Abstract
In angiosperms, flower development requires the combined action of the transcription factor LEAFY (LFY) and the ubiquitin ligase adaptor F-box protein, UNUSUAL FLORAL ORGANS (UFO), but the molecular mechanism underlying this synergy has remained unknown. Here we show in transient assays and stable transgenic plants that the connection to ubiquitination pathways suggested by the UFO F-box domain is mostly dispensable. On the basis of biochemical and genome-wide studies, we establish that UFO instead acts by forming an active transcriptional complex with LFY at newly discovered regulatory elements. Structural characterization of the LFY–UFO–DNA complex by cryo-electron microscopy further demonstrates that UFO performs this function by directly interacting with both LFY and DNA. Finally, we propose that this complex might have a deep evolutionary origin, largely predating flowering plants. This work reveals a unique mechanism of an F-box protein directly modulating the DNA binding specificity of a master transcription factor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The ampDAP-seq data have been deposited at GEO and are publicly available as of the date of publication (GSE204793). The cryo-EM structure determined in this study is deposited in the EM data bank under the reference number EMD-15145. The .pdb file of the model is available in the Supplementary Information. Any additional information required to reanalyse the data reported in this paper is available from the corresponding author upon request. The biological materials generated in this study are available from the corresponding author without restriction. Source data are provided with this paper.
Code availability
All original code has been deposited at GitHub (https://github.com/Bioinfo-LPCV-RDF/LFYUFO_project) and is publicly available as of the date of publication.
References
Moyroud, E., Kusters, E., Monniaux, M., Koes, R. & Parcy, F. LEAFY blossoms. Trends Plant Sci. 15, 346–352 (2010).
Irish, V. F. The flowering of Arabidopsis flower development. Plant J. 61, 1014–1028 (2010).
Parcy, F., Nilsson, O., Busch, M. A., Lee, I. & Weigel, D. A genetic framework for floral patterning. Nature 395, 561–566 (1998).
Wagner, D., Sablowski, R. W. M. & Meyerowitz, E. M. Transcriptional activation of APETALA1 by LEAFY. Science 285, 582–584 (1999).
Lohmann, J. U. et al. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105, 793–803 (2001).
Lee, I., Wolfe, D. S., Nilsson, O. & Weigel, D. A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr. Biol. 7, 95–104 (1997).
Levin, J. Z. & Meyerowitz, E. M. UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7, 529–548 (1995).
Wilkinson & Haughn UNUSUAL FLORAL ORGANS controls meristem identity and organ primordia fate in Arabidopsis. Plant Cell 7, 1485–1499 (1995).
Krizek, B. A. & Meyerowitz, E. M. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122, 11–22 (1996).
Ikeda-Kawakatsu, K., Maekawa, M., Izawa, T., Itoh, J.-I. & Nagato, Y. ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1. Plant J. 69, 168–180 (2012).
Lippman, Z. B. et al. The making of a compound inflorescence in tomato and related nightshades. PLoS Biol. 6, e288 (2008).
Souer, E. et al. Patterning of inflorescences and flowers by the F-box protein DOUBLE TOP and the LEAFY homolog ABERRANT LEAF AND FLOWER of petunia. Plant Cell Online 20, 2033–2048 (2008).
Kuzay, S. et al. WAPO-A1 is the causal gene of the 7AL QTL for spikelet number per spike in wheat. PLoS Genet. 18, e1009747 (2022).
Ingram, G. C. et al. Dual role for fimbriata in regulating floral homeotic genes and cell division in Antirrhinum. EMBO J. 16, 6521–6534 (1997).
Samach, A. et al. The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant J. 20, 433–445 (1999).
Simon, R., Carpenter, R., Doyle, S. & Coen, E. Fimbriata controls flower development by mediating between meristem and organ identity genes. Cell 78, 99–107 (1994).
Wang, X. et al. The COP9 signalosome interacts with SCF UFO and participates in Arabidopsis flower development. Plant Cell 15, 1071–1082 (2003).
Chae, E., Tan, Q. K.-G., Hill, T. A. & Irish, V. F. An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development 135, 1235–1245 (2008).
Geng, F., Wenzel, S. & Tansey, W. P. Ubiquitin and proteasomes in transcription. Annu. Rev. Biochem. 81, 177–201 (2012).
