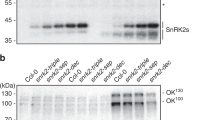
Abstract
The root:shoot ratio has long been known to be enhanced in plants under drought stress. Here we discovered that osmotic stress enhances long-distance sucrose transport to increase the root:shoot ratio in an abscisic-acid-dependent manner. The Arabidopsis sucrose transporters SWEET11 and 12, key players in phloem loading, are rapidly phosphorylated upon drought and abscisic acid treatments. The drought- and abscisic-acid-activated SnRK2 protein kinases phosphorylate the carboxy-terminal cytosolic regions of SWEET11 and 12. This phosphorylation enhances the oligomerization and sucrose transport activity of SWEETs, which results in elevated sucrose contents in roots and improved root growth under drought stress, leading to the enhanced root:shoot ratio of biomass and drought resistance. Notably, the expression of phospho-mimic SWEETs led to improved root growth even under non-stressed conditions. The phosphorylation of sucrose transporters provides an explanation for the long-standing observation that drought stress enhances the root:shoot ratio in plants and suggests a strategy for engineering drought-resistant crops.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All materials are available from the corresponding author upon request. Source data are provided with this paper.
References
Gupta, A., Rico-Medina, A. & Cano-Delgado, A. I. The physiology of plant responses to drought. Science 368, 266–269 (2020).
Oldroyd, G. E. D. & Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 368, eaba0196 (2020).
Ogura, T. et al. Root system depth in Arabidopsis is shaped by EXOCYST70A3 via the dynamic modulation of auxin transport. Cell 178, 400–412e16 (2019).
Chang, J. et al. Asymmetric distribution of cytokinins determines root hydrotropism in Arabidopsis thaliana. Cell Res. 29, 984–993 (2019).
Orosa-Puente, B. et al. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362, 1407–1410 (2018).
Markhart, A. H. Comparative water relations of Phaseolus vulgaris L. and Phaseolus acutifolius Gray. Plant Physiol. 77, 113–117 (1985).
Li, Y. et al. Root growth adaptation is mediated by PYLs ABA receptor-PP2A protein phosphatase complex. Adv. Sci. (Weinh.) 7, 1901455 (2020).
Zhao, Y. et al. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci. Signal. 7, ra53 (2014).
Dietrich, D. et al. Root hydrotropism is controlled via a cortex-specific growth mechanism. Nat. Plants 3, 17057 (2017).
Yu, S. M., Lo, S. F. & Ho, T. H. Source–sink communication: regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 20, 844–857 (2015).
Chen, L. Q. et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335, 207–211 (2012).
Ayre, B. G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 4, 377–394 (2011).
Zhang, C. & Turgeon, R. Mechanisms of phloem loading. Curr. Opin. Plant Biol. 43, 71–75 (2018).
Durand, M. et al. Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 170, 1460–1479 (2016).
Lemoine, R. et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 4, 272 (2013).
Thalmann, M. et al. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 28, 1860–1878 (2016).
Rodrigues, J., Inze, D., Nelissen, H. & Saibo, N. J. M. Source–sink regulation in crops under water deficit. Trends Plant Sci. 24, 652–663 (2019).
Zhu, J. K. Abiotic stress signaling and responses in plants. Cell 167, 313–324 (2016).
Chen, K. et al. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 62, 25–54 (2020).
Park, S. Y. et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071 (2009).
Ma, Y. et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324, 1064–1068 (2009).
Fujii, H. & Zhu, J. K. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl Acad. Sci. USA 106, 8380–8385 (2009).
Chen, L. Q., Cheung, L. S., Feng, L., Tanner, W. & Frommer, W. B. Transport of sugars. Annu. Rev. Biochem. 84, 865–894 (2015).
Kumar, S., Lee, I. H. & Plamann, M. Cytoplasmic dynein ATPase activity is regulated by dynactin-dependent phosphorylation. J. Biol. Chem. 275, 31798–31804 (2000).
Xuan, Y. H. et al. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc. Natl Acad. Sci. USA 110, E3685–E3694 (2013).
Han, L. et al. Molecular mechanism of substrate recognition and transport by the AtSWEET13 sugar transporter. Proc. Natl Acad. Sci. USA 114, 10089–10094 (2017).
Tao, Y. et al. Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature 527, 259–263 (2015).
Hochholdinger, F. & Tuberosa, R. Genetic and genomic dissection of maize root development and architecture. Curr. Opin. Plant Biol. 12, 172–177 (2009).
Den Herder, G., Van Isterdael, G., Beeckman, T. & De Smet, I. The roots of a new green revolution. Trends Plant Sci. 15, 600–607 (2010).
