Abstract
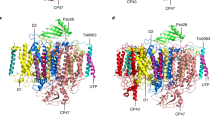
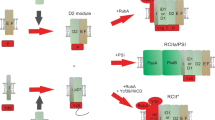
Biogenesis of photosystem II (PSII), nature’s water-splitting catalyst, is assisted by auxiliary proteins that form transient complexes with PSII components to facilitate stepwise assembly events. Using cryo-electron microscopy, we solved the structure of such a PSII assembly intermediate from Thermosynechococcus elongatus at 2.94 Å resolution. It contains three assembly factors (Psb27, Psb28 and Psb34) and provides detailed insights into their molecular function. Binding of Psb28 induces large conformational changes at the PSII acceptor side, which distort the binding pocket of the mobile quinone (QB) and replace the bicarbonate ligand of non-haem iron with glutamate, a structural motif found in reaction centres of non-oxygenic photosynthetic bacteria. These results reveal mechanisms that protect PSII from damage during biogenesis until water splitting is activated. Our structure further demonstrates how the PSII active site is prepared for the incorporation of the Mn4CaO5 cluster, which performs the unique water-splitting reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The cryo-EM density maps are deposited in the Electron Microscopy Data Bank under accession numbers EMD-12335 (PSII-M), EMD-12336 (PSII-I) and EMD-12337 (PSII-I′). The atomic models of the cryo-EM structures are deposited in the Worldwide Protein Data Bank under accession numbers 7NHO (PSII-M), 7NHP (PSII-I) and 7NHQ (PSII-I′). The NMR backbone assignments for Psb28 bound to the C-terminal peptide of CP47 are deposited in the Biological Magnetic Resonance Bank under accession code 50747. Protein sequences with the following accession codes were downloaded from the UniProt database (P0A444, Q8DIQ1, Q8DIF8, Q8CM25, Q8DIP0, Q8DIN9, Q8DJ43, Q8DJZ6, Q9F1K9, Q8DIN8, Q8DHA7, Q8DIQ0, Q9F1R6, Q8DJI1, Q8DHJ2, Q8DG60, Q8DLJ8, Q8DMP8). Source data are provided with this paper. Additional data supporting the findings of this manuscript are available from the corresponding authors on reasonable request.
References
Hohmann-Marriott, M. F. & Blankenship, R. E. Evolution of photosynthesis. Annu. Rev. Plant Biol. 62, 515–548 (2011).
Sanchez-Baracaldo, P. & Cardona, T. On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 225, 1440–1446 (2020).
Vinyard, D. J., Ananyev, G. M. & Dismukes, G. C. Photosystem II: the reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 82, 577–606 (2013).
Boekema, E. J. et al. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc. Natl Acad. Sci. USA 92, 175–179 (1995).
Cox, N., Pantazis, D. A. & Lubitz, W. Current understanding of the mechanism of water oxidation in photosystem II and its relation to XFEL data. Annu. Rev. Biochem. 89, 795–820 (2020).
Shen, J. R. The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol. 66, 23–48 (2015).
Yano, J. et al. Light-dependent production of dioxygen in photosynthesis. Met. Ions Life Sci. 15, 13–43 (2015).
Ferreira, K. N., Iverson, T. M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004).
Cardona, T., Sedoud, A., Cox, N. & Rutherford, A. W. Charge separation in photosystem II: a comparative and evolutionary overview. Biochim. Biophys. Acta 1817, 26–43 (2012).
Holzwarth, A. R. et al. Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proc. Natl Acad. Sci. USA 103, 6895–6900 (2006).
Müh, F., Glöckner, C., Hellmich, J. & Zouni, A. Light-induced quinone reduction in photosystem II. Biochim. Biophys. Acta 1817, 44–65 (2012).
Faller, P. et al. Rapid formation of the stable tyrosyl radical in photosystem II. Proc. Natl Acad. Sci. USA 98, 14368–14373 (2001).
Roose, J. L., Frankel, L. K., Mummadisetti, M. P. & Bricker, T. M. The extrinsic proteins of photosystem II: update. Planta 243, 889–908 (2016).
