Abstract
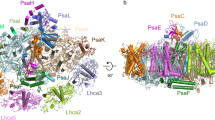
The moss Physcomitrium patens diverged from green algae shortly after the colonization of land by ancient plants. This colonization posed new environmental challenges, which drove evolutionary processes. The photosynthetic machinery of modern flowering plants is adapted to the high light conditions on land. Red-shifted Lhca4 antennae are present in the photosystem I light-harvesting complex of many green-lineage plants but absent in P. patens. The cryo-EM structure of the P. patens photosystem I light-harvesting complex I supercomplex (PSI–LHCI) at 2.8 Å reveals that Lhca4 is replaced by a unique Lhca2 paralogue in moss. This PSI–LHCI supercomplex also retains the PsaM subunit, present in Cyanobacteria and several algal species but lost in vascular plants, and the PsaO subunit responsible for binding light-harvesting complex II. The blue-shifted Lhca2 paralogue and chlorophyll b enrichment relative to flowering plants make the P. patens PSI–LHCI spectroscopically unique among other green-lineage supercomplexes. Overall, the structure represents an evolutionary intermediate PSI with the crescent-shaped LHCI common in vascular plants, and contains a unique Lhca2 paralogue that facilitates the moss’s adaptation to low-light niches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The models and maps of the final model (PDB ID: 7SKQ; EMDB: EMD-23023); the PSI–LHCI model, excluding PsaO (PDB ID: 7KUX; EMDB: EMD-23040); and the PsaO alone (PDB ID: 7KU5; EMDB: EMD-23034) are all deposited in the Protein Databank and Electron Microscopy Database, respectively. Source data are provided with this paper.
References
Fischer, W. W., Hemp, J. & Johnson, J. E. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 44, 647–683 (2016).
Yoon, H. S., Hackett, J. D., Ciniglia, C., Pinto, G. & Bhattacharya, D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 21, 809–818 (2004).
Heckman, D. S. et al. Molecular evidence for the early colonization of land by fungi and plants. Science 293, 1129–1133 (2001).
Rensing, S. A. et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319, 64–69 (2008).
Alboresi, A., Caffarri, S., Nogue, F., Bassi, R. & Morosinotto, T. In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: identification of subunits which evolved upon land adaptation. PLoS ONE 3, e2033 (2008).
Alboresi, A., Gerottob, C., Giacomettib, G. M., Bassia, R. & Morosinotto, T. Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc. Natl Acad. Sci. USA 107, 11128–11133 (2010).
Blankenship, R. E. Molecular Mechanisms of Photosynthesis 2nd edn (Wiley-Blackwell, 2014).
Nelson, N. & Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 84, 659–683 (2015).
Paul, M. Photosynthesis. Plastid biology, energy conversion and carbon assimilation. Ann. Bot. 111, ix (2013).
Nelson, N. & Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 5, 971–982 (2004).
Amunts, A., Toporik, H., Borovikova, A. & Nelson, N. Structure determination and improved model of plant photosystem I. J. Biol. Chem. 285, 3478–3486 (2010).
Mazor, Y., Borovikova, A., Caspy, I. & Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 3, 17014 (2017).
Caspy, I. & Nelson, N. Structure of the plant photosystem I. Biochem. Soc. Trans. 46, 285–294 (2018).
Croce, R. & van Amerongen, H. Light-harvesting in photosystem I. Photosynth. Res. 116, 153–166 (2013).
Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 (1999).
Busch, A. & Hippler, M. The structure and function of eukaryotic photosystem I. Biochim. Biophys. Acta Bioenerg. 1807, 864–877 (2011).
Rochaix, J. D. & Bassi, R. LHC-like proteins involved in stress responses and biogenesis/repair of the photosynthetic apparatus. Biochem. J. 476, 581–593 (2019).
Jensen, P. E. et al. Structure, function and regulation of plant photosystem I. Biochim. Biophys. Acta Bioenerg. 1767, 335–352 (2007).
Pan, X. et al. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 360, 1109–1113 (2018).
Qin, X., Suga, M., Kuang, T. & Shen, J.-R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348, 989–995 (2015).
