Abstract
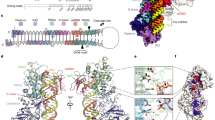
MicroRNAs (miRNAs) are short non-coding RNAs that inhibit the expression of target genes by directly binding to their mRNAs. In animals, pri-miRNAs are cleaved by Drosha to generate pre-miRNAs, which are subsequently cleaved by Dicer to generate mature miRNAs. Instead of being cleaved by two different enzymes, both cleavages in plants are performed by Dicer-like 1 (DCL1). With a similar domain architecture as human Dicer, it is mysterious how DCL1 recognizes pri-miRNAs and performs two cleavages sequentially. Here, we report the single-particle cryo-electron microscopy structures of Arabidopsis DCL1 complexed with a pri-miRNA and a pre-miRNA, respectively, in cleavage-competent states. These structures uncover the plasticity of the PAZ domain, which is critical for the recognition of both pri-miRNA and pre-miRNA. These structures suggest that the helicase module serves as an engine that transfers the substrate between two sequential cleavage events. This study lays a foundation for dissecting the regulation mechanism of miRNA biogenesis in plants and provides insights into the dicing state of human Dicer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The accession codes for the cryo-EM density maps reported in this paper are Electron Microscopy Data Bank: EMD-31181 (https://www.emdataresource.org/EMD-31181) (DCL1–pri-miRNA complex) and EMD-31182 (https://www.emdataresource.org/EMD-31182) (DCL1–pre-miRNA complex). The accession codes for the atomic coordinates reported in this paper are Protein Data Bank: 7ELD (https://doi.org/10.2210/pdb7ELD/pdb) (DCL1–pri-miRNA complex) and 7ELE (https://doi.org/10.2210/pdb7ELE/pdb) (DCL1–pre-miRNA complex). The accession codes for the atomic coordinates used in this study are PDB 4NHA (https://doi.org/10.2210/pdb4NHA/pdb), PDB 5ZAK (https://doi.org/10.2210/pdb5ZAK/pdb), PDB 5ZAL (https://doi.org/10.2210/pdb5ZAL/pdb), PDB 6BU9 (https://doi.org/10.2210/pdb6BU9/pdb) and PDB 6LXD (https://doi.org/10.2210/pdb6LXD/pdb). Source data are provided with this paper. Other data are available from the corresponding author on reasonable request..
References
Bartel, D. P. Metazoan microRNAs. Cell 173, 20–51 (2018).
Gebert, L. F. R. & MacRae, I. J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 20, 21–37 (2019).
O’Brien, J., Hayder, H., Zayed, Y. & Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 9, 402 (2018).
Treiber, T., Treiber, N. & Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 20, 5–20 (2019).
Lee, Y. et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060 (2004).
Cai, X., Hagedorn, C. H. & Cullen, B. R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10, 1957–1966 (2004).
Lee, Y., Jeon, K., Lee, J. T., Kim, S. & Kim, V. N. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 21, 4663–4670 (2002).
Lee, Y. et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 (2003).
Grishok, A. et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34 (2001).
Hutvagner, G. et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838 (2001).
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Zhang, H., Kolb, F. A., Jaskiewicz, L., Westhof, E. & Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 118, 57–68 (2004).
Liu, Z. et al. Cryo-EM structure of human Dicer and its complexes with a pre-miRNA substrate. Cell 173, 1549–1550 (2018).
Bologna, N. G. & Voinnet, O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 65, 473–503 (2014).
Yu, Y., Jia, T. & Chen, X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 216, 1002–1017 (2017).
Wang, J., Mei, J. & Ren, G. Plant microRNAs: biogenesis, homeostasis, and degradation. Front. Plant Sci. 10, 360 (2019).
Margis, R. et al. The evolution and diversification of Dicers in plants. FEBS Lett. 580, 2442–2450 (2006).
Golden, T. A. et al. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol. 130, 808–822 (2002).