Müh, F. & Zouni, A. Structural basis of light-harvesting in the photosystem II core complex. Protein Sci. 29, 1090–1119 (2020).
Shi, L. X., Hall, M., Funk, C. & Schröder, W. P. Photosystem II, a growing complex: updates on newly discovered components and low molecular mass proteins. Biochim. Biophys. Acta 1817, 13–25 (2012).
Stewart, D. H. & Brudvig, G. W. Cytochrome b559 of photosystem II. Biochim. Biophys. Acta 1367, 63–87 (1998).
Cox, N. et al. Electronic structure of the oxygen-evolving complex in photosystem II prior to O-O bond formation. Science 345, 804–808 (2014).
Kern, J. et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563, 421–425 (2018).
Kupitz, C. et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 513, 261–265 (2014).
Suga, M. et al. An oxyl/oxo mechanism for oxygen–oxygen coupling in PSII revealed by an X-ray free-electron laser. Science 366, 334–338 (2019).
Umena, Y., Kawakami, K., Shen, J. R. & Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011).
Krieger-Liszkay, A., Fufezan, C. & Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 98, 551–564 (2008).
Vass, I. Molecular mechanisms of photodamage in the photosystem II complex. Biochim. Biophys. Acta 1817, 209–217 (2012).
Shevela, D. et al. ‘Birth defects’ of photosystem II make it highly susceptible to photodamage during chloroplast biogenesis. Physiol. Plant. 166, 165–180 (2019).
Heinz, S., Liauw, P., Nickelsen, J. & Nowaczyk, M. Analysis of photosystem II biogenesis in cyanobacteria. Biochim. Biophys. Acta 1857, 274–287 (2016).
Nickelsen, J. & Rengstl, B. Photosystem II assembly: from cyanobacteria to plants. Annu. Rev. Plant Biol. 64, 609–635 (2013).
Nixon, P. J., Michoux, F., Yu, J., Boehm, M. & Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 106, 1–16 (2010).
Komenda, J. et al. Cleavage after residue Ala352 in the C-terminal extension is an early step in the maturation of the D1 subunit of photosystem II in Synechocystis PCC 6803. Biochim. Biophys. Acta 1767, 829–837 (2007).
Komenda, J. et al. The cyanobacterial homologue of HCF136/YCF48 is a component of an early photosystem II assembly complex and is important for both the efficient assembly and repair of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 283, 22390–22399 (2008).
Boehm, M. et al. Subunit composition of CP43-less photosystem II complexes of Synechocystis sp. PCC 6803: implications for the assembly and repair of photosystem II. Philos. Trans. R. Soc. Lond. B 367, 3444–3454 (2012).
Dobáková, M., Sobotka, R., Tichy, M. & Komenda, J. Psb28 protein is involved in the biogenesis of the photosystem II inner antenna CP47 (PsbB) in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 149, 1076–1086 (2009).
Komenda, J. et al. The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 158, 476–486 (2012).
Mamedov, F., Nowaczyk, M. M., Thapper, A., Rögner, M. & Styring, S. Functional characterization of monomeric photosystem II core preparations from Thermosynechococcus elongatus with or without the Psb27 protein. Biochemistry 46, 5542–5551 (2007).
Nowaczyk, M. M. et al. Psb27, a cyanobacterial lipoprotein, is involved in the repair cycle of photosystem II. Plant Cell 18, 3121–3131 (2006).
Roose, J. L. & Pakrasi, H. B. The Psb27 protein facilitates manganese cluster assembly in photosystem II. J. Biol. Chem. 283, 4044–4050 (2008).
Bao, H. & Burnap, R. L. Photoactivation: the light-driven assembly of the water oxidation complex of photosystem II. Front. Plant Sci. 7, 578–591 (2016).
Becker, K., Cormann, K. U. & Nowaczyk, M. M. Assembly of the water-oxidizing complex in photosystem II. J. Photochem. Photobiol. B 104, 204–211 (2011).
