimated using a linear mixed-effects modeling approach with the PRO as the outcome, fixed effects for each time point, and a random intercept for each participant. A corresponding four-degree-of-freedom test was used to summarize the overall change in the first 24 months.
The time to discontinuation of endocrine therapy was calculated as the time from study enrollment to endocrine therapy discontinuation. Participants who stopped the endocrine therapy initiated at enrollment due to recurrence, new primary breast cancer, completion of at least 5 years of endocrine therapy or who switched to another type of endocrine therapy after less than 6 weeks interruption were censored. All other participants were censored at the date of the last clinic visit before the database was locked May 15, 2020. Time to discontinuation was estimated for the entire cohort and according to type of endocrine therapy (AI versus Tamoxifen) using the Kaplan-Meier method.
To assess how variables measured at baseline and during follow-up were associated with time to discontinuation, we fit Cox proportional hazards models using a time-dependent covariate structure. Non-time dependent demographic variables considered in the models included age at enrollment, neighborhood poverty rate, and race. Neighborhood poverty rate, the percentage of persons living in a zip code with a family income below the federal poverty line based on US census data, was used as a surrogate for socioeconomic status (SES) with >15% considered indicative of low SES90. Clinical characteristics in the models included stage, HER2 status, type of surgery, receipt of chemotherapy, receipt of radiation, number of self-reported concomitant medications at enrollment and type of endocrine therapy. Time-dependent covariates included change in PRO scores compared to baseline based on MIDs, specified as worsening of scores at any time up to 60 months on the PROMIS measures in 4-point increments, on the Endocrine Subscale of the FACT-ES in 5-point increments, and on the MOS-SP in 8-point increments. First, associations between variables with time to endocrine therapy discontinuation were estimated with univariate models. We then used a forward and backward stepwise selection approach based on Akaike’s Information Criterion (AIC) to identify a final multivariate Cox proportional hazards model for the association of treatment-emergent symptoms, assessed by minimal important changes in PRO scores up to 5 years, and clinico-demographic variables with time to endocrine therapy discontinuation91,92. Analyses were completed with R version 4.0.093.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are not publicly available as they contain information that could compromise individual patient privacy, however they are available upon reasonable request from the corresponding author.
References
Davies, C. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378, 771–784 (2011).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Goss, P. E. et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 349, 1793–1802 (2003).
Goss, P. E. et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N. Engl. J. Med. 375, 209–219 (2016).
Blok, E. J. et al. Optimal Duration of Extended Adjuvant Endocrine Therapy for Early Breast Cancer; Results of the IDEAL Trial (BOOG 2006-05). J. Natl. Cancer Inst. 110 https://doi.org/10.1093/jnci/djx134 (2018).
Tjan-Heijnen, V. C. G. et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 18, 1502–1511 (2017).
Hershman, D. L. et al. Randomized Trial of Text Messaging to Reduce Early Discontinuation of Adjuvant Aromatase Inhibitor Therapy in Women With Early-Stage Breast Cancer: SWOG S1105. J. Clin. Oncol. 38, 2122–2129 (2020).
Hershman, D. L. et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J. Clin. Oncol. 28, 4120–4128 (2010).
Hershman, D. L. et al. Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res. Treat. 157, 133–143 (2016).
Henry, N. L. et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J. Clin. Oncol. 30, 936–942 (2012).
Murphy, C. C., Bartholomew, L. K., Carpentier, M. Y., Bluethmann, S. M. & Vernon, S. W. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res. Treat. 134, 459–478 (2012).
Kadakia, K. C. et al. Patient-Reported Outcomes and Early Discontinuation in Aromatase Inhibitor-Treated Postmenopausal Women With Early Stage Breast Cancer. Oncologist 21, 539–546 (2016).
Partridge, A. H., Wang, P. S., Winer, E. P. & Avorn, J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J. Clin. Oncol. 21, 602–606 (2003).
Partridge, A. H. et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J. Clin. Oncol. 26, 556–562 (2008).
Lambert-Cote, L. et al. Adherence trajectories of adjuvant endocrine therapy in the five years after its initiation among women with non-metastatic breast cancer: a cohort study using administrative databases. Breast Cancer Res. Treat. 180, 777–790 (2020).
Shinn, E. H. et al. Simulating Time-Dependent Patterns of Nonadherence by Patients With Breast Cancer to Adjuvant Oral Endocrine Therapy. JCO Clin. Cancer Inf. 3, 1–9 (2019).
Hershman, D. L. et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 126, 529–537 (2011).
Makubate, B., Donnan, P. T., Dewar, J. A., Thompson, A. M. & McCowan, C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br. J. Cancer 108, 1515–1524 (2013).