Tagami, Y., Motose, H. & Watanabe, Y. A dominant mutation in DCL1 suppresses the hyl1 mutant phenotype by promoting the processing of miRNA. RNA 15, 450–458 (2009).
Liu, C., Axtell, M. J. & Fedoroff, N. V. The helicase and RNaseIIIa domains of Arabidopsis Dicer-Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol. 159, 748–758 (2012).
Willmann, M. R., Mehalick, A. J., Packer, R. L. & Jenik, P. D. MicroRNAs regulate the timing of embryo maturation in Arabidopsis. Plant Physiol. 155, 1871–1884 (2011).
Jacobsen, S. E., Running, M. P. & Meyerowitz, E. M. Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126, 5231–5243 (1999).
Kurihara, Y. & Watanabe, Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc. Natl Acad. Sci. USA 101, 12753–12758 (2004).
Zhu, H. et al. Bidirectional processing of pri-miRNAs with branched terminal loops by Arabidopsis Dicer-like1. Nat. Struct. Mol. Biol. 20, 1106–1115 (2013).
Dong, Z., Han, M. H. & Fedoroff, N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl Acad. Sci. USA 105, 9970–9975 (2008).
Kwon, S. C. et al. Structure of human Drosha. Cell 164, 81–90 (2016).
Sinha, N. K., Iwasa, J., Shen, P. S. & Bass, B. L. Dicer uses distinct modules for recognizing dsRNA termini. Science 359, 329–334 (2018).
Macrae, I. J. et al. Structural basis for double-stranded RNA processing by Dicer. Science 311, 195–198 (2006).
Fang, Y. & Spector, D. L. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 17, 818–823 (2007).
Burdisso, P. et al. Second double-stranded RNA binding domain of dicer-like ribonuclease 1: structural and biochemical characterization. Biochemistry 51, 10159–10166 (2012).
Liu, Q. et al. Complementation of HYPONASTIC LEAVES1 by double-strand RNA-binding domains of DICER-LIKE1 in nuclear dicing bodies. Plant Physiol. 163, 108–117 (2013).
Tian, Y. et al. A phosphate-binding pocket within the platform-PAZ-connector helix cassette of human Dicer. Mol. Cell 53, 606–616 (2014).
Partin, A. C. et al. Cryo-EM structures of human Drosha and DGCR8 in complex with primary microRNA. Mol. Cell 78, 411–422 (2020).
Jin, W., Wang, J., Liu, C. P., Wang, H. W. & Xu, R. M. Structural basis for pri-miRNA recognition by Drosha. Mol. Cell 78, 423–433 (2020).
Park, J. E. et al. Dicer recognizes the 5′ end of RNA for efficient and accurate processing. Nature 475, 201–205 (2011).
Kowalinski, E. et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147, 423–435 (2011).
Luo, D. et al. Structural insights into RNA recognition by RIG-I. Cell 147, 409–422 (2011).
Bologna, N. G., Mateos, J. L., Bresso, E. G. & Palatnik, J. F. A loop-to-base processing mechanism underlies the biogenesis of plant microRNAs miR319 and miR159. EMBO J. 28, 3646–3656 (2009).
Wang, L. et al. NOT2 proteins promote polymerase II-dependent transcription and interact with multiple MicroRNA biogenesis factors in Arabidopsis. Plant Cell 25, 715–727 (2013).
Machida, S., Chen, H. Y. & Yuan, Y. A. Molecular insights into miRNA processing by Arabidopsis thaliana SERRATE. Nucleic Acids Res. 39, 7828–7836 (2011).
Yu, B. et al. The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc. Natl Acad. Sci. USA 105, 10073–10078 (2008).
Qin, H. et al. Structure of the Arabidopsis thaliana DCL4 DUF283 domain reveals a noncanonical double-stranded RNA-binding fold for protein–protein interaction. RNA 16, 474–481 (2010).