Cheniae, G. & Martin, I. Photoactivation of the manganese catalyst of O2 evolution. I-Biochemical and kinetic aspects. Biochim. Biophys. Acta 253, 167–181 (1971).
Dasgupta, J., Ananyev, G. M. & Dismukes, G. C. Photoassembly of the water-oxidizing complex in photosystem II. Coord. Chem. Rev. 252, 347–360 (2008).
Radmer, R. & Cheniae, G. M. Photoactivation of the manganese catalyst of O2 evolution. II. A two-quantum mechanism. Biochim. Biophys. Acta 253, 182–186 (1971).
Nixon, P. J. & Diner, B. A. Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry 31, 942–948 (1992).
Regel, R. E. et al. Deregulation of electron flow within photosystem II in the absence of the PsbJ protein. J. Biol. Chem. 276, 41473–41478 (2001).
Nowaczyk, M. M. et al. Deletion of psbJ leads to accumulation of Psb27-Psb28 photosystem II complexes in Thermosynechococcus elongatus. Biochim. Biophys. Acta 1817, 1339–1345 (2012).
Sakata, S., Mizusawa, N., Kubota-Kawai, H., Sakurai, I. & Wada, H. Psb28 is involved in recovery of photosystem II at high temperature in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1827, 50–59 (2013).
Weisz, D. A. et al. Mass spectrometry-based cross-linking study shows that the Psb28 protein binds to cytochrome b559 in Photosystem II. Proc. Natl Acad. Sci. USA 114, 2224–2229 (2017).
Bečková, M. et al. Association of Psb28 and Psb27 proteins with PSII–PSI supercomplexes upon exposure of Synechocystis sp. PCC 6803 to high light. Mol. Plant 10, 62–72 (2017).
Bentley, F. K., Luo, H., Dilbeck, P., Burnap, R. L. & Eaton-Rye, J. J. Effects of inactivating psbM and psbT on photodamage and assembly of photosystem II in Synechocystis sp. PCC 6803. Biochemistry 47, 11637–11646 (2008).
Grasse, N. et al. Role of novel dimeric photosystem II (PSII)–Psb27 protein complex in PSII repair. J. Biol. Chem. 286, 29548–29555 (2011).
Liu, H., Roose, J. L., Cameron, J. C. & Pakrasi, H. B. A genetically tagged Psb27 protein allows purification of two consecutive photosystem II (PSII) assembly intermediates in Synechocystis 6803, a cyanobacterium. J. Biol. Chem. 286, 24865–24871 (2011).
Weisz, D. A. et al. A novel chlorophyll protein complex in the repair cycle of photosystem II. Proc. Natl Acad. Sci. USA 116, 21907–21913 (2019).
Mabbitt, P. D., Wilbanks, S. M. & Eaton-Rye, J. J. Structure and function of the hydrophilic photosystem II assembly proteins: Psb27, Psb28 and Ycf48. Plant Physiol. Biochem. 81, 96–107 (2014).
Broser, M. et al. Crystal structure of monomeric photosystem II from Thermosynechococcus elongatus at 3.6-Å resolution. J. Biol. Chem. 285, 26255–26262 (2010).
Komenda, J. & Sobotka, R. Cyanobacterial high-light-inducible proteins—protectors of chlorophyll-protein synthesis and assembly. Biochim. Biophys. Acta 1857, 288–295 (2016).
Mulo, P. et al. Mutagenesis of the D-E loop of photosystem II reaction centre protein D1. Function and assembly of photosystem II. Plant Mol. Biol. 33, 1059–1071 (1997).
Eaton-Rye, J. J. & Govindjee. Electron transfer through the quinone acceptor complex of photosystem II in bicarbonate-depleted spinach thylakoid membranes as a function of actinic flash number and frequency. Biochim. Biophys. Acta 935, 237–247 (1988).
Allen, J. F. & Nield, J. Redox tuning in photosystem II. Trends Plant Sci. 22, 97–99 (2017).
