Abstract
Reactive oxygen species (ROS) are essential for life and are involved in the regulation of almost all biological processes. ROS production is critical for plant development, response to abiotic stresses and immune responses. Here, we focus on recent discoveries in ROS biology emphasizing abiotic and biotic stress responses. Recent advancements have resulted in the identification of one of the first sensors for extracellular ROS and highlighted waves of ROS production during stress signalling in Arabidopsis. Enzymes that produce ROS, including NADPH oxidases, exhibit precise regulation through diverse post-translational modifications. Discoveries highlight the importance of both amino- and carboxy-terminal regulation of NADPH oxidases through protein phosphorylation and cysteine oxidation. Here, we discuss advancements in ROS compartmentalization, systemic ROS waves, ROS sensing and post-translational modification of ROS-producing enzymes and identify areas where foundational gaps remain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data used to generate Fig. 3a,b and Supplementary Fig. 1a,b, as well as detailed methodology and scripts, are available in the GitHub repository (https://github.com/DanielleMStevens/ROS_production_review). The NOX C-terminal alignments are available in wasabi (http://was.bi?id=gedS1F).
References
Mhamdi, A. & Van Breusegem, F. Reactive oxygen species in plant development. Development 145, dev164376 (2018).
Mittler, R. ROS are good. Trends Plant Sci. 22, 11–19 (2017).
Dietz, K. J., Turkan, I. & Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 171, 1541–1550 (2016).
Huang, S., Van Aken, O., Schwarzländer, M., Belt, K. & Millar, A. H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 171, 1551–1559 (2016).
Sandalio, L. M. & Romero-Puertas, M. C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 116, 475–485 (2015).
Huang, H., Ullah, F., Zhou, D. X., Yi, M. & Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10, 800 (2019).
Zhao, C., Zhang, H., Song, C., Zhu, J.-K. & Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 1, 100017 (2020).
El-Shetehy, M. et al. Nitric oxide and reactive oxygen species are required for systemic acquired resistance in plants. Plant Signal. Behav. 10, e998544 (2015).
Schmidt, R., Kunkowska, A. B. & Schippers, J. H. Role of reactive oxygen species during cell expansion in leaves. Plant Physiol. 172, 2098–2106 (2016).
Kaya, H. et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26, 1069–1080 (2014).
Takeda, S. et al. Local positive feedback regulation determines cell shape in root hair cells. Science 319, 1241–1244 (2008).
Mangano, S. et al. Molecular link between auxin and ROS-mediated polar growth. Proc. Natl Acad. Sci. USA 114, 5289–5294 (2017).
Lee, Y., Rubio, M. C., Alassimone, J. & Geldner, N. A mechanism for localized lignin deposition in the endodermis. Cell 153, 402–412 (2013).
Cheval, C. et al. Chitin perception in plasmodesmata characterizes submembrane immune-signaling specificity in plants. Proc. Natl Acad. Sci. USA 117, 9621–9629 (2020).
Han, J. P. et al. Fine-tuning of RBOHF activity is achieved by differential phosphorylation and Ca. New Phytol. 221, 1935–1949 (2019).
Smirnoff, N. & Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 221, 1197–1214 (2019).
Waszczak, C., Carmody, M. & Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 69, 209–236 (2018).
Kadota, Y., Shirasu, K. & Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 56, 1472–1480 (2015).
Vaahtera, L., Brosché, M., Wrzaczek, M. & Kangasjärvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal. 21, 1422–1441 (2014).
Willems et al. The ROS wheel: refining ROS transcriptional footprints. Plant Physiol. 171, 1720–1733 (2016).
Mielecki, J., Gawroński, P. & Karpiński, S. Retrograde signaling: understanding the communication between organelles. Int. J. Mol. Sci. 21, 6173 (2020).
Ng, S. et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25, 3450–3471 (2013).
Shapiguzov, A. et al. Arabidopsis RCD1 coordinates chloroplast and mitochondrial functions through interaction with ANAC transcription factors. eLife 8, e43284 (2019).