Yang, S. W. et al. Structure of Arabidopsis HYPONASTIC LEAVES1 and its molecular implications for miRNA processing. Structure 18, 594–605 (2010).
Ma, E., MacRae, I. J., Kirsch, J. F. & Doudna, J. A. Autoinhibition of human Dicer by its internal helicase domain. J. Mol. Biol. 380, 237–243 (2008).
Zhang, H., Kolb, F. A., Brondani, V., Billy, E. & Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21, 5875–5885 (2002).
Tsutsumi, A., Kawamata, T., Izumi, N., Seitz, H. & Tomari, Y. Recognition of the pre-miRNA structure by Drosophila Dicer-1. Nat. Struct. Mol. Biol. 18, 1153–1158 (2011).
Jiang, F. et al. Dicer-1 and R3D1-L catalyze microRNA maturation in Drosophila. Genes Dev. 19, 1674–1679 (2005).
Liu, Q. et al. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science 301, 1921–1925 (2003).
Cenik, E. S. et al. Phosphate and R2D2 restrict the substrate specificity of Dicer-2, an ATP-driven ribonuclease. Mol. Cell 42, 172–184 (2011).
Sinha, N. K., Trettin, K. D., Aruscavage, P. J. & Bass, B. L. Drosophila Dicer-2 cleavage is mediated by helicase- and dsRNA termini-dependent states that are modulated by Loquacious-PD. Mol. Cell 58, 406–417 (2015).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Cardone, G., Heymann, J. B. & Steven, A. C. One number does not fit all: mapping local variations in resolution in cryo-EM reconstructions. J. Struct. Biol. 184, 226–236 (2013).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Shi, J. et al. Transcription activation by a sliding clamp. Nat. Commun. 12, 1131 (2021).
Shi, J. et al. Structural basis of Mfd-dependent transcription termination. Nucleic Acids Res. 48, 11762–11772 (2020).
Acknowledgements
We thank S. Chang at the Center of Cryo Electron Microscopy in Zhejiang University School of Medicine for help with cryo-EM data collection. We are thankful for technical support by the Core Facilities, Zhejiang University School of Medicine. This work was funded by National Natural Science Foundation of China (grant nos. 31970040 to Y.F. and 32000025 to J.S.) and Natural Science Foundation of Zhejiang Province (grant no. LR21C010002 to Y.F.).
Author information
Authors and Affiliations
Contributions
X.W., H.K., A.W., B.G. and J.S. performed the experiments. Y.F. supervised the experiments. All authors contributed to the analysis of the data and the interpretation of the results. Y.F. wrote the manuscript with contributions from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Seong Wook Yang, Peng Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification and characterization of Arabidopsis DCL1.
a, SDS–PAGE of full-length (FL) and nuclear localization signal truncated (ΔNLS) DCL1. Experiments were repeated independently three times with similar results. b, Electrophoretic mobility shift assay shows that Arabidopsis DCL1 binds to pri-miRNA 166f. The assay is performed in 20 μL reaction mixtures containing 1 μM DCL1, 1 μM pri-miRNA, 20 mM Tris-HCl, pH 7.0, 50 mM NaCl and 2 mM DTT. Experiments were repeated independently three times with similar results. c, Arabidopsis DCL1 cuts pri-miRNA 166f efficiently. The positions of the substrate and cleavage products are indicated. Bottom, schematic illustration of the final cleavage products of pri-miRNA 166f. Cleavage sites are indicated by black triangles. Experiments were repeated independently three times with similar results.
Extended Data Fig. 2
Data processing pipeline for the dataset of DCL1–pri-miRNA complex.
Extended Data Fig. 3 Data validation for DCL1–pri-miRNA complex.
a, A representative micrograph of DCL1–pri-miRNA complex. 4,759 micrographs were collected with similar results. b, Typical 2D class averages of DCL1–pri-miRNA complex. 100 classes were obtained with similar results. c, The gold-standard FSC of DCL1–pri-miRNA complex. The gold-standard FSC is calculated by comparing the two independently determined half-maps from RELION. The dashed line represents the 0.143 FSC cutoff. d, Cryo-EM density map coloured by local resolution. View orientations as in Fig. 1c. e, Angular distribution of particle projections. View orientations as in Fig. 1c.