Croce, R., Morosinotto, T., Castelletti, S., Breton, J. & Bassi, R. The Lhca antenna complexes of higher plants photosystem I. Biochim. Biophys. Acta 1556, 29–40 (2002).
Ganeteg, U., Klimmek, F. & Jansson, S. Lhca5—an LHC-type protein associated with photosystem I. Plant Mol. Biol. 54, 641–651 (2004).
Kato, Y., Odahara, M., Fukao, Y. & Shikanai, T. Stepwise evolution of supercomplex formation with photosystem I is required for stabilization of chloroplast NADH dehydrogenase-like complex: Lhca5-dependent supercomplex formation in Physcomitrella patens. Plant J. 96, 937–948 (2018).
Otani, T., Kato, Y. & Shikanai, T. Specific substitutions of light‐harvesting complex I proteins associated with photosystem I are required for supercomplex formation with chloroplast NADH dehydrogenase‐like complex. Plant J. 94, 122–130 (2018).
Otani, T., Yamamoto, H. & Shikanai, T. Stromal loop of Lhca6 is responsible for the linker function required for the NDH–PSI supercomplex formation. Plant Cell Physiol. 58, 851–861 (2017).
Ballottari, M., Dall’Osto, L., Morosinotto, T. & Bassi, R. Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J. Biol. Chem. 282, 8947–8958 (2007).
Wientjes, E., Oostergetel, G. T., Jansson, S., Boekema, E. J. & Croce, R. The role of Lhca complexes in the supramolecular organization of higher plant photosystem I. J. Biol. Chem. 284, 7803–7810 (2009).
Kubota-Kawai, H. et al. Ten antenna proteins are associated with the core in the supramolecular organization of the photosystem I supercomplex in Chlamydomonas reinhardtii. J. Biol. Chem. 294, 4304–4314 (2019).
Ozawa, S. I. et al. Configuration of ten light-harvesting chlorophyll a/b complex I subunits in Chlamydomonas reinhardtii photosystem I. Plant Physiol. 178, 583–595 (2018).
Mozzo, M. et al. Functional analysis of photosystem I light-harvesting complexes (Lhca) gene products of Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1797, 212–221 (2010).
Perez-Boerema, A. et al. Structure of a minimal photosystem I from the green alga Dunaliella salina. Nat. Plants 6, 321–327 (2020).
Caspy, I. et al. Structure and energy transfer pathways of the Dunaliella salina photosystem I supercomplex. Biochim. Biophys. Acta Bioenerg. 1861, 148253 (2020).
Suga, M. et al. Structure of the green algal photosystem I supercomplex with a decameric light-harvesting complex I. Nat. Plants 5, 626–636 (2019).
Su, X. et al. Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex. Nat. Plants 5, 273–281 (2019).
Qin, X. et al. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants 5, 263–272 (2019).
Busch, A. et al. Composition and structure of photosystem I in the moss Physcomitrella patens. J. Exp. Bot. 64, 2689–2699 (2013).
Iwai, M. & Yokono, M. Light-harvesting antenna complexes in the moss Physcomitrella patens: implications for the evolutionary transition from green algae to land plants. Curr. Opin. Plant Biol. 37, 94–101 (2017).
Neilson, J. A. D. & Durnford, D. G. Evolutionary distribution of light-harvesting complex-like proteins in photosynthetic eukaryotes. Genome 53, 68–78 (2010).
Neilson, J. A. D. & Durnford, D. G. Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes. Photosynth. Res. 106, 57–71 (2010).
Pinnola, A. et al. A LHCB9-dependent photosystem I megacomplex induced under low light in Physcomitrella patens. Nat. Plants 4, 910–919 (2018).
Iwai, M., Grob, P., Nogales, E. & Niyogi, K. A unique supramolecular organization of photosystem I in the moss Physcomitrella patens. Nat. Plants 4, 904–909 (2018).
Iwai, M. et al. Light-harvesting complex Lhcb9 confers a green alga-type photosystem I supercomplex to the moss Physcomitrella patens. Nat. Plants 1, 14008 (2015).