Chan, K. X., Phua, S. Y., Crisp, P., McQuinn, R. & Pogson, B. J. Learning the languages of the chloroplast: retrograde signaling and beyond. Annu. Rev. Plant Biol. 67, 25–53 (2016).
Op den Camp, R. G. et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15, 2320–2332 (2003).
Daudi, A. et al. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24, 275–287 (2012).
Fujita, S. et al. SCHENGEN receptor module drives localized ROS production and lignification in plant roots. EMBO J. 39, e103894 (2020).
Lassig, R., Gutermuth, T., Bey, T. D., Konrad, K. R. & Romeis, T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 78, 94–106 (2014).
Müller, K., Carstens, A. C., Linkies, A., Torres, M. A. & Leubner-Metzger, G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol. 184, 885–897 (2009).
Lolle, S., Stevens, D. & Coaker, G. Plant NLR-triggered immunity: from receptor activation to downstream signaling. Curr. Opin. Immunol. 62, 99–105 (2020).
O’Brien, J. A. et al. A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 158, 2013–2027 (2012).
Wang, C. et al. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 4, eaar4509 (2018).
Li, X. et al. Tomato SlRbohB, a member of the NADPH oxidase family, is required for disease resistance against Botrytis cinerea and tolerance to drought stress. Front. Plant Sci. 6, 463 (2015).
Wong, H. L. et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19, 4022–4034 (2007).
Tian, S. et al. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 171, 1635–1650 (2016).
Bienert, G. P. & Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604 (2014).
Grondin, A. et al. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27, 1945–1954 (2015).
Möller, M. N., Cuevasanta, E., Orrico, F., Lopez, A. C., Thomson, L. & Denicola, A.Diffusion and transport of reactive species across cell membranes. Adv. Exp. Med. Biol. 1127, 3–19 (2019).
Lynch, R. E. & Fridovich, I. Permeation of the erythrocyte stroma by superoxide radical. J. Biol. Chem. 253, 4697–4699 (1978).
Reithmeier, R. A. et al. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta 1858, 1507–1532 (2016).
De Rezende, F. F. et al. Integrin α7β1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic. Biol. Med. 53, 521–531 (2012).
Noctor, G. & Foyer, C. H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 171, 1581–1592 (2016).
Bononi, A. et al. Mitochondria-associated membranes (MAMs) as hotspot Ca2+ signaling units. Adv. Exp. Med. Biol. 740, 411–437 (2012).
Hancock, J. T. Considerations of the importance of redox state for reactive nitrogen species action. J. Exp. Bot. 70, 4323–4331 (2019).
Thordal-Christensen, H., Zhang, Z., Wei, Y. & Collinge, D. B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 11, 1187–1194 (1997).
Dikalov, S. I. & Harrison, D. G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 20, 372–382 (2014).
Kauffman, M. E. et al. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React. Oxyg. Species (Apex) 2, 361–370 (2016).
Fichman, Y., Miller, G. & Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 12, 1203–1210 (2019).
Nietzel, T. et al. The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol. 221, 1649–1664 (2019).
Fichman, Y. & Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: is the ROS wave master of all trades? Plant J. 102, 887–896 (2020).
Gilroy, S. et al. ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol. 171, 1606–1615 (2016).
Wu, F. et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578, 577–581 (2020).
Cheval, C. & Faulkner, C. Plasmodesmal regulation during plant–pathogen interactions. New Phytol. 217, 62–67 (2018).
Mittler, R. & Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27, 64–70 (2015).
Zandalinas, S. I., Fichman, Y. & Mittler, R. Vascular bundles mediate systemic reactive oxygen signaling during light stress. Plant Cell 32, 3425–3435 (2020).
Chen, Y. C. et al. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl Acad. Sci. USA 115, E4920–E4929 (2018).
Návarová, H., Bernsdorff, F., Döring, A. C. & Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24, 5123–5141 (2012).
