Abstract
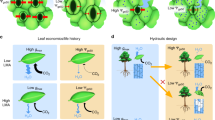
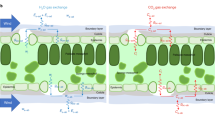
Stomata are orifices that connect the drier atmosphere with the interconnected network of more humid air spaces that surround the cells within a leaf. Accurate values of the humidities inside the substomatal cavity, wi, and in the air, wa, are needed to estimate stomatal conductance and the CO2 concentration in the internal air spaces of leaves. Both are vital factors in the understanding of plant physiology and climate, ecological and crop systems. However, there is no easy way to measure wi directly. Out of necessity, wi has been taken as the saturation water vapour concentration at leaf temperature, wsat, and applied to the whole leaf intercellular air spaces. We explored the occurrence of unsaturation by examining gas exchange of leaves exposed to various magnitudes of wsat − wa, or Δw, using a double-sided, clamp-on chamber, and estimated degrees of unsaturation from the gradient of CO2 across the leaf that was required to sustain the rate of CO2 assimilation through the upper surface. The relative humidity in the substomatal cavities dropped to about 97% under mild Δw and as dry as around 80% when Δw was large. Measurements of the diffusion of noble gases across the leaf indicated that there were still regions of near 100% humidity distal from the stomatal pores. We suggest that as Δw increases, the saturation edge retreats into the intercellular air spaces, accompanied by the progressive closure of mesophyll aquaporins to maintain the cytosolic water potential.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data Availability
All generated and analysed data from this study are included in the published article and its Supplementary Information.
References
Jarvis, P. G. & Slatyer, R. O. The role of the mesophyll cell wall in leaf transpiration. Planta 90, 303–322 (1970).
Livingston, B. E. & Brown, W. H. Relation of the daily march of transpiration to variations in the water content of foliage leaves. Bot. Gaz. 53, 309–330 (1912).
Farquhar, G. D. & Raschke, K. On the resistance to transpiration of sites of evaporation within leaf. Plant Physiol. 61, 1000–1005 (1978).
Egorov, V. P. & Karpushkin, L. T. Determination of air humidity over evaporating surface inside a leaf by a compensation method. Photosynthetica 22, 394–404 (1988).
Canny, M. J. & Huang, C. X. Leaf water content and palisade cell size. N. Phytol. 170, 75–85 (2006).
Gaastra, P. Photosynthesis of Crop Plants as Influenced by Light, Carbon Dioxide, Temperature, and Stomatal Diffusion Resistance (Meded. Landbouwhogeschool, 1959).
von Caemmerer, S. & Farquhar, G. D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387 (1981).
Cernusak, L. A. et al. Unsaturation of vapour pressure inside leaves of two conifer species. Sci. Rep. 8, 7667 (2018).
Cernusak, L. A., Goldsmith, G. R., Arend, M. & Siegwolf, R. T. W. Effect of vapor pressure deficit on gas exchange in wild-type and abscisic acid–insensitive plants. Plant Physiol. 181, 1573–1586 (2019).
Holloway-Phillips, M., Cernusak, L. A., Stuart-Williams, H., Ubierna, N. & Farquhar, G. D. Two-source δ18O method to validate the CO18O-photosynthetic discrimination model: implications for mesophyll conductance. Plant Physiol. 181, 1175–1190 (2019).
Buckley, T. N. & Sack, L. The humidity inside leaves and why you should care: implications of unsaturation of leaf intercellular airspaces. Am. J. Bot. 106, 618–621 (2019).
Rockwell, F. E., Holbrook, N. M. & Stroock, A. D. The competition between liquid and vapor transport in transpiring leaves. Plant Physiol. 164, 1741–1758 (2014).
Moss, D. N. & Rawlins, S. L. Concentration of carbon dioxide inside leaves. Nature 197, 1320–1321 (1963).
Sharkey, T. D., Imai, K., Farquhar, G. D. & Cowan, I. R. A direct confirmation of the standard method of estimating intercellular partial pressure of CO2. Plant Physiol. 69, 657–659 (1982).
Parkhurst, D. F., Wong, S. C., Farquhar, G. D. & Cowan, I. R. Gradients of intercellular CO2 levels across the leaf mesophyll. Plant Physiol. 86, 1032–1037 (1988).
Mott, K. A. & Parkhurst, D. F. Stomatal responses to humidity in air and helox. Plant Cell Environ. 14, 509–515 (1991).
Márquez, D. A., Stuart-Williams, H. & Farquhar, G. D. An improved theory for calculating leaf gas exchange more precisely accounting for small fluxes. Nat. Plants 7, 317–326 (2021).
Hsiao, T. C. & Xu, L. K. Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. Exp. Bot. 51, 1595–1616 (2000).
Kramer, P. J. & Boyer, J. S. in Water Relations of Plants and Soils 42–83 (Academic Press, 1995).
Ye, D. et al. Resonant soft X-ray scattering reveals cellulose microfibril spacing in plant primary cell walls. Sci. Rep. 8, 12449 (2018).
Kohonen, M. M. in Contact Angle, Wettability and Adhesion Vol. 5 (ed. Mittal, K. L.) 47–57 (CRC, 2008).
Taiz, L., Zeiger, E., Møller, I. M. & Murphy, A. in Plant Physiology and Development (ed. Sinauer, A. D.) 379–405 (Sinauer Associates, 2015).
Buckley, T. N. The control of stomata by water balance. N. Phytol. 168, 275–292 (2005).
Damour, G., Simonneau, T., Cochard, H. & Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 33, 1419–1438 (2010).