Risseeuw, E. et al. An activated form of UFO alters leaf development and produces ectopic floral and inflorescence meristems. PLoS ONE 8, e83807 (2013).
Krizek, B. A., Lewis, M. W. & Fletcher, J. C. RABBIT EARS is a second-whorl repressor of AGAMOUS that maintains spatial boundaries in Arabidopsis flowers. Plant J. 45, 369–383 (2006).
Busch, M. A., Bomblies, K. & Weigel, D. Activation of a floral homeotic gene in Arabidopsis. Science 285, 585–587 (1999).
Hill, T. A., Day, C. D., Zondlo, S. C., Thackeray, A. G. & Irish, V. F. Discrete spatial and temporal cis-acting elements regulate transcription of the Arabidopsis floral homeotic gene APETALA3. Development 125, 1711–1721 (1998).
Lamb, R. S., Hill, Ta., Tan, Q. K.-G. & Irish, V. F. Regulation of APETALA3 floral homeotic gene expression by meristem identity genes. Development 129, 2079–2086 (2002).
Goslin, K. et al. Transcription factor interplay between LEAFY and APETALA1/CAULIFLOWER during floral initiation. Plant Physiol. 174, 1097–1109 (2017).
Sayou, C. et al. A SAM oligomerization domain shapes the genomic binding landscape of the LEAFY transcription factor. Nat. Commun. 7, 11222 (2016).
Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859 (1992).
Weigel, D. & Nilsson, O. A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500 (1995).
Hamès, C. et al. Structural basis for LEAFY floral switch function and similarity with helix-turn-helix proteins. EMBO J. 27, 2628–2637 (2008).
Sayou, C. et al. A promiscuous intermediate underlies the evolution of LEAFY DNA binding specificity. Science 343, 645–648 (2014).
Zhao, D., Yu, Q., Chen, M. & Ma, H. The ASK1 gene regulates B function gene expression in cooperation with UFO and LEAFY in Arabidopsis. Development 128, 2735–2746 (2001).
Ni, W. et al. Regulation of flower development in Arabidopsis by SCF complexes. Plant Physiol. 134, 1574–1585 (2004).
Levin, J. Z., Fletcher, J. C., Chen, X. & Meyerowitz, E. M. A genetic screen for modifiers of UFO meristem activity identifies three novel FUSED FLORAL ORGANS genes required for early flower development in Arabidopsis. Genetics 149, 579–595 (1998).
Singh, N. & Bhalla, N. Moonlighting Proteins. Annu. Rev. Genet. 54, 265–285 (2020).
Honma, T. & Goto, K. The Arabidopsis floral homeotic gene PISTILLATA is regulated by discrete cis-elements responsive to induction and maintenance signals. Development 127, 2021–2030 (2000)
Tilly, J. J., Allen, D. W. & Jack, T. The CArG boxes in the promoter of the Arabidopsis floral organ identity gene APETALA3 mediate diverse regulatory effects. Development 125, 1647–1657 (1998).
Liu, C., Xi, W., Shen, L., Tan, C. & Yu, H. Regulation of floral patterning by flowering time genes. Dev. Cell 16, 711–722 (2009).
Gregis, V., Sessa, A., Colombo, L. & Kater, M. M. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell 18, 1373–1382 (2006).
Castillejo, C., Romera-Branchat, M. & Pelaz, S. A new role of the Arabidopsis SEPALLATA3 gene revealed by its constitutive expression. Plant J. 43, 586–596 (2005).
Siggers, T., Duyzend, M. H., Reddy, J., Khan, S. & Bulyk, M. L. Non-DNA-binding cofactors enhance DNA-binding specificity of a transcriptional regulatory complex. Mol. Syst. Biol. 7, 555 (2011).
Babb, R., Huang, C., Aufiero, D. J. & Herr, W. DNA recognition by the herpes simplex virus transactivator VP16: a novel DNA-binding structure. Mol. Cell. Biol. 21, 4700–4712 (2001).
Chahtane, H. et al. LEAFY activity is post-transcriptionally regulated by BLADE ON PETIOLE2 and CULLIN3 in Arabidopsis. N. Phytol. 220, 579–592 (2018).
Blanvillain, R. et al. The Arabidopsis peptide kiss of death is an inducer of programmed cell death. EMBO J. 30, 1173–1183 (2011).