Chirgwin, J. H. et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1-98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J. Clin. Oncol. 34, 2452–2459 (2016).
Smith, S. G., Sestak, I., Howell, A., Forbes, J. & Cuzick, J. Participant-Reported Symptoms and Their Effect on Long-Term Adherence in the International Breast Cancer Intervention Study I (IBIS I). J. Clin. Oncol. 35, 2666–2673 (2017).
Sestak, I., Smith, S. G., Howell, A., Forbes, J. F. & Cuzick, J. Early participant-reported symptoms as predictors of adherence to anastrozole in the International Breast Cancer Intervention Studies II. Ann. Oncol. 29, 504–509 (2018).
Hadji, P. et al. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann. Oncol. 25, 372–377 (2014).
Chim, K. et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer 13, 401 (2013).
Kemp, A. et al. Early discontinuation of endocrine therapy for breast cancer: who is at risk in clinical practice? Springerplus 3, 282 (2014).
van Londen, G. J. et al. Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support Care Cancer 22, 937–945 (2014).
Wagner, L. I. et al. Patient-reported predictors of early treatment discontinuation: treatment-related symptoms and health-related quality of life among postmenopausal women with primary breast cancer randomized to anastrozole or exemestane on NCIC Clinical Trials Group (CCTG) MA.27 (E1Z03). Breast Cancer Res Treat. 169, 537–548 (2018).
Kidwell, K. M. et al. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer 120, 2403–2411 (2014).
Ribi, K. et al. Treatment-induced symptoms, depression and age as predictors of sexual problems in premenopausal women with early breast cancer receiving adjuvant endocrine therapy. Breast Cancer Res. Treat. 181, 347–359 (2020).
Mausbach, B. T., Schwab, R. B. & Irwin, S. A. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res. Treat. 152, 239–246 (2015).
Fallowfield, L. J., Leaity, S. K., Howell, A., Benson, S. & Cella, D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res. Treat. 55, 189–199 (1999).
Nabieva, N. et al. Influence of side-effects on early therapy persistence with letrozole in post-menopausal patients with early breast cancer: Results of the prospective EvAluate-TM study. Eur. J. Cancer 96, 82–90 (2018).
Henry, N. L. et al. Associations Between Patient and Anthropometric Characteristics and Aromatase Inhibitor Discontinuation. Clin. Breast cancer 17, 350–355.e354 (2017).
Aiello Bowles, E. J. et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J. Oncol. Pract. 8, e149–e157 (2012).
Kuba, S., Ishida, M., Nakamura, Y., Taguchi, K. & Ohno, S. Persistence and discontinuation of adjuvant endocrine therapy in women with breast cancer. Breast Cancer 23, 128–133 (2016).
Kimmick, G. et al. Medication taking behaviors among breast cancer patients on adjuvant endocrine therapy. Breast 24, 630–636 (2015).
Brett, J. et al. Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer. Eur. J. Cancer Care 27, e12601 (2018).
Yusufov, M. et al. Predictors of increased risk for early treatment non-adherence to oral anti-estrogen therapies in early-stage breast cancer patients. Breast Cancer Res Treat, https://doi.org/10.1007/s10549-020-05920-y (2020).
Lash, T. L., Fox, M. P., Westrup, J. L., Fink, A. K. & Silliman, R. A. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 99, 215–220 (2006).
Bell, R. J., Fradkin, P., Schwarz, M. & Davis, S. R. Understanding discontinuation of oral adjuvant endocrine therapy by women with hormone receptor-positive invasive breast cancer nearly 4 years from diagnosis. Menopause 20, 15–21 (2013).
Moscetti, L. et al. Adjuvant aromatase inhibitor therapy in early breast cancer: what factors lead patients to discontinue treatment? Tumori 101, 469–473 (2015).
Stanton, A. L., Petrie, K. J. & Partridge, A. H. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res. Treat. 145, 525–534 (2014).
Bender, C. M. et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol. Nurs. Forum 41, 274–285 (2014).
Liu, Y., Malin, J. L., Diamant, A. L., Thind, A. & Maly, R. C. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res. Treat. 137, 829–836 (2013).
Wulaningsih, W. et al. Determinants of non-adherence to adjuvant endocrine treatment in women with breast cancer: the role of comorbidity. Breast Cancer Res. Treat. 172, 167–177 (2018).
Guth, U., Myrick, M. E., Kilic, N., Eppenberger-Castori, S. & Schmid, S. M. Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Res. Treat. 131, 491–499 (2012).
Oberguggenberger, A. et al. Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res. Treat. 128, 553–561 (2011).
Snyder, C. F., Jensen, R. E., Segal, J. B. & Wu, A. W. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med. Care 51, S73–S79 (2013).