Wu, J. et al. Deficient plastidic fatty acid synthesis triggers cell death by modulating mitochondrial reactive oxygen species. Cell Res. 25, 621–633 (2015).
Gilroy, S. et al. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 19, 623–630 (2014).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature https://doi.org/10.1038/s41586-021-03316-6 (2021).
McConnell, E. W. et al. Proteome-wide analysis of cysteine reactivity during effector-triggered immunity. Plant Physiol. 179, 1248–1264 (2019).
Ngou, B. P. M., Ahn, H.-K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature https://doi.org/10.1038/s41586-021-03315-7 (2021).
Liu, Y. et al. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 51, 941–954 (2007).
Rossi, F. R. et al. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant J. 92, 761–773 (2017).
Zurbriggen, M. D. et al. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J. 60, 962–973 (2009).
Moreau, M. et al. The Arabidopsis oligopeptidases TOP1 and TOP2 are salicylic acid targets that modulate SA-mediated signaling and the immune response. Plant J. 76, 603–614 (2013).
Westlake, T. J., Ricci, W. A., Popescu, G. V. & Popescu, S. C. Dimerization and thiol sensitivity of the salicylic acid binding thimet oligopeptidases TOP1 and TOP2 define their functions in redox-sensitive cellular pathways. Front. Plant Sci. 6, 327 (2015).
Kimura, S., Waszczak, C., Hunter, K. & Wrzaczek, M. Bound by fate: the role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 29, 638–654 (2017).
Akter, S. et al. Cysteines under ROS attack in plants: a proteomics view. J. Exp. Bot. 66, 2935–2944 (2015).
Waszczak, C. et al. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 66, 2923–2934 (2015).
Jacques, S. et al. Protein methionine sulfoxide dynamics in Arabidopsis thaliana under oxidative stress. Mol. Cell Proteom. 14, 1217–1229 (2015).
Jacques, S., Ghesquière, B., Van Breusegem, F. & Gevaert, K. Plant proteins under oxidative attack. Proteomics 13, 932–940 (2013).
Felle, H. H. pH: signal and messenger in plant cells. Plant Biol. 3, 577–591 (2001).
Geilfus, C. M. The pH of the apoplast: dynamic factor with functional impact under stress. Mol. Plant 10, 1371–1386 (2017).
Wojtkowiak, J. W., Verduzco, D., Schramm, K. J. & Gillies, R. J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol. Pharm. 8, 2032–2038 (2011).
Toledano, M. B. et al. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78, 897–909 (1994).
Gaudu, P., Moon, N. & Weiss, B. Regulation of the soxRS oxidative stress regulon reversible oxidation of the Fe–S centers of SoxR in vivo. J. Biol. Chem. 272, 5082–5086 (1997).
Delaunay, A., Pflieger, D., Barrault, M. B., Vinh, J. & Toledano, M. B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481 (2002).
Miao, Y. et al. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18, 2749–2766 (2006).
Ding, Y. et al. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454–1467 (2018).
Dong, X. NPR1, all things considered. Curr. Opin. Plant Biol. 7, 547–552 (2004).
Mou, Z., Fan, W. & Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935–944 (2003).
Tada, Y. et al. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952–956 (2008).
Kinkema, M., Fan, W. & Dong, X. Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12, 2339–2350 (2000).
Zaffagnini, M., Fermani, S., Costa, A., Lemaire, S. D. & Trost, P. Plant cytoplasmic GAPDH: redox post-translational modifications and moonlighting properties. Front. Plant Sci. 4, 450 (2013).
Hancock, J. T. et al. Proteomic identification of glyceraldehyde 3-phosphate dehydrogenase as an inhibitory target of hydrogen peroxide in Arabidopsis. Plant Physiol. Biochem. 43, 828–835 (2005).
Schneider, M., Knuesting, J., Birkholz, O., Heinisch, J. J. & Scheibe, R. Cytosolic GAPDH as a redox-dependent regulator of energy metabolism. BMC Plant Biol. 18, 184 (2018).