Brinkert, K., De Causmaecker, S., Krieger-Liszkay, A., Fantuzzi, A. & Rutherford, A. W. Bicarbonate-induced redox tuning in photosystem II for regulation and protection. Proc. Natl Acad. Sci. USA 113, 12144–12149 (2016).
Cormann, K. U., Möller, M. & Nowaczyk, M. M. Critical assessment of protein cross-linking and molecular docking: an updated model for the interaction between photosystem II and Psb27. Front. Plant Sci. 7, 157 (2016).
Liu, H., Huang, R. Y., Chen, J., Gross, M. L. & Pakrasi, H. B. Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates. Proc. Natl Acad. Sci. USA 108, 18536–18541 (2011).
Wei, L. et al. LPA19, a Psb27 homolog in Arabidopsis thaliana, facilitates D1 protein precursor processing during PSII biogenesis. J. Biol. Chem. 285, 21391–21398 (2010).
Wei, X. et al. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature 534, 69–74 (2016).
Avramov, A. P., Hwang, H. J. & Burnap, R. L. The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc. Natl Acad. Sci. USA 117, 28036–28045 (2020).
Cormann, K. U. et al. Structure of Psb27 in solution: implications for transient binding to photosystem II during biogenesis and repair. Biochemistry 48, 8768–8770 (2009).
Fagerlund, R. D. & Eaton-Rye, J. J. The lipoproteins of cyanobacterial photosystem II. J. Photochem. Photobiol. B 104, 191–203 (2011).
Liu, H. et al. Mass spectrometry-based footprinting reveals structural dynamics of loop E of the chlorophyll-binding protein CP43 during photosystem II assembly in the cyanobacterium Synechocystis 6803. J. Biol. Chem. 288, 14212–14220 (2013).
Michoux, F. et al. Crystal structure of the Psb27 assembly factor at 1.6 Å: implications for binding to photosystem II. Photosynth. Res. 110, 169–175 (2012).
Weisz, D. A., Gross, M. L. & Pakrasi, H. B. The use of advanced mass spectrometry to dissect the life-cycle of photosystem II. Front. Plant Sci. 7, 617 (2016).
Kettunen, R., Tyystjarvi, E. & Aro, E. M. Degradation pattern of photosystem II reaction center protein D1 in intact leaves. The major photoinhibition-induced cleavage site in D1 polypeptide is located amino terminally of the DE loop. Plant Physiol. 111, 1183–1190 (1996).
Mulo, P., Laakso, S., Maenpaa, P. & Aro, E. M. Stepwise photoinhibition of photosystem II. Studies with Synechocystis species PCC 6803 mutants with a modified D-E loop of the reaction center polypeptide D1. Plant Physiol. 117, 483–490 (1998).
Stowell, M. H. et al. Light-induced structural changes in photosynthetic reaction center: implications for mechanism of electron–proton transfer. Science 276, 812–816 (1997).
Wang, X. et al. Is bicarbonate in photosystem II the equivalent of the glutamate ligand to the iron atom in bacterial reaction centers? Biochim. Biophys. Acta 1100, 1–8 (1992).
Cheap, H. et al. M234Glu is a component of the proton sponge in the reaction center from photosynthetic bacteria. Biochim. Biophys. Acta 1787, 1505–1515 (2009).
Burnap, R. L. D1 protein processing and Mn cluster assembly in light of the emerging photosystem II structure. Phys. Chem. Chem. Phys. 6, 4803–4809 (2004).
Tokano, T., Kato, Y., Sugiyama, S., Uchihashi, T. & Noguchi, T. Structural dynamics of a protein domain relevant to the water-oxidizing complex in photosystem II as visualized by high-speed atomic force microscopy. J. Phys. Chem. B 124, 5847–5857 (2020).
Zhang, M. et al. Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. eLife 6, e26933 (2017).
Gisriel, C. J. et al. Cryo-electron microscopy structure of monomeric photosystem II from Synechocystis sp. PCC 6803 lacking the water-oxidation complex. Joule 4, 2131–2148 (2020).