Takeda, S., Matsumoto, N. & Okada, K. RABBIT EARS, encoding a SUPERMAN-like zinc finger protein, regulates petal development in Arabidopsis thaliana. Development 131, 425–434 (2004).
Benlloch, R. et al. Integrating long-day flowering signals: a LEAFY binding site is essential for proper photoperiodic activation of APETALA1. Plant J. 67, 1094–1102 (2011).
Iwata, Y., Lee, M. H. & Koizumi, N. Analysis of a transcription factor using transient assay in Arabidopsis protoplasts. Methods Mol. Biol. 754, 107–117 (2011).
Dümmler, A., Lawrence, A. M. & de Marco, A. Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb. Cell Fact. 4, 34 (2005).
Lai, X. et al. The LEAFY floral regulator displays pioneer transcription factor properties. Mol. Plant 14, 829–837 (2021).
Bartlett, A. et al. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat. Protoc. 12, 1659–1672 (2017).
Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl Acad. Sci. USA 97, 3718–3723 (2000).
Chahtane, H. et al. A variant of LEAFY reveals its capacity to stimulate meristem development by inducing RAX1. Plant J. 74, 678–689 (2013).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Terwilliger, T. C. et al. Improved AlphaFold modeling with implicit experimental information. Nat. Methods 19, 1376–1382 (2022).
Lai, X. et al. Genome-wide binding of SEPALLATA3 and AGAMOUS complexes determined by sequential DNA-affinity purification sequencing. Nucleic Acids Res. 48, 9637–9648 (2020).
Gaspar, J. M. NGmerge: merging paired-end reads via novel empirically-derived models of sequencing errors. BMC Bioinform. 19, 536 (2018).
Berardini, T. Z. et al. The Arabidopsis Information Resource: making and mining the ‘gold standard’ annotated reference plant genome. Genesis 53, 474–485 (2015).
Jalili, V., Matteucci, M., Masseroli, M. & Morelli, M. J. Using combined evidence from replicates to evaluate ChIP-seq peaks. Bioinformatics 31, 2761–2769 (2015).
Machanick, P. & Bailey, T. L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 (2011).
Stigliani, A. et al. Capturing auxin response factors syntax using DNA binding models. Mol. Plant 12, 822–832 (2019).
Moyroud, E. et al. Prediction of regulatory interactions from genome sequences using a biophysical model for the Arabidopsis LEAFY transcription factor. Plant Cell 23, 1293–1306 (2011).
Sachs, M. C. plotROC: A Tool for Plotting ROC Curves. J. Stat. Softw. 79, 1–19 (2017)..
Schmid, M. et al. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37, 501–506 (2005).
Wu J. et al. gcrma: Background Adjustment Using Sequence Information. R package version 2.70.0. (2022). https://bioconductor.org/packages/release/bioc/manuals/gcrma/man/gcrma.pdf
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Jin, R. et al. LEAFY is a pioneer transcription factor and licenses cell reprogramming to floral fate. Nat. Commun. 12, 626 (2021).
Quinlan, A.R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
RStudio Team RStudio: Integrated Development for R. (RStudio, PBC, Boston, MA, 2020). http://www.rstudio.com/
Gagne, J. M., Downes, B. P., Shiu, S. H., Durski, A. M. & Vierstra, R. D. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 11519–11524 (2002).
Zhang, S. et al. Proliferating floral organs (pfo), a Lotus japonicus gene required for specifying floral meristem determinacy and organ identity, encodes an F-box protein. Plant J. 33, 607–619 (2003).
Zhao, Y. et al. Evolutionary co-option of floral meristem identity genes for patterning of the flower-like Asteraceae inflorescence. Plant Physiol. 172, 284–296 (2016).
Chen, Y. et al. CsUFO is involved in the formation of flowers and tendrils in cucumber. Theor. Appl. Genet. 3, 2141–2150 (2021).
Ikeda, K., Ito, M., Nagasawa, N., Kyozuka, J. & Nagato, Y. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant J. 51, 1030–1040 (2007).
Li, F. et al. Reduced expression of CbUFO is associated with the phenotype of a flower-defective Cosmos bipinnatus. Int. J. Mol. Sci. 20, 2503 (2019).
Sasaki, K. et al. Mutation in Torenia fournieri Lind. UFO homolog confers loss of TfLFY interaction and results in a petal to sepal transformation. Plant J. 71, 1002–1014 (2012).