Croce, R. et al. The low-energy forms of photosystem I light-harvesting complexes: spectroscopic properties and pigment–pigment interaction characteristics. Biophys. J. 93, 2418–2428 (2007).
Gobets, B. et al. Time-resolved fluorescence emission measurements of photosystem I particles of various cyanobacteria: a unified compartmental model. Biophys. J. 81, 407–424 (2001).
Alboresi, A., Ballottari, M., Hienerwadel, R., Giacometti, G. M. & Morosinotto, T. Antenna complexes protect Photosystem I from photoinhibition. BMC Plant Biol. 9, 71 (2009).
Carbonera, D., Agostini, G., Morosinotto, T. & Bassi, R. Quenching of chlorophyll triplet states by carotenoids in reconstituted Lhca4 subunit of peripheral light-harvesting complex of photosystem I. Biochemistry 44, 8337–8346 (2005).
Rivadossi, A., Zucchelli, G., Garlaschi, F. M. & Jennings, R. C. The importance of PS I chlorophyll red forms in light-harvesting by leaves. Photosynth. Res. 60, 209–215 (1999).
Castelletti, S. et al. Recombinant LHca2 and LHca3 subunits of the photosystem I antenna system. Biochemistry 42, 4226–4234 (2003).
Morosinotto, T., Castelletti, S., Breton, J., Bassi, R. & Croce, R. Mutation analysis of Lhca1 antenna complex: low energy absorption forms originate from pigment–pigment interactions. J. Biol. Chem. 277, 36253–36261 (2002).
Wientjes, E. & Croce, R. The light-harvesting complexes of higher-plant photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem. J. 433, 477–485 (2011).
Antoshvili, M., Caspy, I., Hippler, M. & Nelson, N. Structure and function of photosystem I in Cyanidioschyzon merolae. Photosynth. Res. 139, 499–508 (2019).
Pi, X. et al. Unique organization of photosystem I–light-harvesting supercomplex revealed by cryo-EM from a red alga. Proc. Natl Acad. Sci. USA 115, 4423–4428 (2018).
Morosinotto, T., Breton, J., Bassi, R. & Croce, R. The nature of a chlorophyll ligand in Lhca proteins determines the far-red fluorescence emission typical of photosystem I. J. Biol. Chem. 278, 49223–49229 (2003).
Morosinotto, T., Mozzo, M., Bassi, R. & Croce, R. Pigment–pigment interactions in Lhca4 antenna complex of higher plants photosystem I. J. Biol. Chem. 280, 20612–20619 (2005).
Mazor, Y., Borovikova, A. & Nelson, N. The structure of plant photosystem I super-complex at 2.8 Å resolution. Elife 4, e07433 (2015).
Natali, A., Roy, L. M. & Croce, R. In vitro reconstitution of light-harvesting complexes of plants and green algae. J. Vis. Exp. https://doi.org/10.3791/51852 (2014).
Boardman, N. K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. https://doi.org/10.1146/annurev.pp.28.060177.002035 (1977).
Hobe, S., Fey, H., Rogl, H. & Paulsen, H. Determination of relative chlorophyll binding affinities in the major light-harvesting chlorophyll a/b complex. J. Biol. Chem. 278, 5912–5919 (2003).
Naithani, S., Hou, J. M. & Chitnis, P. R. Targeted inactivation of the psaK1, psaK2 and psaM genes encoding subunits of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 63, 225–236 (2000).
Iwai, M., Grob, P., Iavarone, A. T., Nogales, E. & Niyogi, K. K. A unique supramolecular organization of photosystem I in the moss Physcomitrella patens. Nat. Plants https://doi.org/10.1038/s41477-018-0271-1 (2018).
Yan, Q. et al. Antenna arrangement and energy-transfer pathways of PSI–LHCI from the moss Physcomitrella patens. Cell Discov. 7, 10 (2021).
Cunningham, A., Ramage, L. & McKee, D. Relationships between inherent optical properties and the depth of penetration of solar radiation in optically complex coastal waters. J. Geophys. Res. Ocean. 118, 2310–2317 (2013).