Revicki, D., Hays, R. D., Cella, D. & Sloan, J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J. Clin. Epidemiol. 61, 102–109 (2008).
Bluethmann, S. M. et al. Deconstructing Decisions to Initiate, Maintain, or Discontinue Adjuvant Endocrine Therapy in Breast Cancer Survivors: A Mixed-Methods Study. Oncol. Nurs. Forum 44, E101–E110 (2017).
Hurtado-de-Mendoza, A., Cabling, M. L., Lobo, T., Dash, C. & Sheppard, V. B. Behavioral Interventions to Enhance Adherence to Hormone Therapy in Breast Cancer Survivors: A Systematic Literature Review. Clin. Breast Cancer 16, 247–255 e243 (2016).
Basch, E., Barbera, L., Kerrigan, C. L. & Velikova, G. Implementation of Patient-Reported Outcomes in Routine Medical Care. Am. Soc. Clin. Oncol. Educ. Book 38, 122–134 (2018).
Gensheimer, S. G., Wu, A. W. & Snyder, C. F., Group, P.-E. U. G. S. & Group, P.-E. U. G. W. Oh, the Places We’ll Go: Patient-Reported Outcomes and Electronic Health Records. Patient 11, 591–598 (2018).
Basch, E. et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA, https://doi.org/10.1001/jama.2017.7156 (2017).
Basch, E. et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 34, 557–565 (2016).
Snyder, C. F. et al. Can patient-reported outcome measures identify cancer patients’ most bothersome issues? J. Clin. Oncol. 29, 1216–1220 (2011).
Snyder, C. F. et al. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients’ supportive care needs. Qual. Life Res. 24, 1207–1216 (2015).
Brett, J. et al. Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer. Eur. J. Cancer Care (Engl) 27, https://doi.org/10.1111/ecc.12601 (2018).
Lowery-Allison, A. E. et al. Sleep problems in breast cancer survivors 1-10 years posttreatment. Palliat. Support Care 16, 325–334 (2018).
Chang, H. Y. et al. Hot flashes in breast cancer survivors: Frequency, severity and impact. Breast 27, 116–121 (2016).
Tomlinson, D., Diorio, C., Beyene, J. & Sung, L. Effect of exercise on cancer-related fatigue: a meta-analysis. Am. J. Phys. Med Rehabil. 93, 675–686 (2014).
Mishra, S. I. et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane database Syst. Rev. 2012, CD007566–CD007566 (2012).
Cheville, A. L. et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J. Pain. Symptom Manag. 45, 811–821 (2013).
Mishra, S. I. et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 2012, Cd008465 (2012).
Payne, J. K., Held, J., Thorpe, J. & Shaw, H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol. Nurs. Forum 35, 635–642 (2008).
Rogers, L. Q. et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr. Cancer Ther. 12, 323–335 (2013).
Roscoe, J. A. et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J. Clin. Oncol. 33, 165–171 (2015).
Johnson, J. A. et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep. Med Rev. 27, 20–28 (2016).
Ramaswami, R. et al. Venlafaxine in management of hot flashes in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 152, 231–237 (2015).
Garland, S. N. et al. Comparative effectiveness of electro-acupuncture versus gabapentin for sleep disturbances in breast cancer survivors with hot flashes: a randomized trial. Menopause 24, 517–523 (2017).
Hadji, P. et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res. Treat. 138, 185–191 (2013).
Farias, A. J. & Du, X. L. Racial Differences in Adjuvant Endocrine Therapy Use and Discontinuation in Association with Mortality among Medicare Breast Cancer Patients by Receptor Status. Cancer Epidemiol. Biomark. Prev. 26, 1266 (2017).
Wheeler, S. B. et al. Endocrine Therapy Nonadherence and Discontinuation in Black and White Women. J. Natl. Cancer Inst. 111, 498–508 (2019).
Lambert, L. K., Balneaves, L. G., Howard, A. F. & Gotay, C. C. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res. Treat. 167, 615–633 (2018).
Calip, G. S. et al. Polypharmacy and Adherence to Adjuvant Endocrine Therapy for Breast Cancer. J. Oncol. Pr. 13, e451–e462 (2017).
van Herk-Sukel, M. P. et al. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res. Treat. 122, 843–851 (2010).
Barbera, L. et al. Impact of Standardized Edmonton Symptom Assessment System Use on Emergency Department Visits and Hospitalization: Results of a Population-Based Retrospective Matched Cohort. Anal. JCO Oncol. Pr. 16, e958–e965 (2020).
Snyder, C. F., Jensen, R., Courtin, S. O. & Wu, A. W., Website for Outpatient, Q. O. L. A. R. N. PatientViewpoint: a website for patient-reported outcomes assessment. Qual. Life Res. 18, 793–800 (2009).