Sharma, B. et al. Homologs of LEAFY and UNUSUAL FLORAL ORGANS promote the transition from inflorescence to floral meristem identity in the cymose Aquilegia coerulea. Front. Plant Sci. 10, 1218 (2019).
Taylor, S., Hofer, J. & Murfet, I. Stamina pistilloida, the pea ortholog of Fim and UFO, is required for normal development of flowers, inflorescences, and leaves. Plant Cell 13, 31–46 (2001).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Acknowledgements
We thank A. M. Boisson for preparing the suspension cells, X. Lai for the ampDAP-seq libraries and technical assistance and R. Koes for sharing data and materials. We acknowledge C. Marondedze, G. Vachon, M. Le Masson, C. Berthollet, B. Orlando Marchesano and J. Bourenane-Vieira for help with the experiments. We thank G. Vert, U. Dolde and R. Dumas for discussion. The electron microscopy facility is supported by the Rhône-Alpes Region, the FRM, the FEDER and the GIS-IBISA. This work used the platforms of the Grenoble Instruct-ERIC centre (ISBG; UAR 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology, supported by FRISBI (ANR-10-INBS-0005-02). We thank C. Mas for assistance and access to the biophysical platform. This work was supported by the GRAL Labex financed within the University Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (ANR-17-EURE-0003), the CEA (PhD fellowship to P.R.) and the ANR-17-CE20-0014-01 Ubiflor project to F.P.
Author information
Authors and Affiliations
Contributions
F.P. and P.R. designed the project. P.R. performed the plant experiments with assistance from G.T. P.R. and E.T. performed the biochemical experiments with assistance from H.C. on the evolutionary analyses. L.T. performed the bioinformatics analyses with assistance from J.L. and R.B.-M. E.Z. and G.S. performed the cryo-EM experiments, and M.N., E.Z., C.Z. and G.S. analysed the data. P.R. and L.T. assembled the figures. P.R. and F.P. wrote the paper with contributions from L.T. and C.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Nobutoshi Yamaguchi, Aiwu Dong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 UFO has SCF-dependent and independent functions.
a-c, pAP3 activation measured by DLRA in Arabidopsis protoplasts. EV = Empty Vector (pRT104-3xHA). UFOΔFbox corresponds to a deletion of the whole N-terminal part comprising the F-box domain (aa. 1-90), while UFOdelF corresponds to a previously-described internal deletion in the F-box domain (aa. 50-62)20. Data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4). One-way ANOVA with Tukey’s multiple comparisons tests. Stars above bars represent a significant statistical difference compared to 3xHA-LFY + EV or 3xHA-LFY-VP16 + EV negative controls (NS: p > 0.05,*: p < 0.05, **: p < 0.01, ***: p < 0.001 and ****: p < 0.0001). d, Western Blot on protein extracts from independent T1 plants from different phenotypic classes described in Fig. 1g (one independent line per lane). 35S::UFO-5xmyc (line 178-#19) and 35S::UFO-3xFLAG (line 177-#6) plants were used as positive controls. Total proteins were extracted from rosette leaves. Note the difference of molecular weight between UFO and UFOΔFbox. Loss-of-function defects are likely due to silencing of both transgene-encoded UFOΔFbox and endogenous UFO. e, Western Blot on protein extracts from F2 plants described in Fig. 1h. Total proteins were extracted from rosette leaves. f, ufo-1 complementation assay with other 35S::UFO and 35S::UFO∆Fbox lines. Rosette leaves (right, scale bar, 1 cm), inflorescence (middle, scale bar 1 mm) and flower (right, scale bar, 0.5 mm) phenotypes are shown. Primary inflorescences were removed to observe rosette phenotype. For each construct, at least 5 plants were analyzed per line. As in Risseeuw et al, our 35S::UFO lines displayed relatively milder phenotypes than the 35S::UFO phenotypes reported by Lee et al.6,20. Note that the 35S::UFO-5xmyc 178-#2 line did not display the serrated leaves phenotype. g, Sequence alignment of UFO N-terminal region. The F-box domain is represented76. In selected species, presented proteins were identified as UFO homologs and their role was confirmed genetically7,11,12,16,77,78,79,80,81,82,83,84. Source data are available in Supplementary Data 4.