Extended Data Fig. 4
Data processing pipeline for the dataset of DCL1–pre-miRNA complex.
Extended Data Fig. 5 Data validation for DCL1–pre-miRNA complex.
a, A representative micrograph of DCL1–pre-miRNA complex. 2,145 micrographs were collected with similar results. b, Typical 2D class averages of DCL1–pre-miRNA complex. 100 classes were obtained with similar results. c, The gold-standard FSC of DCL1–pre-miRNA complex. The gold-standard FSC is calculated by comparing the two independently determined half-maps from RELION. The dashed line represents the 0.143 FSC cutoff. d, Cryo-EM density map coloured by local resolution. View orientations as in Fig. 1d. e, Angular distribution of particle projections. View orientations as in Fig. 1d.
Extended Data Fig. 6 Sequence alignment of the RNase III domains.
At, Arabidopsis thaliana; Hs, Homo sapiens; Dm, Drosophila melanogaster; Gi, Giardia intestinalis; Aa, Aquifex aeolicus. The sequences were aligned using Clustal Omega and the figure was prepared using ESPript 3.0. Black triangles indicate the conserved catalytic residues.
Extended Data Fig. 7 Representative gel images of in vitro cleavage assay and electrophoretic mobility shift assay.
a, Representative gel images of in vitro cleavage assay with pri-miRNA. Experiments were repeated independently three times with similar results. b, Representative gel images of in vitro cleavage assay with pre-miRNA. Experiments were repeated independently three times with similar results. c, Representative gel images of electrophoretic mobility shift assay with pri-miRNA. Experiments were repeated independently three times with similar results. d, Representative gel images of electrophoretic mobility shift assay with pre-miRNA. Experiments were repeated independently three times with similar results.
Extended Data Fig. 8 Sequence alignment of DCL1 PAZ domains.
At, Arabidopsis thaliana; Os, Oryza sativa; Tu, Triticum urartu; Zm, Zea mays; Mp, Mucuna pruriens; Na, Nicotiana attenuate; Sl, Solanum lycopersicum; Ht, Helianthus tuberosus; Sm, Salvia miltiorrhiza; Dc, Dendrobium catenatum; Pt, Pinus tabuliformis. The sequences were aligned using Clustal Omega and the figure was prepared using ESPript 3.0. Black triangles indicate the conserved residues of the internal loop binding groove.
Extended Data Fig. 9 Sequence alignment of the RIG-I helicase module.
At, Arabidopsis thaliana; Hs, Homo sapiens; Dm, Drosophila melanogaster; Ap, Anas platyrhynchos. The sequences were aligned using Clustal Omega and the figure was prepared using ESPript 3.0. Black triangles indicate the conserved Walker A and Walker B residues.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 7
Unprocessed gels.
Rights and permissions
About this article
Cite this article
Wei, X., Ke, H., Wen, A. et al. Structural basis of microRNA processing by Dicer-like 1. Nat. Plants 7, 1389–1396 (2021). https://doi.org/10.1038/s41477-021-01000-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-01000-1
This article is cited by
-
From diagnosis to resistance: a symphony of miRNAs in pheochromocytoma progression and treatment response
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Structural mechanism of R2D2 and Loqs-PD synergistic modulation on DmDcr-2 oligomers
Nature Communications (2023)
-
microRNAs in action: biogenesis, function and regulation
Nature Reviews Genetics (2023)
-
Cofactor-assisted dicing: insights from structural snapshots
Cell Research (2022)
-
Structure of the Dicer-2–R2D2 heterodimer bound to a small RNA duplex
Nature (2022)