Uraji, M. et al. Cooperative function of PLDδ and PLDα1 in abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 159, 450–460 (2012).
Bourdais, G. et al. Large-scale phenomics identifies primary and fine-tuning roles for CRKs in responses related to oxidative stress. PLoS Genet. 11, e1005373 (2015).
Kimura, S. et al. CRK2 and C-terminal phosphorylation of NADPH oxidase RBOHD regulate reactive oxygen species production in Arabidopsis. Plant Cell 32, 1063–1080 (2020).
Vaattovaara, A. et al. Mechanistic insights into the evolution of DUF26-containing proteins in land plants. Commun. Biol. 2, 56 (2019).
Tian, W. et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572, 131–135 (2019).
Byrne, D. P. et al. Aurora A regulation by reversible cysteine oxidation reveals evolutionarily conserved redox control of Ser/Thr protein kinase activity. Sci. Signal. 13, eaax2713 (2020).
Behring, J. B. et al. Spatial and temporal alterations in protein structure by EGF regulate cryptic cysteine oxidation. Sci. Signal. 13, eaay7315 (2020).
Zhang, T., Zhu, M., Song, W. Y., Harmon, A. C. & Chen, S. Oxidation and phosphorylation of MAP kinase 4 cause protein aggregation. Biochim. Biophys. Acta 1854, 156–165 (2015).
Kovtun, Y., Chiu, W. L., Tena, G. & Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl Acad. Sci. USA 97, 2940–2945 (2000).
Xie, G., Sasaki, K., Imai, R. & Xie, D. A redox-sensitive cysteine residue regulates the kinase activities of OsMPK3 and OsMPK6 in vitro. Plant Sci. 227, 69–75 (2014).
Ueoka-Nakanishi, H. et al. Thioredoxin h regulates calcium dependent protein kinases in plasma membranes. FEBS J. 280, 3220–3231 (2013).
Kaya, H. et al. Comparative analysis of the reactive oxygen species-producing enzymatic activity of Arabidopsis NADPH oxidases. Plant J. 98, 291–300 (2019).
Pandey, D., Gratton, J. P., Rafikov, R., Black, S. M. & Fulton, D. J. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharmacol. 80, 407–415 (2011).
Streller, S., Krömer, S. & Wingsle, G. Isolation and purification of mitochondrial Mn-superoxide dismutase from the gymnosperm Pinus sylvestris L. Plant Cell Physiol. 35, 859–867 (1994).
Alscher, R. G., Erturk, N. & Heath, L. S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53, 1331–1341 (2002).
Winterbourn, C. C., Parsons-Mair, H. N., Gebicki, S., Gebicki, J. M. & Davies, M. J. Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem. J. 381, 241–248 (2004).
Zhang, Y. et al. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21, 2357–2377 (2009).
Hu, C. H. et al. NADPH oxidases: the vital performers and center hubs during plant growth and signaling. Cells 9, 437 (2020).
Morales, J., Kadota, Y., Zipfel, C., Molina, A. & Torres, M. A. The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J. Exp. Bot. 67, 1663–1676 (2016).
Chen, D. et al. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 8, 2265 (2017).
Kadota, Y. et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54, 43–55 (2014).
Li, L. et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338 (2014).
Dubiella, U. et al. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl Acad. Sci. USA 110, 8744–8749 (2013).
Gao, X. et al. Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog. 9, e1003127 (2013).
Zhang, M. et al. The MAP4 kinase SIK1 ensures robust extracellular ROS burst and antibacterial immunity in plants. Cell Host Microbe 24, 379–391 (2018).
Kadota, Y. et al. Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector- and PAMP-triggered immunity in plants. New Phytol. 221, 2160–2175 (2019).
Boisson-Dernier, A., Franck, C. M., Lituiev, D. S. & Grossniklaus, U. Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl Acad. Sci. USA 112, 12211–12216 (2015).
Fernando, V. et al. S-nitrosylation: an emerging paradigm of redox signaling. Antioxid. (Basel) 8, 404 (2019).