Bailey-Serres, J., Parker, J. E., Ainsworth, E. A., Oldroyd, G. E. D. & Schroeder, J. I. Genetic strategies for improving crop yields. Nature 575, 109–118 (2019).
Uga, Y. et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 45, 1097–1102 (2013).
Moore, B. et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300, 332–336 (2003).
Arai-Sanoh, Y. et al. Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci. Rep. 4, 5563 (2014).
Blum, A. Plant Breeding for Water-Limited Environments (Springer-Verlag, 2011).
Shen, B. R. et al. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 12, 199–214 (2019).
South, P. F., Cavanagh, A. P., Liu, H. W. & Ort, D. R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363, eaat9077 (2019).
Xu, Q. et al. Carbon export from leaves is controlled via ubiquitination and phosphorylation of sucrose transporter SUC2. Proc. Natl Acad. Sci. USA 117, 6223–6230 (2020).
Wang, H. L., Lee, P. D., Chen, W. L., Huang, D. J. & Su, J. C. Osmotic stress-induced changes of sucrose metabolism in cultured sweet potato cells. J. Exp. Bot. 51, 1991–1999 (2000).
Xu, W. et al. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 37, 9 (2015).
Chen, X. et al. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 63, 53–78 (2021).
Beuchat, G., Xue, X. & Chen, L.-Q. The next steps in crop improvement: adoption of emerging strategies to identify bottlenecks in sugar flux. Plant Sci. 301, 110675 (2020).
Khadilkar, A. S. et al. Constitutive and companion cell-specific overexpression of AVP1, encoding a proton-pumping pyrophosphatase, enhances biomass accumulation, phloem loading, and long-distance transport. Plant Physiol. 170, 401–414 (2016).
Zhao, Y. et al. Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity. Cell Rep. 23, 3340–3351e5 (2018).
Lisec, J., Schauer, N., Kopka, J., Willmitzer, L. & Fernie, A. R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 1, 387–396 (2006).
Tetyuk, O., Benning, U. F. & Hoffmann-Benning, S. Collection and analysis of Arabidopsis phloem exudates using the EDTA-facilitated method. J. Vis. Exp. 23, e51111 (2013).
Yoo, S.-D., Cho, Y.-H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007).
Abelenda, J. A. et al. Source–sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 29, 1178–1186.e6 (2019).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB27040107), the National Natural Science Foundation of China (NSFC grant no. 31970293) and the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Y.Z. conceived and designed the research. Q.C., T.H. and X.L. performed the experiments. Q.C., Y.Z., L.C., C.-P.S. and J.-K.Z. analysed the results. Y.Z., Q.C. and L.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Shuhua Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Reduced root growth and root sugar levels in ABA insensitive mutants and sweet11/12 mutant under osmotic stress.
a, Fresh weight of roots from Col-0 wild-type, pyl duodecuple (pyl), snrk2.2/3/6 (snrk2) and sweet11/12 mutants, and SWEETpro::SWEET-HA transgenic plants expressing wild-type SWEET11 or 12 in the sweet11/12 mutant background, without or with mannitol, PEG or ABA treatments. b, Sucrose contents in roots of Col-0 wild-type, pyl duodecuple (pyl), snrk2.2/3/6 (snrk2) and sweet11/12 mutants, and SWEETpro::SWEET-HA transgenic plants expressing wild-type SWEET11 or 12 in the sweet11/12 mutant background, without or with mannitol or ABA treatments. c-d, Root growth of Col-0 wild-type, snrk2.2/3/6 (snrk2) and pyl duodecuple (pyl) mutants, grown on mannitol plates with (right panel) or without (left panel) exogenous sucrose. The primary root length was quantified (d). e-f, Glucose (e) and fructose (f) levels in roots of Col-0 wild-type, pyl duodecuple (pyl), snrk2.2/3/6 (snrk2) and sweet11/12 mutants, without or with mannitol or ABA treatments. g-h, Root growth of Col-0 wild-type and sweet11/12 mutant grown on mannitol plates with (right panel) or without (left panel) exogenous sucrose. The primary root length was quantified (h). Error bars indicate SEM (n = 10 seedlings) in (a, d, h), SEM (n = 5 seedlings) in (b) and SEM (n = 3 seedlings) in (e, f). Student’s t-test (two-sided). ‘a,’ ‘b,’ and ‘c’, significance evaluated by post hoc Tukey test after one way ANOVA (p < 0.05). The experiments in (c and g) were repeated independently at least three times with similar results.