FJ, K. et al. Decreasing the chlorophyll a/b ratio in reconstituted LHCII: structural and functional consequences. Biochemistry 38, 6587–6596 (1999).
Pagano, A., Cinque, G. & Bassi, R. In vitro reconstitution of the recombinant photosystem II light-harvesting complex CP24 and its spectroscopic characterization. J. Biol. Chem. https://doi.org/10.1074/jbc.273.27.17154 (1998).
Sakuraba, Y., Yokono, M., Akimoto, S., Tanaka, R. & Tanaka, A. Deregulated chlorophyll b synthesis reduces the energy transfer rate between photosynthetic pigments and induces photodamage in Arabidopsis thaliana. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcq050 (2010).
Leong, T. Y. & Anderson, J. M. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth. Res. https://doi.org/10.1007/bf00028524 (1984).
Ben-Shem, A., Frolow, F. & Nelson, N. Crystal structure of plant photosystem I. Nature https://doi.org/10.1038/nature02200 (2003).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. https://doi.org/10.1093/nar/gkh340 (2004).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics https://doi.org/10.1093/bioinformatics/btp033 (2009).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. https://doi.org/10.1093/molbev/msy096 (2018).
de las Rivas, J., Abadía, A. & Abadía, J. A new reversed phase-HPLC method resolving all major higher plant photosynthetic pigments. Plant Physiol. https://doi.org/10.1104/pp.91.1.190 (1989).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. https://doi.org/10.1016/j.jsb.2005.07.007 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife https://doi.org/10.7554/eLife.42166 (2018).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ https://doi.org/10.1107/S205225251801463X (2019).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ https://doi.org/10.1107/s2052252520000081 (2020).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods https://doi.org/10.1038/nmeth.2727 (2014).
Naydenova, K. & Russo, C. J. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat. Commun. https://doi.org/10.1038/s41467-017-00782-3 (2017).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. https://doi.org/10.1107/s2059798319011471 (2019).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank Prof. N. Ohad and Dr R. Yaari for help with moss strains, cultivation and protocols; the Arizona State University for use of the Titan Krios at the Erying Materials Center; and the National Science Foundation (No. MRI 1531991) for funding of this instrument. This study was funded by a startup grant from Arizona State University and supported by grant number 2034021 from the National Science Foundation to Y.M.
Author information
Authors and Affiliations
Contributions
C.G. and R.R. performed sample preparation and measurements. Z. Da and Z. Dobson performed measurements. D.W. performed cryo-EM data acquisition. Y.M., C.G. and R.R. analysed the data and performed structural modelling. C.G., H.T., R.R. and Y.M. planned the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Toshiharu Shikanai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, uncropped blots for Fig. 5c and Tables 1–3.
Source data
Source Data Fig. 1
Fig. 1d,e source data – absorption and emission data.
Source Data Fig. 5
Fig. 5c,d source data – HPLC chromatograms and absorption data.
Source Data Table 1
Table 1 source data – HPLC chromatograms.
Rights and permissions
About this article
Cite this article
Gorski, C., Riddle, R., Toporik, H. et al. The structure of the Physcomitrium patens photosystem I reveals a unique Lhca2 paralogue replacing Lhca4. Nat. Plants 8, 307–316 (2022). https://doi.org/10.1038/s41477-022-01099-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01099-w
This article is cited by
-
Architecture of symbiotic dinoflagellate photosystem I–light-harvesting supercomplex in Symbiodinium
Nature Communications (2024)
-
Uncovering the photosystem I assembly pathway in land plants
Nature Plants (2024)
-
Structural insights into a unique PSI–LHCI–LHCII–Lhcb9 supercomplex from moss Physcomitrium patens
Nature Plants (2023)
-
Structural insights into the assembly and energy transfer of the Lhcb9-dependent photosystem I from moss Physcomitrium patens
Nature Plants (2023)
-
Comparative transcriptomics reveals key genes contributing to the differences in drought tolerance among three cultivars of foxtail millet (Setaria italica)
Plant Growth Regulation (2023)