Heinrich, T. A. et al. Biological nitric oxide signalling: chemistry and terminology. Br. J. Pharmacol. 169, 1417–1429 (2013).
Astier, J., Gross, I. & Durner, J. Nitric oxide production in plants: an update. J. Exp. Bot. 69, 3401–3411 (2017).
Gupta, K. J. et al. Recommendations on terminology and experimental best practice associated with plant nitric oxide research. New Phytol. 225, 1828–1834 (2020).
Lindermayr, C. & Durner, J. S-nitrosylation in plants: pattern and function. J. Proteom. 73, 1–9 (2009).
Yun, B. W. et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478, 264–268 (2011).
Beaumel, S. et al. Down-regulation of NOX2 activity in phagocytes mediated by ATM-kinase dependent phosphorylation. Free Radic. Biol. Med. 113, 1–15 (2017).
Qian, J. et al. Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radic. Biol. Med. 52, 1806–1819 (2012).
Shen, J. et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 32, 1000–1017 (2020).
Lee, D. et al. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 11, 1838 (2020).
Swatek, K. N. & Komander, D. Ubiquitin modifications. Cell Res. 26, 399–422 (2016).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Mithoe, S. C. & Menke, F. L. Regulation of pattern recognition receptor signalling by phosphorylation and ubiquitination. Curr. Opin. Plant Biol. 45, 162–170 (2018).
Lu, D. et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439–1442 (2011).
Ma, X. et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 581, 199–203 (2020).
Liao, D. et al. Arabidopsis E3 ubiquitin ligase PLANT U-BOX13 (PUB13) regulates chitin receptor LYSIN MOTIF RECEPTOR KINASE5 (LYK5) protein abundance. New Phytol. 214, 1646–1656 (2017).
Li, Q.-Y., Li, P., Myint Phyu Sin Htwe, N., Shangguan, K.-K. & Liang, Y. Antepenultimate residue at the C-terminus of NADPH oxidase RBOHD is critical for its function in the production of reactive oxygen species in Arabidopsis. J. Zhejiang Univ. Sci. B 20, 713–727 (2019).
Acknowledgements
B.C. and G.C. are supported by a grant from the National Institutes of Health (NIH 1R35GM136402). B.C. is partially supported by the University of California, Davis Dean’s Distinguished Graduate Fellowship. M.W. acknowledges funding from the Academy of Finland (Decision 323917). S.K. is supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant 20K05831).
Author information
Authors and Affiliations
Contributions
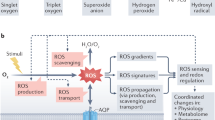
All authors contributed to writing the manuscript and conceiving the ideas presented. G.C. and M.W. led the structure and ideas in the manuscript and finalized the writing. S.K. generated Fig. 1. B.C. generated Fig. 2 and portions of Fig. 3. D.M.S. generated Fig. 3a,b and Supplementary Fig. 1a,b. M.C. generated the models in Fig. 3 and Supplementary Fig. 1.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks John Hancock and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1, methods and references.
Rights and permissions
About this article
Cite this article
Castro, B., Citterico, M., Kimura, S. et al. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 7, 403–412 (2021). https://doi.org/10.1038/s41477-021-00887-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00887-0
This article is cited by
-
Commensal lifestyle regulated by a negative feedback loop between Arabidopsis ROS and the bacterial T2SS
Nature Communications (2024)
-
Decoding early stress signaling waves in living plants using nanosensor multiplexing
Nature Communications (2024)
-
Glucosinolate O-methyltransferase mediated callus formation and affected ROS homeostasis in Arabidopsis thaliana
Physiology and Molecular Biology of Plants (2024)
-
Protective role of manganese, proline and rice straw extract in wheat against drought driven oxidative stress
Acta Physiologiae Plantarum (2024)
-
Drought and heat stress: insights into tolerance mechanisms and breeding strategies for pigeonpea improvement
Planta (2024)