Kolling, D. R., Cox, N., Ananyev, G. M., Pace, R. J. & Dismukes, G. C. What are the oxidation states of manganese required to catalyze photosynthetic water oxidation? Biophys. J. 103, 313–322 (2012).
Zaltsman, L., Ananyev, G. M., Bruntrager, E. & Dismukes, G. C. Quantitative kinetic model for photoassembly of the photosynthetic water oxidase from its inorganic constituents: requirements for manganese and calcium in the kinetically resolved steps. Biochemistry 36, 8914–8922 (1997).
Stengel, A. et al. Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell 24, 660–675 (2012).
Tyryshkin, A. M. et al. Spectroscopic evidence for Ca2+ involvement in the assembly of the Mn4Ca cluster in the photosynthetic water-oxidizing complex. Biochemistry 45, 12876–12889 (2006).
Campbell, K. A. et al. Dual-mode EPR detects the initial intermediate in photoassembly of the photosystem II Mn cluster: the influence of amino acid residue 170 of the D1 polypeptide on Mn coordination. J. Am. Chem. Soc. 122, 3754–3761 (2000).
Cohen, R. O., Nixon, P. J. & Diner, B. A. Participation of the C-terminal region of the D1-polypeptide in the first steps in the assembly of the Mn4Ca cluster of photosystem II. J. Biol. Chem. 282, 7209–7218 (2007).
Kuhl, H. et al. Towards structural determination of the water-splitting enzyme. Purification, crystallization, and preliminary crystallographic studies of photosystem II from a thermophilic cyanobacterium. J. Biol. Chem. 275, 20652–20659 (2000).
Iwai, M., Katoh, H., Katayama, M. & Ikeuchi, M. Improved genetic transformation of the thermophilic cyanobacterium, Thermosynechococcus elongatus BP-1. Plant Cell Physiol. 45, 171–175 (2004).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Biyani, N. et al. Focus: the interface between data collection and data processing in cryo-EM. J. Struct. Biol. 198, 124–133 (2017).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. A Bayesian view on cryo-electron microscopy structure determination. J. Mol. Biol. 415, 406–418 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife 3, e03665 (2014).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Pettersen, E. F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Bialek, W. et al. Crystal structure of the Psb28 accessory factor of Thermosynechococcus elongatus photosystem II at 2.3 Å. Photosynth. Res. 117, 375–383 (2013).
Michoux, F. et al. Crystal structure of CyanoQ from the thermophilic cyanobacterium Thermosynechococcus elongatus and detection in isolated photosystem II complexes. Photosynth. Res. 122, 57–67 (2014).
Schwede, T., Kopp, J., Guex, N. & Peitsch, M. C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31, 3381–3385 (2003).
Wu, S. & Zhang, Y. LOMETS: a local meta-threading-server for protein structure prediction. Nucleic Acids Res. 35, 3375–3382 (2007).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Dobson, L., Remenyi, I. & Tusnady, G. E. CCTOP: a consensus constrained TOPology prediction web server. Nucleic Acids Res. 43, W408–W412 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of coot. Acta Crystallogr D 66, 486–501 (2010).
Trabuco, L. G., Villa, E., Schreiner, E., Harrison, C. B. & Schulten, K. Molecular dynamics flexible fitting: a practical guide to combine cryo-electron microscopy and X-ray crystallography. Methods 49, 174–180 (2009).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graphics 14, 33–38 (1996).
Ribeiro, J. V. et al. QwikMD—integrative molecular dynamics toolkit for novices and experts. Sci. Rep. 6, 26536 (2016).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Leaver-Fay, A. et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 487, 545–574 (2011).
Lindert, S. & McCammon, J. A. Improved cryoEM-guided iterative molecular dynamics—rosetta protein structure refinement protocol for high precision protein structure prediction. J. Chem. Theory Comput. 11, 1337–1346 (2015).
Guo, Q. et al. In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell 172, 696–705 (2018).
