Abstract
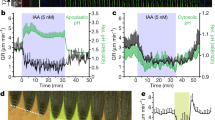
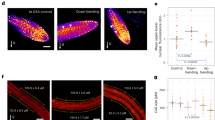
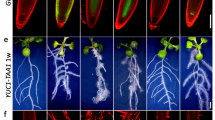
The membrane potential reflects the difference between cytoplasmic and apoplastic electrical potentials and is essential for cellular operation. The application of the phytohormone auxin (3-indoleacetic acid (IAA)) causes instantaneous membrane depolarization in various cell types1,2,3,4,5,6, making depolarization a hallmark of IAA-induced rapid responses. In root hairs, depolarization requires functional IAA transport and TIR1–AFB signalling5, but its physiological importance is not understood. Specifically in roots, auxin triggers rapid growth inhibition7,8,9 (RGI), a process required for gravitropic bending. RGI is initiated by the TIR1–AFB co-receptors, with the AFB1 paralogue playing a crucial role10,11. The nature of the underlying rapid signalling is unknown, as well as the molecular machinery executing it. Even though the growth and depolarization responses to auxin show remarkable similarities, the importance of membrane depolarization for root growth inhibition and gravitropism is unclear. Here, by combining the DISBAC2(3) voltage sensor with microfluidics and vertical-stage microscopy, we show that rapid auxin-induced membrane depolarization tightly correlates with RGI. Rapid depolarization and RGI require the AFB1 auxin co-receptor. Finally, AFB1 is essential for the rapid formation of the membrane depolarization gradient across the gravistimulated root. These results clarify the role of AFB1 as the central receptor for rapid auxin responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All raw images used in this study are available on Zenodo (https://doi.org/10.5281/zenodo.4922659). All the measured datasets are available as an Excel file in the Supplementary Information. Source data are provided with this paper.
Code availability
All the script used in this study are available at https://sourceforge.net/projects/lbopsis/ and https://sourceforge.net/projects/gravifast/.
Change history
07 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41477-022-01287-8
References
Göering, H., Polevoy, V. V., Stahlberg, R. & Stumpe, G. Depolarization of transmembrane potential of corn and wheat coleoptiles under reduced water potential and after IAA application. Plant Cell Physiol. https://doi.org/10.1093/oxfordjournals.pcp.a075851 (1979).
Bates, G. W. & Goldsmith, M. H. Rapid response of the plasma-membrane potential in oat coleoptiles to auxin and other weak acids. Planta 159, 231–237 (1983).
Tretyn, A., Wagner, G. & Felle, H. H. Signal transduction in Sinapis alba root hairs: auxins as external messengers. J. Plant Physiol. 139, 187–193 (1991).
Felle, H., Peters, W. & Palme, K. The electrical response of maize to auxins. Biochim. Biophy. Acta 1064, 199–204 (1991).
Dindas, J. et al. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat. Commun. 9, 1174 (2018).
Papanov, I. A. et al. Auxin-induced plasma membrane depolarization is regulated by auxin transport and not by AUXIN BINDING PROTEIN1. Front. Plant Sci. 9, 1953 (2019).
Evans, M. L., Mulkey, T. J. & Vesper, M. J. Auxin action on proton influx in corn roots and its correlation with growth. Planta 148, 510–512 (1980).
Monshausen, G. B., Miller, N. D., Murphy, A. S. & Gilroy, S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis: calcium and auxin signaling. Plant J. 65, 309–318 (2011).
Scheitz, K., Lüthen, H. & Schenck, D. Rapid auxin-induced root growth inhibition requires the TIR and AFB auxin receptors. Planta 238, 1171–1176 (2013).
Fendrych, M. et al. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 4, 453–459 (2018).
Prigge, M. J. et al. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. eLife 9, e54740 (2020).
Renier, M. et al. Use of a membrane potential-sensitive probe to assess biological expression of the cystic fibrosis transmembrane conductance regulator. Hum. Gene Ther. 6, 1275–1283 (1995).
von Wangenheim, D. et al. Live tracking of moving samples in confocal microscopy for vertically grown roots. eLife 6, e26792 (2017).
Baunsgaard, L. et al. The 14-3-3 proteins associate with the plant plasma membrane H+-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J. 13, 661–671 (1998).
Senn, A. P. & Goldsmith, M. H. M. Regulation of electrogenic proton pumping by auxin and fusicoccin as related to the growth of Avena coleoptiles. Plant Physiol. 88, 131–138 (1988).
Ullrich, C. I. & Novacky, A. J. Extra- and intracellular pH and membrane potential changes induced by K+, Cl−, H2 PO4−, and NO3− uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiol. 94, 1561–1567 (1990).
Labady, A., Thomas, D., Shvetsova, T. & Volkov, A. G. Plant bioelectrochemistry: effects of CCCP on electrical signaling in soybean. Bioelectrochemistry 57, 47–53 (2002).
Merlot, S. et al. Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 26, 3216–3226 (2007).
Ren, H., Park, M. Y., Spartz, A. K., Wong, J. H. & Gray, W. M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 14, e1007455 (2018).
Shabala, S. N. & Lew, R. R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 129, 290–299 (2002).