Snyder, C. F. et al. Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psychooncology 22, 895–901 (2013).
Wu, A. W. et al. Improving an electronic system for measuring PROs in routine oncology practice. J. Cancer Surviv 10, 573–582 (2016).
Jensen, R. E. et al. Responsiveness of 8 Patient-Reported Outcomes Measurement Information System (PROMIS) measures in a large, community-based cancer study cohort. Cancer 123, 327–335 (2017).
Jensen, R. E. et al. United States Population-Based Estimates of Patient-Reported Outcomes Measurement Information System Symptom and Functional Status Reference Values for Individuals With Cancer. J. Clin. Oncol. 35, 1913–1920 (2017).
Schalet, B. D. et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J. Clin. Epidemiol. 73, 119–127 (2016).
Teresi, J. A., Ocepek-Welikson, K., Kleinman, M., Ramirez, M. & Kim, G. Measurement Equivalence of the Patient Reported Outcomes Measurement Information System. Psychol. Test. Assess. Model 58, 183–219 (2016).
Bartula, I. & Sherman, K. A. Screening for sexual dysfunction in women diagnosed with breast cancer: systematic review and recommendations. Breast Cancer Res Treat. 141, 173–185 (2013).
Burwell, S. R., Case, L. D., Kaelin, C. & Avis, N. E. Sexual problems in younger women after breast cancer surgery. J. Clin. Oncol. 24, 2815–2821 (2006).
Ganz, P. A. et al. Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 387, 857–865 (2016).
Sherbourne, C. D. Social functioning: sexual problems measures. In: Stewart A. L., Ware J. E. (eds) Measuring functioning and well-being: the medical outcomes study approach. 194-204 (Duke University Press, 1992).
Yost, K. J., Eton, D. T., Garcia, S. F. & Cella, D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J. Clin. Epidemiol. 64, 507–516 (2011).
Sloan, J. A. & Dueck, A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J. Biopharm. Stat. 14, 73–96 (2004).
Zager, S. et al. Neighborhood poverty rate and mortality in patients receiving critical care in the academic medical center setting. Chest 139, 1368–1379 (2011).
Hastie, T. J. & Pregibon, D. in Statistical Models in S (eds J. M. Chambers & T. J. Hastie) Ch. 6, (Wadsworth Brooks/Cole, 1992).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S (4th Edition). (Springer, 2002).
R: A language and environment for statistical computing. R Foundation for Statistical Computing. (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2020).
Acknowledgements
This work was supported by Susan G. Komen Foundation and National Institutes of Health [P30 CA006973]
Author information
Authors and Affiliations
Contributions
Conceptualization/Design: K.L.S., V.S., K.W., C.S. Funding Acquisition: V.S. Resources: C.S. Data Acquisition: K.L.S., J.F., A.C.W., D.J., R.S.M., R.C., D.K.A., R.N., K.V., C.R., K.P., N.Z., V.S. Data Curation: J.L., N.V. Analysis: D.L., A.B. Interpretation of Data: All authors. Writing-original draft: K.L.S., N.V. Writing-review and editing: All authors. Approval of final manuscript: All authors. Agreement to be accountable for all aspects of work: All authors. Accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Corresponding author
Ethics declarations
Competing interests
Karen Lisa Smith has received research support (to institution) from Pfizer. Karen Lisa Smith’s spouse has stock ownership in ABT Labs and Abbvie. Jennifer Y. Sheng has received research support (to institution) from Pfizer. Roisin Connolly has received an unrestricted educational grant to her institution from Pfizer. Vered Stearns has received research grants (to institution) from Pfizer and Novartis Claire Snyder has research funding (to institution) from Pfizer and Genentech. The following authors declare that they have no conflicts of interest related to the work presented in this manuscript: Neha Verma, Amanda Blackford, Jennifer Lehman, Kelly Westbrook, David Lim, John Fetting, Antonio Wolff, Danijela Jelovac, Robert Miller, Deborah Armstrong, Raquel Nunes, Kala Visvanathan, Carol Riley, Katie Papathakis, Nelli Zafman.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, K.L., Verma, N., Blackford, A.L. et al. Association of treatment-emergent symptoms identified by patient-reported outcomes with adjuvant endocrine therapy discontinuation. npj Breast Cancer 8, 53 (2022). https://doi.org/10.1038/s41523-022-00414-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-022-00414-0
This article is cited by
-
Concurrent factors associated with adherence to adjuvant endocrine therapy among women with non-metastatic breast cancer
Journal of Cancer Survivorship (2024)
-
Factors associated with weight gain in pre- and post-menopausal women receiving adjuvant endocrine therapy for breast cancer
Journal of Cancer Survivorship (2023)