Extended Data Fig. 2 pAP3 DEE LFYBS is not required for LFY-UFO-dependent pAP3 activation.
a, Schematic representation of pAP3. Top row represents WT pAP3 with regulatory regions and cis-elements. Orange triangle represents LFYBS. The second row represents the scores for the best LFYBS obtained by scanning WT pAP3 sequence with LFY PWM68 (the best binding sites correspond to the less negative score values). Other rows represent the different pAP3 versions used in (b) and (c). LFYBS mutation corresponds to the previously described site1m-site2m mutation24. b,c, pAP3 activation with promoter versions described in (a) and indicated effectors. For bar charts, data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4). Unpaired t-tests (b,c). (NS: p > 0.05,*: p < 0.05, **: p < 0.01, ***: p < 0.001). Source data are available in Supplementary Data 4.
Extended Data Fig. 3 Analysis of pAP3 activation by LFY-UFO.
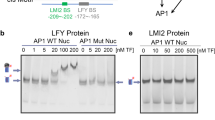
a, Description of pAP3. Top line represents WT pAP3 with regulatory regions and cis-elements. Coordinates are relative to AP3 start codon. TSS: Transcription Start Site. Orange triangle represents LFYBS. Other rows show the promoter versions used in (b) and (c). Green rectangles in swapped versions correspond to the same random sequence. b,c, pAP3 LFY-UFO response element mapping with pAP3 versions described in (a) by DLRA in Arabidopsis protoplasts. Data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4). One-way ANOVA with Tukey’s multiple comparisons test (c). One-way ANOVA was performed with data from the same effector, and stars represent a statistical difference compared to WT pAP3. Unpaired t-tests (b). (NS: p > 0.05,*: p < 0.05, **: p < 0.01; ***: p < 0.001). d, EMSA with ASK1-UFO, LFY-DBD and LUBS0 DNA probe. Different competitor DNA concentrations were tested as indicated. e, Molecular mass determination for ASK1-UFO-LFY-DBD in complex with LUBS0 DNA by SEC-MALLS (top). Elution profiles correspond to absorbance at 280 nm and 260 nm (left ordinate axis, A.U: Arbitrary Unit). The black line shows the molecular mass distribution (right ordinate axis). A mass of 102 ± 3.3 kDa was found for this ASK1-UFO-LFY-DBD-LUBS0 complex, consistent with one copy of each protein per DNA molecule (theoretical mass of 108 kDa). Coomassie-stained SDS-PAGE gel of the different SEC-MALLS fractions (bottom). Each lane corresponds to a 0.5 mL fraction. Molecular weights of the protein standards are indicated (BioRad Precision Plus). Faint bands above UFO likely correspond to contaminants. f, EMSA with ASK1-UFO, LFY-DBD and indicated DNA probes. Sequences with coordinates relative to AP3 start codon (left). Red letters indicate mutated bases. Bars under sequences represent the regions required for ASK1-UFO-LFY-DBD binding. EMSA with described DNA probes (right). Each DNA probe was mixed with the same ASK1-UFO-LFY-DBD protein mix. Note that the LUBS0 mutation also reduced pAP3 activation in protoplasts (Fig. 2b). Source data are available in Supplementary Data 4.
Extended Data Fig. 4 Genome-wide analysis of LFY-UFO binding.