Buckley, T. N. & Mott, K. A. Modelling stomatal conductance in response to environmental factors. Plant Cell Environ. 36, 1691–1699 (2013).
Martre, P. et al. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 130, 2101–2110 (2002).
Postaire, O. et al. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 152, 1418–1430 (2009).
Robinson, D. G., Sieber, H., Kammerloher, W. & Schaffner, A. R. PIP1 aquaporins are concentrated in plasmalemmasomes of Arabidopsis thaliana mesophyll. Plant Physiol. 111, 645–649 (1996).
Morillon, R. & Chrispeels, M. J. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proc. Natl Acad. Sci. USA 98, 14138–14143 (2001).
Canny, M. Water loss from leaf mesophyll stripped of the epidermis. Funct. Plant Biol. 39, 421–434 (2012).
Israel, D., Khan, S., Warren, C. R., Zwiazek, J. J. & Robson, T. M. The contribution of PIP2-type aquaporins to photosynthetic response to increased vapour pressure deficit. J. Exp. Bot. 72, 5066–5078 (2021).
Jain, P. et al. A minimally disruptive method for measuring water potential in planta using hydrogel nanoreporters. Proc. Natl Acad. Sci. USA 118, e2008276118 (2021).
Uehlein, N. et al. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20, 648–657 (2008).
Ermakova, M. et al. Expression of a CO2-permeable aquaporin enhances mesophyll conductance in the C4 species Setaria viridis. eLife 10, e70095 (2021).
Zhao, M. et al. Association between water and carbon dioxide transport in leaf plasma membranes: assessing the role of aquaporins. Plant Cell Environ. 40, 789–801 (2017).
Evans, J. R. & von Caemmerer, S. Carbon dioxide diffusion inside leaves. Plant Physiol. 110, 339–346 (1996).
Evans, J. R., Sharkey, T. D., Berry, J. A. & Farquhar, G. D. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust. J. Plant Physiol. 13, 281–292 (1986).
Cernusak, L. A., Farquhar, G. D., Wong, S. C. & Stuart-Williams, H. Measurement and interpretation of the oxygen isotope composition of carbon dioxide respired by leaves in the dark. Plant Physiol. 136, 3350–3363 (2004).
Farquhar, G. D. & Cernusak, L. A. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant Cell Environ. 35, 1221–1231 (2012).
Acknowledgements
We thank P. Groeneveld for his assistance in building the leaf chamber and parts for the gas exchange system. G.D.F. and L.A.C. acknowledge ARC support in the form of a Discovery Grant (no. DP210103186). We thank W. Stiller, CSIRO Agriculture and Food, Narrabri, Australia, for providing the cotton seeds.
Author information
Authors and Affiliations
Contributions
S.C.W. conducted the gas exchange measurements and developed the concept. The late M.J.C. conceived the initial concept. M.H.-P. worked on the oxygen isotopes and analysis. H.S.-W. worked on the noble gases and isotopes. L.A.C. worked on the oxygen isotopes and analysis. D.A.M. measured the water potentials. G.D.F. and D.A.M. worked on the theory and modelling. All authors contributed to concept development and writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Michael Blatt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Uncorrected gas exchange measurements in a sunflower leaf using a double-sided clamp-on chamber.
(a) CO2 assimilation rate, A, transpiration rate, E, and leaf conductance to water vapour, g, as functions of increasing Δw = wsat - wa. (b) The internal CO2 concentration, ci, of upper and lower substomatal cavities, and the ci difference (upper minus lower) between them are plotted as functions of Δw = wsat - wa. Dotted line denotes zero difference between upper (adaxial) ci and lower (abaxial) ci. Photosynthetically active radiation was fixed at 1000 µmol m−2 s−1.
Extended Data Fig. 2 Relative humidity inside (a) two cotton leaves and (b) five sunflower leaves.
Relative humidity inside (a) two cotton leaves and (b) five sunflower leaves as estimated by the ci difference technique and simultaneously (within minutes) by the oxygen isotope method.
Extended Data Fig. 3 Resistances to water diffusion.
Resistances to water diffusion estimated using routine gas exchange calculations (\(R_{{{{\mathrm{H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\)), corrected values (\(cR_{{{{\mathrm{H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\)) and using noble gases (\(R_{{{{\mathrm{argon - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\),\(R_{{{{\mathrm{neon - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\) and \(R_{{{{\mathrm{helium - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\)). Estimations of the unsaturated mesophyll air space resistance as \(R_{{{{\mathrm{unsat}}}}} = R_{{{{\mathrm{H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}} - cR_{{{{\mathrm{H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\) and the intercellular air space resistance to water as \(R_{{{{\mathrm{ias - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}} = R_{{{{\mathrm{x - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}} - cR_{{{{\mathrm{H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\). Three leaves are presented as examples for the noble gases experiments, cotton leaf using argon (a and b), sunflower using neon (c and d) and cotton using helium (e and f).
Extended Data Fig. 4 Comparison of \(R_{{{{\mathrm{ias - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\)derived from noble gas measurements.
Comparison of \(R_{{{{\mathrm{ias - H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}}\)derived from noble gas measurements, with that derived more crudely but simply as 2(ciu-cil)/A.
Supplementary information
Supplementary Information
Supplementary Sections 1–3.
Supplementary Data 1
Measurements for the ten species.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, S.C., Canny, M.J., Holloway-Phillips, M. et al. Humidity gradients in the air spaces of leaves. Nat. Plants 8, 971–978 (2022). https://doi.org/10.1038/s41477-022-01202-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01202-1
This article is cited by
-
Nature-inspired interfacial engineering for energy harvesting
Nature Reviews Electrical Engineering (2024)
-
Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants
Nature Communications (2023)