Wehmer, M. et al. Structural insights into the functional cycle of the ATPase module of the 26S proteasome. Proc. Natl Acad. Sci. USA 114, 1305–1310 (2017).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Acknowledgements
We thank C. König, M. Völkel and R. Oworah-Nkruma for excellent technical assistance, K. Becker for cloning of the pIVEXPsb28His plasmid, B. Erjavec for preparation of the scheme in Fig. 1 and N. Cox for helpful discussion. J.M.S. is grateful to E. Conti for scientific independence and great mentorship and to J. M. Plitzko and W. Baumeister for access to the cryo-EM infrastructure and early career support. M.M.N. is grateful to his mentor M. Rögner for generous support. Financial support was provided by the Max Planck Society, the Helmholtz Zentrum München, the Deutsche Forschungsgemeinschaft (DFG) Research Unit FOR2092 (grant nos. EN 1194/1-1 to B.D.E. and 836/3-2 to M.M.N.), the DFG priority programme 2002 (grant no. 836/4-1 to M.M.N. and grant no. 3542/1-1 to J.D.L.), National Institutes of Health (NIH) grant no. NIH P41-GM104601 (to E.T.) and an Emmy-Noether fellowship (SCHU 3364/1-1 to J.M.S). A.K.-L. was supported by the LabEx Saclay Plant Sciences-SPS (grant no. ANR-10-LABX-0040-SPS) and the French Infrastructure for Integrated Structural Biology (FRISBI; grant no. ANR-10-INSB-05). R.S. gratefully acknowledges support from the DFG (grant nos. INST 213/757-1 FUGG and INST 213/843-1 FUGG).
Author information
Authors and Affiliations
Contributions
B.D.E., T.R., J.M.S. and M.M.N. conceived the research, prepared the figures and wrote the manuscript with the contribution of J.Z. and all other authors. M.M.N. coordinated the activities. Preparation of mutants, PSII isolation and biochemical analysis were performed by J.Z., M.M, P.L. and M.M.N. Mass spectrometry analysis was done by J.M.-C. and J.D.L. J.M.S., S.B. and B.D.E. performed the cryo-EM analysis. T.R. built the structural model with the help of S.K.S., A.C. and E.T. Fluorescence spectroscopy was carried out by J.Z. and M.M.N. EPR experiments were conducted by A.K.-L. NMR experiments were conducted and analysed by O.A. and R.S. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks R. Burnap, N. Nelson, A. Rutherford and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Discussion, Figs. 1–7, Tables 1 and 2, descriptions for Videos 1–4 and references.
Supplementary Video 1
Interpolated trajectory between PSII-I and PSII-M.
Supplementary Video 2
Interpolated trajectory between PSII-I and PSII-M.
Supplementary Video 3
Interpolated trajectory between PSII-I and PSII-M.
Supplementary Video 4
Interpolated trajectory between PSII-I and mature PSII (PDB-ID 3WU2).
Source data
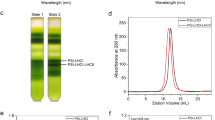
Source Data Fig. 2
Unprocessed two-dimensional polyacrylamide gel electrophoresis.
Rights and permissions
About this article
Cite this article
Zabret, J., Bohn, S., Schuller, S.K. et al. Structural insights into photosystem II assembly. Nat. Plants 7, 524–538 (2021). https://doi.org/10.1038/s41477-021-00895-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00895-0
This article is cited by
-
Computational dissection of genetic variation modulating the response of multiple photosynthetic phenotypes to the light environment
BMC Genomics (2024)
-
Thylakoid protein FPB1 synergistically cooperates with PAM68 to promote CP47 biogenesis and Photosystem II assembly
Nature Communications (2024)
-
Unveiling large charge transfer character of PSII in an iron-deficient cyanobacterial membrane: A Stark fluorescence spectroscopy study
Photosynthesis Research (2024)
-
Indirect interactions involving the PsbM or PsbT subunits and the PsbO, PsbU and PsbV proteins stabilize assembly and activity of Photosystem II in Synechocystis sp. PCC 6803
Photosynthesis Research (2024)
-
The photosystem-II repair cycle: updates and open questions
Planta (2024)