a, Western Blot after DNA elution during ampDAP-seq experiment. After DNA elution, 20 µL of 1X SDS-PAGE Protein Sample Buffer was added to the remaining beads to run WB. Each lane represents one replicate. b, Assessment of experimental reproducibility of ampDAP-seq experiment through the comparison of replicates datasets 2 by 2. c, Effect of the LFY KARA mutation (K303A-R233A)51 on pAP3 activation in Arabidopsis protoplasts. Data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4). Unpaired t-tests (**: p < 0.01; ****: p < 0.0001). d, The LFY KARA mutation (K303A-R233A) does not disrupt LFY-UFO interaction in Yeast-Two-Hybrid (Y2H). EV = Empty Vector. LFY-40 is a LFY version lacking the first 40 aa and better tolerated by yeast cells. Values correspond to the different dilutions (OD = 7, 0.7 and 0.07). Top picture corresponds to the non-selective plate lacking Leucine and Tryptophan (SD -L-W), and bottom picture to the selective plate lacking Leucine, Tryptophan, Histidine and Adenine (SD -L-W-A-H). Pictures were taken at day + 4. e, Receiver operating characteristics (ROC) curves for mLUBS, dLUBS and LFY using the top 20% high-CFC LFY-UFO-specific peaks. Area under the curve (AUC) values are shown. TPR: True Positive Rate, FPR: False Positive Rate. f, Score distribution of LFY PWM with dependencies68 within dLUBS (best site on 20% most LFY-UFO-specific genomic regions, high CFC, n = 3843 genomic regions) and in canonical LFYBS (best site on 20% most LFY-specific genomic regions, low CFC, n = 3843 genomic regions). Best sites were selected within ±25 bp around the peak maximum. Wilcoxon rank sum test (****: p < 0.0001). Median (solid line), interquartile range (box edges), ±1.5 × interquartile range (whiskers) and outliers (black dot) are shown. g, De novo identification of URM from LFY ChIP-seq data25. Motifs identified at a fixed distance from LFY canonical binding sites in 298 regions harboring high LFY ChIP-seq to LFY ampDAP-seq coverage ratio. The text above each motif gives the motif’s start position relative to the canonical LFYBS, its length and the number of sites used to build the motif. h, EMSA with mLUBS and dLUBS highest score sequences. 6xHis-LFY-DBD is recombinant. UFO* refers to either recombinant ASK1-UFO-3xFLAG complex (top gel) or in vitro produced UFO-3xFLAG (bottom gel). Drawings represent the different types of complexes involving LFY-DBD (pale blue) and ASK1-UFO (red) on DNA. LFY-DBD binds as a monomer as previously reported29. The fact that in vitro produced UFO-3xFLAG shifts DNA in the presence of LFY indicates that ASK1 is not required for the UFO-LFY-DNA complex formation in vitro. i, EMSA with DNA probes corresponding to pAP1 and pAP3 DEE LFYBS and indicated proteins. Note that probes used here have the same length as those used to study LUBS. Source data are available in Supplementary Data 4.
Extended Data Fig. 5 pAP3 LUBS are required for LFY-UFO-dependent activation.
a, EMSA with indicated probes and proteins. LUBS3 is the third highest-score pAP3 LUBS. Because LUBS0 is bound with a lower affinity by LFY-UFO compared to LUBS1 and LUBS2, we then focused on LUBS1 and LUBS2. b, EMSA with pAP3 LUBS1 and LUBS2 DNA probes and indicated proteins. LFYH383A-R386A (LFYHARA) is a LFY mutated version affected in its ability to dimerize29,51. Note the absence of the complex with a slower mobility on LUBS1 with LFYHARA. c, EMSA with pAP3 LUBS1 and LUBS2 DNA probes and indicated proteins. LFY* refers to either in vitro-produced 5xmyc-LFY (top) or recombinant 6xHis-LFY-DBD (bottom). Note the difference of complex size between UFO and UFOΔFbox. d, Same as in (c) except that UFO-3xFLAG and UFO∆Fbox-3xFLAG were produced in vitro. Note that in vitro produced UFO-3xFLAG and UFO∆Fbox-3xFLAG behave similarly as recombinant UFO versions. e, EMSA with indicated proteins and DNA probes corresponding to pAP3 LUBS1 (left) and LUBS2 (right), WT or with URM mutated. f, Promoter activation measured by DLRA in Arabidopsis protoplasts with indicated effectors. Different promoter versions were tested as indicated under x-axis. Either 2 bp (high-informative CA) or 6 bp (whole URM) of pAP3 LUBS1 and LUBS2 URM were mutated. Data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4). One-way ANOVA with Tukey’s multiple comparisons tests. One-way ANOVA were performed with data from the same effector and stars represent a statistical difference compared to WT pAP3 promoter. (NS: p > 0.05,*: p < 0.05, **: p < 0.01, ***: p < 0.001 and ****: p < 0.0001). g, In vivo analysis of pAP3LUBS1-2m::GUS fusions. Same as in Fig. 3d, except that staining incubation time was increased to 17 h (4 h incubation in Fig. 3d). Representative pictures are shown (top scale bar, 100 µm, bottom scale bar, 50 µm). The faint AP3 pattern suggests that other LUBS (such as LUBS0) may take over but less efficiently. Note that with this staining incubation time, all plants expressing pAP3::GUS showed a highly saturated staining. Source data are available in Supplementary Data 4.
