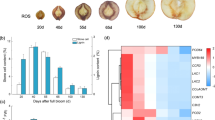
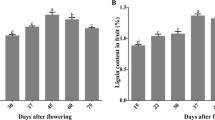
Abstract
At present, a cooperative process hypothesis is used to explain the supply of enzyme (class III peroxidases and/or laccases) and substrates during lignin polymerization. However, it remains elusive how xylem cells meet the needs of early lignin rapid polymerization during secondary cell wall formation. Here we provide evidence that a mitochondrial ascorbate peroxidase (PtomtAPX) is responsible for autonomous lignification during the earliest stage of secondary cell wall formation in Populus tomentosa. PtomtAPX was relocated to cell walls undergoing programmed cell death and catalysed lignin polymerization in vitro. Aberrant phenotypes were caused by altered PtomtAPX expression levels in P. tomentosa. These results reveal that PtomtAPX is crucial for catalysing lignin polymerization during the early stages of secondary cell wall formation and xylem development, and describe how xylem cells provide autonomous enzymes needed for lignin polymerization during rapid formation of the secondary cell wall by coupling with the programmed cell death process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in Science Data Bank at http://cstr.cn/31253.11.sciencedb.j00001.00446. Sequence data in this article can be found in the GenBank database under accession number PtomtAPX (MG921618.1) at https://www.ncbi.nlm.nih.gov/nuccore/MG921618.1/. Source data are provided with this paper.
References
Yordanov, Y. S., Regan, S. & Busov, V. Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell 22, 3662–3677 (2010).
Rojas-Murcia, N. et al. High-order mutants reveal an essential requirement for peroxidases but not laccases in Casparian strip lignification. Proc. Natl Acad. Sci. USA 117, 29166–29177 (2020).
Zhao, Q. Lignification: flexibility, biosynthesis and regulation. Trends Plant Sci. 21, 713–721 (2016).
Hoffmann, N., Benske, A., Betz, H., Schuetz, M. & Samuels, A. L. Laccases and peroxidases co-localize in lignified secondary cell walls throughout stem development. Plant Physiol. 184, 806–822 (2020).
Tobimatsu, Y. & Schuetz, M. Lignin polymerization: how do plants manage the chemistry so well? Curr. Opin. Biotechnol. 56, 75–81 (2019).
Barros, J., Serk, H., Granlund, I. & Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 115, 1053–1074 (2015).
Pesquet, E. et al. Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 25, 1314–1328 (2013).
Smith, R. A. et al. Defining the diverse cell populations contributing to lignification in Arabidopsis stems. Plant Physiol. 174, 1028–1036 (2017).
Smith, R. A. et al. Neighboring parenchyma cells contribute to Arabidopsis xylem lignification, while lignification of interfascicular fibers is cell autonomous. Plant Cell 25, 3988–3999 (2013).
Zhang, B. et al. PIRIN2 suppresses S-type lignin accumulation in a noncell-autonomous manner in Arabidopsis xylem elements. New Phytol. 225, 1923–1935 (2020).
Yin, B. et al. PtomtAPX, a mitochondrial ascorbate peroxidase, plays an important role in maintaining the redox balance of Populus tomentosa Carr. Sci. Rep. 9, 19541 (2019).
Elstein, K. H. & Zucker, R. M. Comparison of cellular and nuclear flow cytometric techniques for discriminating apoptotic subpopulations. Exp. Cell. Res. 211, 322–331 (1994).
Van Aken, O. & Van Breusegem, F. Licensed to kill: mitochondria, chloroplasts, and cell death. Trends Plant Sci. 20, 754–766 (2015).
Courtois-Moreau, C. L. et al. A unique program for cell death in xylem fibers of Populus stem. Plant J. 58, 260–274 (2009).
Zhang, D. et al. The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 26, 2939–2961 (2014).
Miao, Y. C. & Liu, C. J. ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc. Natl Acad. Sci. USA 107, 22728–22733 (2010).
Voxeur, A., Wang, Y. & Sibout, R. Lignification: different mechanisms for a versatile polymer. Curr. Opin. Plant Biol. 23, 83–90 (2015).
Arimura, S. I. Fission and fusion of plant mitochondria, and genome maintenance. Plant Physiol. 176, 152–161 (2018).
Twig, G. et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 (2008).
Minibayeva, F., Dmitrieva, S., Ponomareva, A. & Ryabovol, V. Oxidative stress-induced autophagy in plants: the role of mitochondria. Plant Physiol. Biochem. 59, 11–19 (2012).
Sugiura, A., McLelland, G. L., Fon, E. A. & McBride, H. M. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33, 2142–2156 (2014).