Extended Data Fig. 2 SnRK2s interact with and phosphorylate SWEET11 and 12.
a, SnRK2s are co-immunoprecipitated with SWEET12 in SWEET12pro::SWEET12-GFP transgenic plants. SWEET12 protein was immunoprecipitated using SWEET12pro::SWEET12-GFP transgenic plants treated without or with 100 μM ABA. b, Coimmunoprecipitation (coIP) assay in Arabidopsis protoplast. Total proteins were extracted from protoplasts co-transfected with HA-SnRK2.6 and FLAG-SWEET11, FLAG-SWEET12 or FLAG empty vector control, and immunoprecipitated using anti-FLAG beads. Coimmunoprecipitated HA-SnRK2.6 was detected with anti-HA antibody. The experiments in (b) were repeated independently at least two times with similar results.
Extended Data Fig. 3 SnRK2s phosphorylate SWEET11 and 12.
a, Phosphopeptides containing Ser248 from the C-terminus of SWEET12 were detected in SWEET12pro::SWEET12-GFP transgenic plants following ABA treatment. ‘pep score’, peptide score, which determines the probability that the peptide fragments detected by the mass spectrum. b, Phosphorylation of Ser248 of SWEET11 and 12 in Col-0 wild-type and sweet11/12 mutant seedlings, with or without mannitol or ABA treatment. Anti-SWEET11P and anti-SWEET12P indicate anti-phospho-Ser248-SWEET11 and 12 antibodies, respectively. Anti-phospho-Ser248-SWEET11 and 12 antibodies were used to detect phosphorylation of SWEET11 and 12, respectively. Actin was used as a loading control. c,d, Protein abundance of HA-tagged SWEET11 and SWEET12 in SWEET11pro::SWEET11-HA (c) and SWEET12pro::SWEET12-HA (d) transgenic plants. Anti-HA antibody was used for western blot. Total proteins were detected by Ponceau S staining. e-f, Phosphorylation of SWEET11 and SWEET12 under mannitol or ABA treatment in SWEET11pro::SWEET11-HA (e) and SWEET12pro::SWEET12-HA (f) transgenic plants. Total proteins were separated in a Phos-tag gel, and HA-tagged SWEET were detected with anti-HA antibody. g, Phosphorylation of SWEET11 and SWEET12 under ABA treatment in protoplasts of Col-0 and snrk2.2/3/6 (snrk2). Total proteins were separated in a Phos-tag gel, and FLAG-tagged SWEET were detected with an anti-FLAG antibody. Actin was used as a loading control. The experiments in (b-g) were repeated independently at least two times with similar results.
Extended Data Fig. 4 Phosphorylation enhances oligomerization of SWEET11 and 12.
a, Prediction of cytosolic regions in SWEET11 and SWEET12 proteins using TMHMM Server v. 2.0. b-c, Result matrix of homo- and hetero-oligomerization between combinations of wild-type, phospho-mimic (S-to-D/E) or non-phosphorylatable (S-to-A) SWEET11 and 12 LUC-fusion proteins without (left half of box) or with (right half of box) ABA treatment in protoplasts of sweet11/12 (b) and snrk2.2/3/6 (snrk2) mutants (c). The strength of the interaction is categorized from light blue to dark red as indicated below or to the right of the matrix. We define the hetero-interaction strength of wild type SWEET11 and SWEET12 as n.s., and determines the interaction strength of other combinations according to the mathematical multiple relation with n.s.. No experiment was conducted with combinations depicted as an empty box. The right histogram in (b) shows the illustrative rough data. Error bars indicate SEM (n = 3 biologically independent samples). d-e, Interactions between C-terminal and internal cytosolic regions of SWEETs in the yeast two-hybrid assay. Interactions was determined by yeast growth in media lacking Leu, Trp and His (-L-W-H) with dilutions (10−1, 10−2, and 10−3) of saturated cultures.
Extended Data Fig. 5 Genotyping of SWEETpro::SWEETs-HA transgenic plants.
a, Schematic representation of the SWEET11 and 12 loci and the respective T-DNA insertion sites. Genomic DNA sequence (upper panel) and protein sequence (lower panel). b, Amplification of native SWEET11 and SWEET12 in SWEETpro:: SWEET-HA transgenic plants in sweet11/12 mutant background using primers that bind to the 3’UTR of SWEETs as reverse primers. Col-0 wild-type, and sweet11, sweet12 and sweet11/12 mutants were used as controls. c, Amplification of recombinant SWEET11 and SWEET12 in SWEETpro:: SWEET-HA transgenic plants in sweet11/12 mutant background using a primer binding to HA as reverse primer. d, Sanger sequencing chromatograms indicate mutations on SWEETs. The experiments in (b, c) were repeated independently at least two times with similar results.