Verbelen, J.-P., Cnodder, T. D., Le, J., Vissenberg, K. & Baluška, F. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities: meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signal. Behav. 1, 296–304 (2006).
Rigas, S. et al. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 197, 1130–1141 (2013).
Dharmasiri, N. et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9, 109–119 (2005).
Uchida, N. et al. Chemical hijacking of auxin signaling with an engineered auxin–TIR1 pair. Nat. Chem. Biol. 14, 299–305 (2018).
Yamada, R. et al. A super strong engineered auxin–TIR1 pair. Plant Cell Physiol. 59, 1538–1544 (2018).
Shih, H.-W., DePew, C. L., Miller, N. D. & Monshausen, G. B. The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr. Biol. 25, 3119–3125 (2015).
Behrens, H. M., Gradmann, D. & Sievers, A. Membrane-potential responses following gravistimulation in roots of Lepidium sativum L. Planta 163, 463–472 (1985).
Evans, M. L., Ishikawak, H. & Estelle, M. A. Responses of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxin-response mutants. Planta 194, 215–222 (1994).
Savaldi-Goldstein, S. et al. New auxin analogs with growth-promoting effects in intact plants reveal a chemical strategy to improve hormone delivery. Proc. Natl Acad. Sci. USA 105, 15190–15195 (2008).
Lindsey, B. E., Rivero, L., Calhoun, C. S., Grotewold, E. & Brkljacic, J. Standardized method for high-throughput sterilization of arabidopsis seeds. J. Vis. Exp. https://doi.org/10.3791/56587 (2017).
Shabala, L., Cuin, T. A., Newman, I. A. & Shabala, S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222, 1041–1050 (2005).
Shabala, S. et al. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Aarabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 141, 1653–1665 (2006).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Parslow, A., Cardona, A. & Bryson-Richardson, R. J. Sample drift correction following 4D confocal time-lapse imaging. J. Vis. Exp. https://doi.org/10.3791/51086 (2014).
Briz-Redón, Á. & Serrano-Aroca, Á. Novel pedagogical tool for simultaneous learning of plane geometry and R programming. RIO 4, e25485 (2018).
Bivand, R. S. & Wong, D. W. S. Comparing implementations of global and local indicators of spatial association. Test 27, 716–748 (2018).
Hatzinger, R., Hornik, K., Nagel, H. & Maier, M. J. R: Einführung durch angewandte Statistik (Pearson, 2014).
Wickham, H. Reshaping data with the reshape package. J. Stat. Soft. https://doi.org/10.18637/jss.v021.i12 (2007).
Konietschke, F., Placzek, M., Schaarschmidt, F. & Hothorn, L. A. nparcomp: an R software package for nonparametric multiple comparisons and simultaneous confidence intervals. J. Stat. Soft. https://doi.org/10.18637/jss.v064.i09 (2015).
Acknowledgements
This work was supported by the European Research Council (grant no. 803048), Charles University Primus (grant no. PRIMUS/19/SCI/09), and in the initial stages by the Czech Science Foundation (GAČR grant no. 18-10116Y). S.S. acknowledges support from the Australian Research Council and China National Distinguished Expert Project (WQ20174400441). The authors thank E. Medvecká for technical support, M. Estelle, M. Prigge and W. Gray for Arabidopsis seeds, J. Friml for the initial aux1 pin2 cross, and J. Merrin for guidance in microfluidics.
Author information
Authors and Affiliations
Contributions
N.B.C.S. and M.F. conceived the project, performed the experimental work and analysed and interpreted the data. P.Y. and S.S. conceived the impaling electrode membrane potential experiment and P.Y. measured and analysed the data. D.K., Z.S. and M.F. conceived the microfluidic chip design and D.K. fabricated and optimized it. N.B.C.S., M.F. and S.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Julian Dindas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Supplementary Methods.
Supplementary Table 1
Reported membrane potential values for various plant species and tissues under an array of physiologically relevant conditions published in the literature.
Supplementary Video 1
Membrane potential of a Col0 root growing in microfluidic channels and treated with 100 nM IAA. Scale bar, 50 μm. Treatment starts at time 0. Time is min:s.
Supplementary Video 2
Col0 root tip angle measurements over 30 min after 90° gravistimulation. Angle value between (in degrees) the orange and blue line is indicated in the top left corner. Time in minutes is indicated in the bottom left corner.
Supplementary Video 3
afb1-3 root tip angle measurements over 30 min after 90° gravistimulation. Angle value (in degrees) between the orange and blue line is indicated in the top left corner. Time in minutes is indicated in the bottom left corner.
Supplementary Data 1
Statistical tests and results.
Supplementary Data 2
Measured data.
Supplementary Data 3
Measured data.
Supplementary Data 4
Measured data.
Supplementary Data 5
Measured data.
Source data
Source Data Fig. 1
Measured data.
Source Data Fig. 2
Measured data.
Source Data Fig. 3
Measured data.
Source Data Fig. 4
Measured data.
Source Data Fig. 5
Measured data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Serre, N.B.C., Kralík, D., Yun, P. et al. AFB1 controls rapid auxin signalling through membrane depolarization in Arabidopsis thaliana root. Nat. Plants 7, 1229–1238 (2021). https://doi.org/10.1038/s41477-021-00969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00969-z