He, F. et al. The in vivo impact of MsLAC1, a Miscanthus laccase isoform, on lignification and lignin composition contrasts with its in vitro substrate preference. BMC Plant Biol. 19, 552 (2019).
Sterjiades, R., Dean, J. F. D., Gamble, G., Himmelsbach, D. S. & Eriksson, K.-E. L. Extracellular laccases and peroxidases from sycamore maple (Acer pseudoplatanus) cell-suspension cultures. Planta 190, 75–87 (1993).
Wang, X. et al. Substrate specificity of LACCASE8 facilitates polymerization of caffeyl alcohol for C-lignin biosynthesis in the seed coat of Cleome hassleriana. Plant Cell 32, 3825–3845 (2020).
Xie, T., Liu, Z. & Wang, G. Structural basis for monolignol oxidation by a maize laccase. Nat. Plants 6, 231–237 (2020).
Bao, W., O’Malley, D. M., Whetten, R. & Sederoff, R. R. A laccase associated with lignification in loblolly pine xylem. Science 260, 672–674 (1993).
Sato, Y. et al. Isolation and characterization of a novel peroxidase gene ZPO-C whose expression and function are closely associated with lignification during tracheary element differentiation. Plant Cell Physiol. 47, 493–503 (2006).
Herrero, J. et al. Bioinformatic and functional characterization of the basic peroxidase 72 from Arabidopsis thaliana involved in lignin biosynthesis. Planta 237, 1599–1612 (2013).
Fernandez-Perez, F., Pomar, F., Pedreno, M. A. & Novo-Uzal, E. The suppression of AtPrx52 affects fibers but not xylem lignification in Arabidopsis by altering the proportion of syringyl units. Physiol. Plant. 154, 395–406 (2015).
Barros, J. et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 10, 1994 (2019).
Sterjiades, R., Dean, J. F. & Eriksson, K. E. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 99, 1162–1168 (1992).
Soniya, E. V. & Das, M. R. In vitro organogenesis and genetic transformation in popular Cucumis sativus L. through Agrobacterium tumefaciens. Indian J. Exp. Biol. 40, 329–333 (2002).
Printz, B. et al. An improved protocol to study the plant cell wall proteome. Front. Plant Sci. 6, 237 (2015).
Millar, A. H., Sweetlove, L. J., Giege, P. & Leaver, C. J. Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol. 127, 1711–1727 (2001).
Schmid, M., Simpson, D. & Gietl, C. Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc. Natl Acad. Sci. USA 96, 14159–14164 (1999).
Tsuda, K., Ito, Y., Sato, Y. & Kurata, N. Positive autoregulation of a KNOX gene is essential for shoot apical meristem maintenance in rice. Plant Cell 23, 4368–4381 (2011).
Shigeto, J., Kiyonaga, Y., Fujita, K., Kondo, R. & Tsutsumi, Y. Putative cationic cell-wall-bound peroxidase homologues in Arabidopsis, AtPrx2, AtPrx25, and AtPrx71, are involved in lignification. J. Agric. Food Chem. 61, 3781–3788 (2013).
Lapierre, C., Pollet, B. & Rolando, C. New insights into the molecular architecture of hardwood lignins by chemical degradative methods. Res. Chem. Intermed. 21, 397–412 (1995).
Acknowledgements
This work was supported by the Natural Science Foundation of China (31971618) and National Key Research and Development Program of China (2021YFD2200900).
Author information
Authors and Affiliations
Contributions
H. Lu designed the experiments; J.Z., Y.L. and C.L. performed the experiments; X.L., X.G., C.Z., D.L. and B.Y. performed statistical analysis; H. Lu, J.Z., H. Li and I.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Video 1
Relocation of PtomtAPX-GFP fusion protein in transgenic tobacco suspension cells during PCD.
Source data
Source Data Fig. 1
Unprocessed western blots and gels for Fig. 1.
Source Data Fig. 2
Unprocessed western blots and gels for Fig. 2.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Fig. 6
Unprocessed western blots and gels for Fig. 6.
Source Data Table 1
Statistical source data for Table 1.
Source Data Table 2
Statistical source data for Table 2.
Rights and permissions
About this article
Cite this article
Zhang, J., Liu, Y., Li, C. et al. PtomtAPX is an autonomous lignification peroxidase during the earliest stage of secondary wall formation in Populus tomentosa Carr. Nat. Plants 8, 828–839 (2022). https://doi.org/10.1038/s41477-022-01181-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01181-3
This article is cited by
-
Toward the identification of class III peroxidases potentially involved in lignification in the model C4 grass Setaria viridis
Theoretical and Experimental Plant Physiology (2023)