Extended Data Fig. 6 pRBE LUBS is required for LFY-UFO-dependent activation.
a, IGB view of pRBE showing LFY ChIP-seq in inflorescences (light blue)25 or seedlings (dark blue)26, LFY-UFO ampDAP-seq (yellow), LFY ampDAP-seq (pink)48, numbers indicate read number range (top). Identification of LUBS in pRBE (bottom). Predicted binding sites using dLUBS and mLUBS models from Fig. 2e and LFY PWM with dependencies68, y-axis represents score values (bottom). The best binding sites correspond to the less negative score values. Studied LUBS is indicated with a purple square. b, EMSA with probes corresponding to pRBE LUBS, WT or with URM mutated. c, pRBE activation in Arabidopsis protoplasts. Effect of mutations (underlined) in URM (red) and in LFYBS (blue) bases of pRBE LUBS were assayed. Data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4). One-way ANOVA with Tukey’s multiple comparisons test. One-way ANOVA were performed with data from the same effector, and stars represent a statistical difference compared to WT promoters (****: p < 0.0001). d, In vivo analysis of pRBE::GUS fusions. The percentage of transgenic lines with RBE pattern, unusual pattern or absence of staining was scored (top; χ² test, **: p < 0.01). n = number of independent lines. Unusual pattern refers to staining in unexpected tissues, each pattern seen in a single line. Representative pictures of plants with no staining (bottom left) and a RBE pattern (bottom right) are shown (scale bar, 50 µm). e, In vivo analysis of pRBE::GUS fusions. Same as in (d), with another view showing staining in the four petal primordia (scale bar, 50 µm). Source data are available in Supplementary Data 4.
Extended Data Fig. 7 LFY and UFO likely regulate other genes in Arabidopsis.
a, List of candidate LFY-UFO target genes selected as i) present in regions specifically bound by LFY-UFO in ampDAP-seq (high CFC) ii) bound in vivo in LFY ChIP-seq experiments (A25; B26; C68; D73) and iii) deregulated in ufo inflorescences70. b, IGB view of PISTILLATA promoter region showing LFY ChIP-seq in inflorescences (light blue)25 or seedlings (dark blue)26, LFY-UFO ampDAP-seq (yellow), LFY ampDAP-seq (pink)48, numbers indicate read number range (top). Predicted binding sites using the dLUBS, mLUBS models from Fig. 2e and LFY PWM with dependencies68, y-axis represents score values (bottom). c, IGB view of selected genes showing LFY ChIP-seq in inflorescences (light blue)25, LFY-UFO ampDAP-seq (yellow), LFY ampDAP-seq (pink)48, numbers indicate read number range. Genes in red are deregulated in ufo inflorescences70. ChIP-seq peaks better explained by LFY-UFO than by LFY alone are shaded in grey. Source data are available in Supplementary Data 4.
Extended Data Fig. 8 The LFY K249 is essential for LFY-UFO-LUBS complex formation.
a, Structure of LFY-DBD29. Residues were colored by conservation using Consurf with default parameters85. K249 residues on each LFY monomer are represented as sticks and indicated with arrows. Note that the K249-containing loop is highly conserved. b,c, Promoter activation measured by DLRA in Arabidopsis protoplasts with indicated effectors (right). EV = Empty Vector (pRT104-3xHA). Tested promoters are indicated below each graph. Note that for 3xHA-LFY + UFO-3xFLAG on pAG only n = 3 biological replicates are shown. Data represent averages of independent biological replicates and are presented as mean ± SD, each dot representing one biological replicate (n = 4 unless specified). One-way ANOVA with Tukey’s multiple comparisons tests (b) or Welch’s ANOVA with Games-Howell post-hoc test (c). In (c), stars above bars represent a statistical difference compared to GFP. Other comparisons are indicated with brackets. (NS: p > 0.05,*: p < 0.05, **: p < 0.01, ***: p < 0.001 and ****: p < 0.0001). d, Effect of the LFYK249R mutation on LFY-UFO interaction in Y2H. EV = Empty Vector. LFY-40 is a LFY version lacking the first 40 aa and better tolerated by yeast cells. Values correspond to the different dilutions (OD = 7, 0.7 and 0.07). Top picture corresponds to the non-selective plate lacking Leucine and Tryptophan (SD -L-W), and bottom picture corresponds to the selective plate lacking