Extended Data Fig. 6 Phosphorylation of SWEET11 and 12 Enhances Sucrose transport and accumulation in Roots.
a,b, Protein abundance of wild-type and mutated SWEET11 (a) and SWEET12 (b) in protoplasts without or with 5 μM ABA treatment overnight. Anti-FLAG antibody was used to detect SWEET11 and 12. Actin was used as a loading control. c-d, Protein abundance of SWEET11 and 12 in SWEETpro::SWEET-HA transgenic plants, without or with 20 μM ABA (c) or 200 mM mannitol (d) treatments for the indicated time points. Anti-HA antibody was used to detect SWEET11 and 12. Actin was used as a loading control. e, Sucrose levels in phloem exudates of Col-0 wild-type, sweet11/12 mutant, and SWEET12pro::SWEET12-HA transgenic plants expressing wild-type and mutated SWEET12 in the sweet11/12 mutant background, with or without ABA treatment. f, Sucrose levels in roots of Col-0 wild-type, sweet11/12 mutant and SWEETpro::SWEET-HA transgenic plants expressing wild-type and mutated SWEET11 and SWEET12 in the sweet11/12 mutant background, without or with mannitol or ABA treatments. Error bars indicate SEM (n = 3 biologically independent samples) in (e), and SEM (n = 5 seedlings) in (f). Student’s t-test (two-sided). The experiments in (a-d) were repeated independently at least two times with similar results.
Extended Data Fig. 7 Phosphorylation of SWEET11 contributes to root growth under osmotic stress.
Root growth of Col-0 wild-type, sweet11/12 mutant and SWEETpro::SWEET-HA transgenic plants expressing wild-type and mutated SWEETs in the sweet11/12 mutant background, grown on mannitol plates with (right panel) or without (left panel) exogenous sucrose. The primary root length was quantified (b). Values are mean ± SEM (n = 15 seedlings). ‘a,’ ‘b,’ and ‘c’, significance evaluated by post hoc Tukey test after one way ANOVA (p < 0.05). The experiments in (a) were repeated independently at least three times with similar results.
Extended Data Fig. 8 Phosphorylation of SWEET11 contributes to root growth under drought stress.
a, Vertical sections of representative root systems of Col-0 wild-type, sweet11/12 mutant, and SWEET11pro::SWEET11-HA transgenic plants expressing wild-type and mutated SWEET11 in the sweet11/12 mutant background, grown in well-watered or arid soil for 30 days. Red lines: traced roots from the image; red bars, 5 cm. The experiments in (a) were repeated independently at least three times with similar results. b-e, Fresh and dry weight in roots (b,d) and shoots (c,e) of Col-0 wild-type, sweet11/12 mutant and SWEET11pro::SWEET11-HA transgenic plants expressing wild-type and mutated SWEET11 in the sweet11/12 mutant background, without or with mannitol, PEG or ABA treatments. Error bars indicate SEM (n = 10 seedlings). Student’s t-test (two-sided). f, Representative images of Col-0 wild-type, sweet11/12 mutant and SWEET11pro::SWEET11-HA transgenic plants expressing wild-type and mutated SWEET11 in the sweet11/12 mutant background, grown under drought stress in soil. Water was withheld from 10-day-old Arabidopsis plants for 21 d under short-day conditions. The experiments in (f) were repeated independently at least three times with similar results.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Supplementary Data 1
IP-MS data for SWEET12.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Chen, Q., Hu, T., Li, X. et al. Phosphorylation of SWEET sucrose transporters regulates plant root:shoot ratio under drought. Nat. Plants 8, 68–77 (2022). https://doi.org/10.1038/s41477-021-01040-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-01040-7
This article is cited by
-
Dynamic simulation of photosynthate distribution parameters and biomass of summer maize under water stress
Irrigation Science (2024)
-
ABA functions in low phosphate-induced anthocyanin accumulation through the transcription factor ABI5 in Arabidopsis
Plant Cell Reports (2024)
-
SCAB1 coordinates sequential Ca2+ and ABA signals during osmotic stress induced stomatal closure in Arabidopsis
Science China Life Sciences (2024)
-
Sugar transporter ZmSWEET1b is responsible for assimilate allocation and salt stress response in maize
Functional & Integrative Genomics (2023)
-
Foliar application of strigolactones improves the desiccation tolerance, grain yield and water use efficiency in dryland wheat through modulation of non-hydraulic root signals and antioxidant defense
Stress Biology (2023)