Leucine, Tryptophan, Histidine and Adenine (SD -L-W-A-H). Pictures were taken at day + 4. e, EMSA with DNA probes corresponding to pAP3 DEE LFYBS and pAP3 LUBS1 and indicated proteins. pAP3 DEE LFYBS DNA probe was used as a control for binding on canonical LFYBS. f, WB after DNA elution during ampDAP-seq experiment. After DNA elution, 20 µL of 1X SDS-PAGE Protein Sample Buffer was added to the remaining beads to run WB. Each lane represents one replicate. g, Reproducibility of ampDAP-seq experiments with LFYK249R (left) and LFYK249R-UFO (right) through the comparison of replicates datasets 2 by 2. h, Comparison of peak coverage in LFYK249R (y-axis, this study) and LFY (x-axis)48 ampDAP-seq experiments. i, Integrated Genome Browser (IGB) view of pAP3 showing LFY ChIP-seq in inflorescences (light blue)25 or seedlings (dark blue)26, LFY-UFO ampDAP-seq (yellow; this study), LFY ampDAP-seq (pink)48 and LFYK249R ampDAP-seq (purple; this study). Numbers indicate read number range. j, Pictures of WT and representative transgenic plants expressing 35S::LFY or 35S::LFYK249R (scale bar, 1 cm). The white arrows indicate ectopic rosette flowers. 35S::LFY was obtained previously26. 42 T1 plants expressing 35S::LFYK249R were analyzed; the percentage of plants with a LFY overexpressing phenotype is comparable to the one obtained with 35S::LFY26. Source data are available in Supplementary Data 4.
Extended Data Fig. 9 UFO binds DNA and LFY DBD.
a, A representative micrograph of the ASK1-UFO-LFY-DNA complex in vitreous ice (scale bar, 20 nm). b, Selected 2D class averages of the particles submitted to ab initio reconstruction and heterogeneous refinement for 3D classification. c, Intermediate reconstructions of the 3D classes after heterogeneous refinement. d, Final reconstructions of ASK1-UFO-LFY-DNA complexes (involving either a LFY-DBD monomer (pink) or a LFY-DBD dimer (gray)) after Non-Uniform refinement. e, Unprocessed AlphaFold2 model for ASK1 (top, purple; uniprot ID, Q39255), UFO (middle, red; uniprot ID, Q39090) and the LFY-DBD dimer/DNA crystallographic structure (bottom, pale and dark blue for the LFY-DBD dimer and green for the DNA; PDB, 2VY1). f, Cryo-EM density map color-coded by fitted molecule. Note the kink on DNA induced by the presence of UFO. g, Heat map of the angular distribution of particle projections contributing for the final reconstruction of the complete ASK1-UFO-LFY-DNA complex (with a LFY-DBD dimer). h, Gold-standard Fourier shell correlation (FSC) curves. The dotted line represents the 0.143 FSC threshold, which indicates a nominal resolution of 6.4 Å for the unmasked (red) and 4.3 Å for the masked (blue) reconstruction. i, View of the post-processed map of the complete ASK1-UFO-LFY-DNA complex, colored according to the local resolution.
Supplementary information
Supplementary Data 1
List of plasmids used in this study.
Supplementary Data 2
List of oligonucleotides used in this study.
Supplementary Data 3
Cryo-EM data collection and refinement statistics.
Supplementary Data 4
Statistical source data for Figs. 1–6 and Extended Data Figs. 1–8.
Supplementary File 1
Model for the LFY–ASK1–UFO–DNA complex.
Source data
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 6
Unprocessed gels.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 3
Unprocessed gels.
Source Data Extended Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 5
Unprocessed gels.
Source Data Extended Data Fig. 6
Unprocessed gels.
Source Data Extended Data Fig. 8
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rieu, P., Turchi, L., Thévenon, E. et al. The F-box protein UFO controls flower development by redirecting the master transcription factor LEAFY to new cis-elements. Nat. Plants 9, 315–329 (2023). https://doi.org/10.1038/s41477-022-01336-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01336-2
This article is cited by
-
Genome-wide analysis of plant specific YABBY transcription factor gene family in carrot (Dacus carota) and its comparison with Arabidopsis
BMC Genomic Data (2024)
-
Identification of microRNAs and their target genes associated with chasmogamous and cleistogamous flower development in Viola prionantha
Planta (2024)